Effect of Oral Administration with Lactobacillus plantarum CAM6 on the Hematological Profile, Relative Weight of Digestive Organs, and Cecal Traits in Growing Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Probiotic Preparation, Animals, and Experimental Treatments
2.3. Housing and Experimental Conditions
2.4. Blood Profiles
2.5. Intestinal Tissue Sampling and Histomorphology Measurement
2.6. Cecal Microbiology
2.7. Statistical Analysis
3. Results
3.1. Hematological Parameters
3.2. Relative Weight of the Digestive Organs
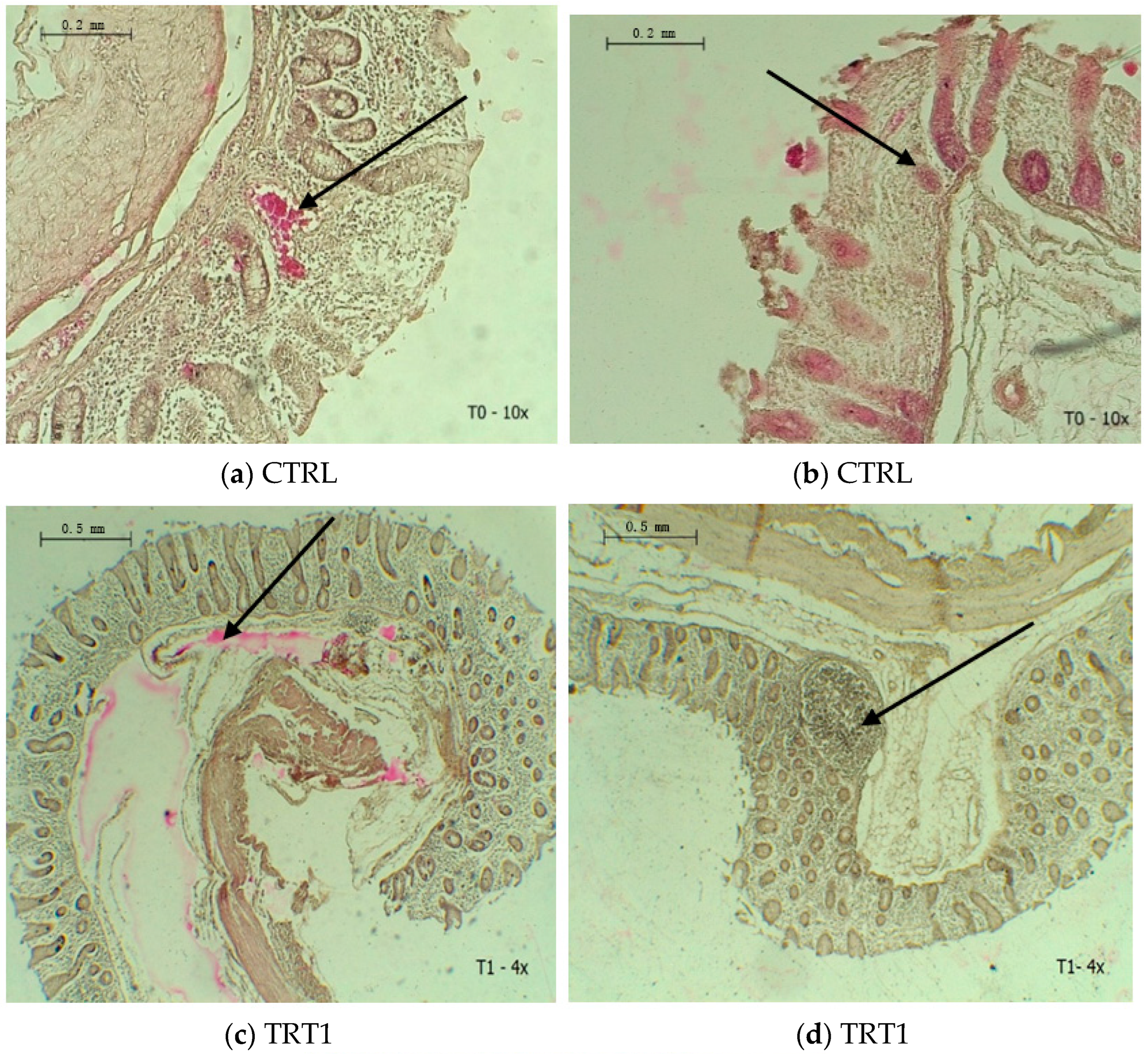
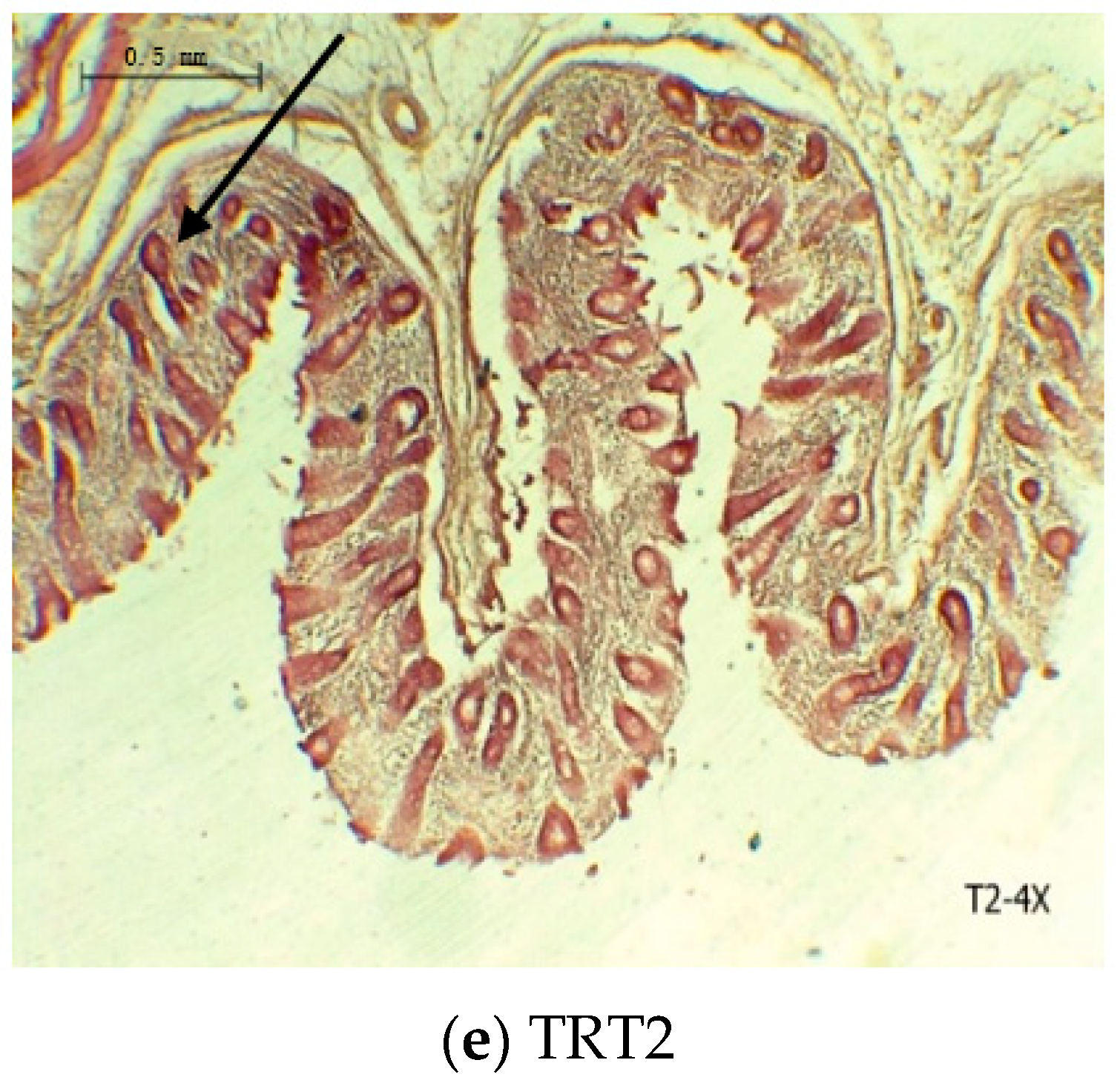
3.3. Cecal Histomorphology
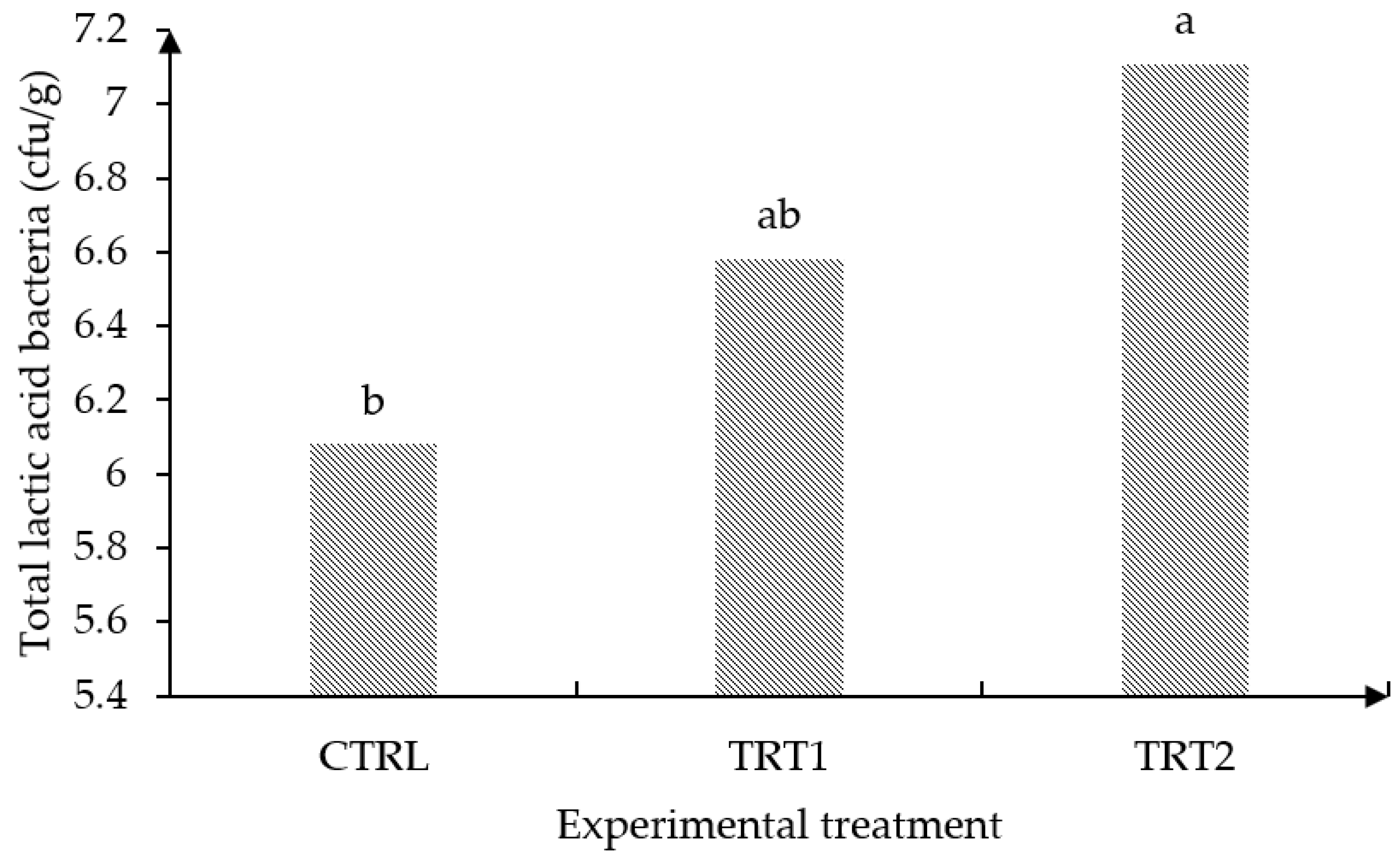
3.4. Cecal Microbiology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassaletta, L.; Estellés, F.; Beusen, A.; Bouwman, L.; Calvet, S.; van Grinsven, A.; Doelman, J.; Stehfest, E.; Uwizeye, A.; Westhoek, H. Future global pig production systems according to the Shared Socioeconomic Pathways. Sci. Total Environ. 