Effects of Dietary Lactic Acid Supplementation on the Activity of Digestive and Antioxidant Enzymes, Gene Expressions, and Bacterial Communities in the Intestine of Common Carp, Cyprinus carpio
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Experimental Protocol
2.3. Sampling and Preservation