2019, 665, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, B.C.; Hayes, M.D.; Condotta, I.C.; Leonard, S.M. Impact of housing environment and management on pre-/post-weaning piglet productivity. J. Anim. Sci. 2022, 100, skac142. [Google Scholar] [CrossRef]
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Sec. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Tsukahara, T.; Inoue, R.; Nakatani, M.; Fukuta, K.; Kishino, E.; Ito, T. Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine. Anim. Sci. J. 2015, 87, 67–75. [Google Scholar] [CrossRef]
- Dlamini, Z.C.; Langa, R.L.S.; Aiyegoro, O.A.; Okoh, A.I. Effects of probiotics on growth performance, blood parameters, and antibody stimulation in piglets. S. Afr. J. Anim. Sci. 2017, 47, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Pohl, C.S.; Medland, J.E.; Mackey, E.; Edwards, L.L.; Bagley, K.D.; DeWilde, M.P. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. J. Neurogastroenterol. Motil. 2017, 29, 1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speight, R.E.; Navone, L.; Gebbie, L.K.; Blinco, J.A.L.; Bryden, W.L. Platforms to accelerate biomanufacturing of enzyme and probiotic animal feed supplements: Discovery considerations and manufacturing implications. Anim. Prod. Sci. 2022, 62, 1113–1128. [Google Scholar] [CrossRef]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.Y.; Choi, Y.J.; Lee, H.K.; Hur, J.; Park, B.-Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [Green Version]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Martín Orúe, S.M.; Castillejos, L. Practical aspects of the use of probiotics in pig production: A review. Livest. Sci. 2019, 223, 84–96. [Google Scholar] [CrossRef]
- Betancur, C.; Martínez, Y.; Merino-Guzman, R.; Hernandez-Velasco, X.; Castillo, R.; Rodríguez, R.; Tellez-Isaias, G. Evaluation of oral administration of Lactobacillus plantarum CAM6 strain as an alternative to antibiotics in weaned pigs. Animals 2020, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Betancur, C.; Martínez, Y.; Tellez-Isaias, G.; Castillo, R.; Ding, X. Effect of oral administration with Lactobacillus plantarum CAM6 strain on sows during gestation-lactation and the derived impact on their progeny performance. Mediat. Inflamm. 2021, 2021, 6615960. [Google Scholar] [CrossRef]
- Betancur, C.A.; Rondon, A.J.; Martinez, Y.; Rodriguez, R. Formulation and characterization of a bioprepration with Lactobacillus plantarum CAM6 from the gastrointestinal tract of Colombian native pigs. Cuban J. Agric. Sci. 2020, 54, 1–10. [Google Scholar]
- Betancur, C.; Martínez, Y.; Tellez-Isaias, G.; Avellaneda, C.; Velázquez-Martí, B. In vitro characterization of indigenous probiotic strains isolated from colombian creole pigs. Animals 2020, 10, 1204. [Google Scholar] [CrossRef]
- NRC. National Research Council. The Nutrient Requirements of Swine; Eighth Revised Edition; National Academy Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef] [Green Version]
- SPSS. IBM-International Business Machine. Statistics Standard Edition 21; Armonk: Nueva York, NY, USA, 2014; Available online: http://www-03.ibm.com/press/us/en/background.wss (accessed on 15 February 2022).
- Perri, A.M.; O’Sullivan, T.L.; Harding, J.C.; Wood, R.D.; Friendship, R.M. Hematology and biochemistry reference intervals for Ontario commercial nursing pigs close to the time of weaning. Can. Vet. J. Rev. 2017, 58, 371–376. [Google Scholar]
- Vasquez, R.; Oh, J.K.; Song, J.H.; Kang, D.K. Gut microbiome-produced metabolites in pigs: A review on their biological functions and the influence of probiotics. J. Anim. Sci. Techn. 2022, 64, 671. [Google Scholar] [CrossRef] [PubMed]
- Larivière-Gauthier, G.; Thibodeau, A.; Letellier, A.; Yergeau, E.; Fravalo, P.; Larrivière-Gauthier, G. Salmonella shedding status of the sow affects the microbiota of their piglets at weaning. J. Appl. Microbiol. 2018, 126, 411–423. [Google Scholar] [CrossRef]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The diversity of escherichia coli pathotypes and vaccination strategies against this versatile bacterial pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Fuglsang, E.; Jiang, P.; Birck, M.M.; Pan, X.; Kamal, S.B.; Sangild, P.T. Oral antibiotics increase blood neutrophil maturation and reduce bacteremia and necrotizing enterocolitis in the immediate postnatal period of preterm pigs. Innate Immun. 2015, 22, 51–62. [Google Scholar] [CrossRef]
- Foster, N.; Lovell, M.A.; Marston, K.L.; Hulme, S.D.; Frost, A.J.; Bland, P.; Barrow, P.A. Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leukocytes by avirulent Salmonella enterica. Infect. Imm. 2012, 71, 2182–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.W.; Lee, T.T.; Shih, Y.C.; Yu, B. Effects of dietary supplementation of Chinese medicinal herbs on polymorphonuclear neutrophil immune activity and small intestinal morphology in weanling pigs. J. Anim. Physiol. Anim. Nutr. 2012, 96, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufarelli, V.; Crovace, A.; Rossi, G.; Laudadio, V. Effect of a dietary probiotic blend on performance, blood characteristics, meat quality and faecal microbial shedding in growing-finishing pigs. South Afr. J. Anim. Sci. 2017, 47, 875. [Google Scholar] [CrossRef] [Green Version]
- Thorn, C.E.; Bowman, A.S.; Eckersall, D. Hematology of Pigs. In Schalm’s Veterinary Hematology, 7th ed.; Wiley Library: Saint Paul, MN, USA, 2022; pp. 1019–1025. [Google Scholar]
- Niewold, T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007, 86, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, D.C. Facing a new challenge: The adverse effects of antibiotics on gut microbiota and host immunity. Chin. Med. J. 2019, 132, 1135–1138. [Google Scholar] [CrossRef]
- Méhi, O.; Bogos, B.; Csörgő, B.; Pál, F.; Nyerges, Á.; Papp, B.; Pál, C. Perturbation of iron homeostasis promotes the evolution of antibiotic resistance. Mol. Biol. Evol. 2014, 31, 2793–2804. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Kim, I.H. Effect of probiotics supplementation in diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, faecal microbial population and faecal noxious gas content in growing pigs. J. App. Anim. Res. 2013, 41, 23–28. [Google Scholar] [CrossRef]
- Ayala, D.I.; Chen, J.C.; Bugarel, M.; Loneragan, G.H.; den Bakker, H.C.; Kottapalli, K.R.; Nightingale, K.K. Molecular detection and quantification of viable probiotic strains in animal feedstuffs using the commercial direct fed microbial Lactobacillus animalis NP51 as a model. J. Microbiol. Methods 2018, 149, 36–43. [Google Scholar] [CrossRef]
- Hou, C.; Zeng, X.; Yang, F.; Liu, H.; Hou, S.Q. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. J. Anim. Sci. Biotechnol. 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilieborg, M.S.; Thymann, T.; Siggers, R.; Boye, M.; Bering, S.B.; Jensen, B.B.; Sangild, P.T. The incidence of necrotizing enterocolitis is increased following probiotic administration to preterm pigs. J. Nutr. Sci. 2011, 141, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Matur, E.; Eraslan, E. The impact of probiotics on the gastrointestinal physiology. In New Advances in the Basic and Clinical Gastroenterology; Brzozowski, T., Ed.; IntechOpen: London, UK, 2012; Volume 1, pp. 51–74. [Google Scholar]
- Pu, G.; Hou, L.; Du, T.; Zhou, W.; Liu, C.; Niu, P.; Li, P. Increased proportion of fiber-degrading microbes and enhanced cecum development jointly promote host to digest appropriate high-fiber diets. Msystems 2022, 8, e00937-22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gao, M.; Zhang, R. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell. Fact. 2017, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Ko, H.; Hosseindoust, A.; Choi, Y.; Chae, B.; Yu, D. Lactobacillus-based fermentation product and lactose level in the feed for weanling pigs: Effects on intestinal morphology, microbiota, gas emission, and targeted intestinal coliforms. Livest. Sci. 2019, 227, 90–96. [Google Scholar] [CrossRef]
- Verdile, N.; Mirmahmoudi, R.; Brevini, T.A.L.; Gandolfi, F. Evolution of pig intestinal stem cells from birth to weaning. Animal 2019, 13, 2830–2839. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Kiesz, M. Dietary fermented rapeseed or/and soybean meal additives on performance and intestinal health of piglets. Sci. Rep. 2021, 11, 16952. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Houy, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef]
- Giang, H.H.; Viet, T.Q.; Ogle, B.; Lindberg, J.E. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with a complex of lactic acid bacteria alone or in combination with Bacillus subtilis and Saccharomyces boulardii. Livest. Sci. 2012, 143, 132–141. [Google Scholar] [CrossRef]
- Yoshida, Y.; Tsukahara, T.; Ushida, K. Oral administration of Lactobacillus plantarum Lq80 and Megasphaera elsdenii iNP-001 induces efficient recovery from mucosal atrophy in the small and the large intestines of weaning piglets. Anim. Sci. J. 2009, 80, 709–715. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Pérez, C.; Vasco, B.; Colina, I.; Machado, I.; Rossini, M.; Arrieta, D. Effect of the addition of enzyme complexes in sorghum (Sorghum bicolor) based diets on intestinal integrity of broilers. Rev. Cient. Fac. Cienc. Vet. Univ. Zulia. 2013, 1, 59–66. [Google Scholar]
- Abo-Shaban, T.; Sharna, S.S.; Hosie, S.; Lee, C.Y.Q.; Balasuriya, G.K.; McKeown, S.J.; Hill-Yardin, E.L. Issues for patchy tissues: Defining roles for gut-associated lymphoid tissue in neurodevelopment and disease. J. Neural Transm. 2022, 130, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bosque, A.; Polo, J.; Torrallardona, D. Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety. Porc. Health Managt. 2016, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechn. 2013, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Baikui, W.; Gong, L.; Yuanhao, Z.; Tang, J.; Zeng, Z.; Zou, P.; Dongyou, Y.; Weifen, L.; Wang, Q. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021, 7, 829–840. [Google Scholar] [CrossRef]
- Vigors, S.; O’Doherty, J.V.; Kelly, A.K.; O’Shea, C.J.; Sweeney, T. The effect of divergence in feed efficiency on the intestinal microbiota and the intestinal immune response in both unchallenged and lipopolysaccharide challenged ileal and colonic explants. PLoS ONE 2016, 11, e0148145. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Zhou, H.; Wang, L.; Ding, S.; Wang, Y.; Li, A. Effects of microencapsulated Lactobacillus plantarum and fructooligosaccharide on growth performance, blood immune parameters, and intestinal morphology in weaned piglets. Food Agric. Immunol. 2018, 29, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Vera, R.; Ormaza, J.; Muñoz, J.; Arteaga, F.; Sánchez, L. Lactobacillus plantarum strains with probiotic potentials isolated from creole pigs. Rev. Salud Anim. 2018, 40, 1–12. [Google Scholar]
- Peng, Q.; Zeng, X.F.; Zhu, J.L.; Wang, S.; Liu, X.T.; Hou, C.L.; Thacker, P.A.; Qia, S.Y. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult. Sci. 2016, 95, 893–900. [Google Scholar] [CrossRef]
- Suo, C.; Yin, Y.; Wang, X.; Lou, X.; Song, D.; Wang, X.; Gu, Q. Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet. Res. 2012, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Lähteinen, T.; Rinttilä, T.; Koort, J.M.K.; Kant, R.; Levonen, K.; Jakava-Viljanen, M.; Palva, A. Effect of a multispecies Lactobacillus formulation as a feeding supplement on the performance and immune function of piglets. Liv. Sci. 2015, 180, 164–171. [Google Scholar] [CrossRef]
- Takahashi, S.; Egawa, Y.; Simojo, N.; Tsukahara, T.; Ushida, K. Oral administration of Lactobacillus plantarum strain Lq80 to weaning piglets stimulates the growth of indigenous lactobacilli to modify the lactobacillal population. J. Gen. Appl. Microbiol. 2007, 53, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Ingredients | % |
|---|---|
| Cornmeal | 41.24 |
| Full-fat soybean meal | 16.00 |
| Wheat bran | 18.29 |
| Defatted soybean meal | 13.47 |
| Sorghum | 7.00 |
| Premix 1 | 4.00 |
| Nutritional contributions | |
| Crude protein, % | 18.0 |
| Calcium, % | 1.13 |
| Phosphorus total, % | 0.95 |
| Metabolizable energy, MJ/kg | 13.39 |
| Experimental Treatments | ||||||
|---|---|---|---|---|---|---|
| Items | CTRL | TRT1 | TRT2 | SEM± | p Value | Normal Parameters [18] |
| Leukocytes, 109/L | 14.36 b | 17.78 a | 15.17 b | 1.1008 | 0.037 | 11.6–32.9 |
| Lymphocytes, 109/L | 4.49 b | 5.58 a | 4.67 b | 0.264 | 0.019 | 3.6–18.5 |
| Monocytes, 109/L | 1.27 | 1.53 | 1.25 | 0.150 | 0.587 | 0.0–4.9 |
| Granulocytes, 109/L | 8.60 b | 10.66 a | 9.25 ab | 0.651 | 0.012 | 0.3–15.2 |
| Eosinophils, 109/L | 0.38 | 0.46 | 0.38 | 0.102 | 0.086 | 0.0–2.5 |
| Erythrocytes, 1012/L | 8.99 | 8.08 | 8.56 | 0.369 | 0.060 | 5.7–8.3 |
| Hb, g/dL | 13.18 a | 11.42 b | 13.09 a | 0.495 | 0.050 | 10–15 |
| Hto, % | 45.27 | 40.66 | 44.73 | 1.593 | 0.133 | 29–46 |
| MCV, f/L | 50.25 | 50.13 | 52.25 | 0.799 | 0.092 | 44–56 |
| MCH, pg | 14.70 | 14.16 | 15.25 | 0.608 | 0.447 | 15–20 |
| MCHC, g/dL | 29.16 | 28.07 | 29.21 | 0.511 | 0.657 | 32–38 |
| Platelets, 109/L | 798.75 | 742.38 | 656.38 | 50.107 | 0.507 | 171–833 |
| MPV, f/L | 14.79 | 14.86 | 13.70 | 0.417 | 0.183 | 7.2–15.6 |
| Treatments | |||||
|---|---|---|---|---|---|
| Indicators | CTRL | TRT1 | TRT2 | SEM± | p Value |
| Small intestine, g/100 g | 2.51 | 2.80 | 2.55 | 0.192 | 0.181 |
| Large intestine, g/100 g | 3.82 | 4.10 | 3.95 | 0.115 | 0.247 |
| Cecum, g/100 g | 0.28 b | 0.27 b | 0.35 a | 0.018 | 0.026 |
| Small intestine, cm | 16.51 b | 17.58 a | 17.24 a | 0.262 | 0.045 |
| Treatments | |||||
|---|---|---|---|---|---|
| Items | CTRL | TRT1 | TRT2 | SEM± | p Value |
| Mucous thickness, μm | 36.17 b | 37.75 b | 45.08 a | 0.414 | ˂0.001 |
| Muscle thickness, μm | 17.67 c | 25.67 b | 30.33 a | 0.417 | ˂0.001 |
| Crypts depth, μm | 20.08 c | 26.92 b | 36.25 a | 0.811 | ˂0.001 |
| Crypts width, μm | 7.00 c | 8.75 b | 10.42 a | 0.278 | ˂0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancur, C.; Martínez, Y. Effect of Oral Administration with Lactobacillus plantarum CAM6 on the Hematological Profile, Relative Weight of Digestive Organs, and Cecal Traits in Growing Pigs. Animals 2023, 13, 1915. https://doi.org/10.3390/ani13121915
Betancur C, Martínez Y. Effect of Oral Administration with Lactobacillus plantarum CAM6 on the Hematological Profile, Relative Weight of Digestive Organs, and Cecal Traits in Growing Pigs. Animals. 2023; 13(12):1915. https://doi.org/10.3390/ani13121915
Chicago/Turabian StyleBetancur, Cesar, and Yordan Martínez. 2023. "Effect of Oral Administration with Lactobacillus plantarum CAM6 on the Hematological Profile, Relative Weight of Digestive Organs, and Cecal Traits in Growing Pigs" Animals 13, no. 12: 1915. https://doi.org/10.3390/ani13121915





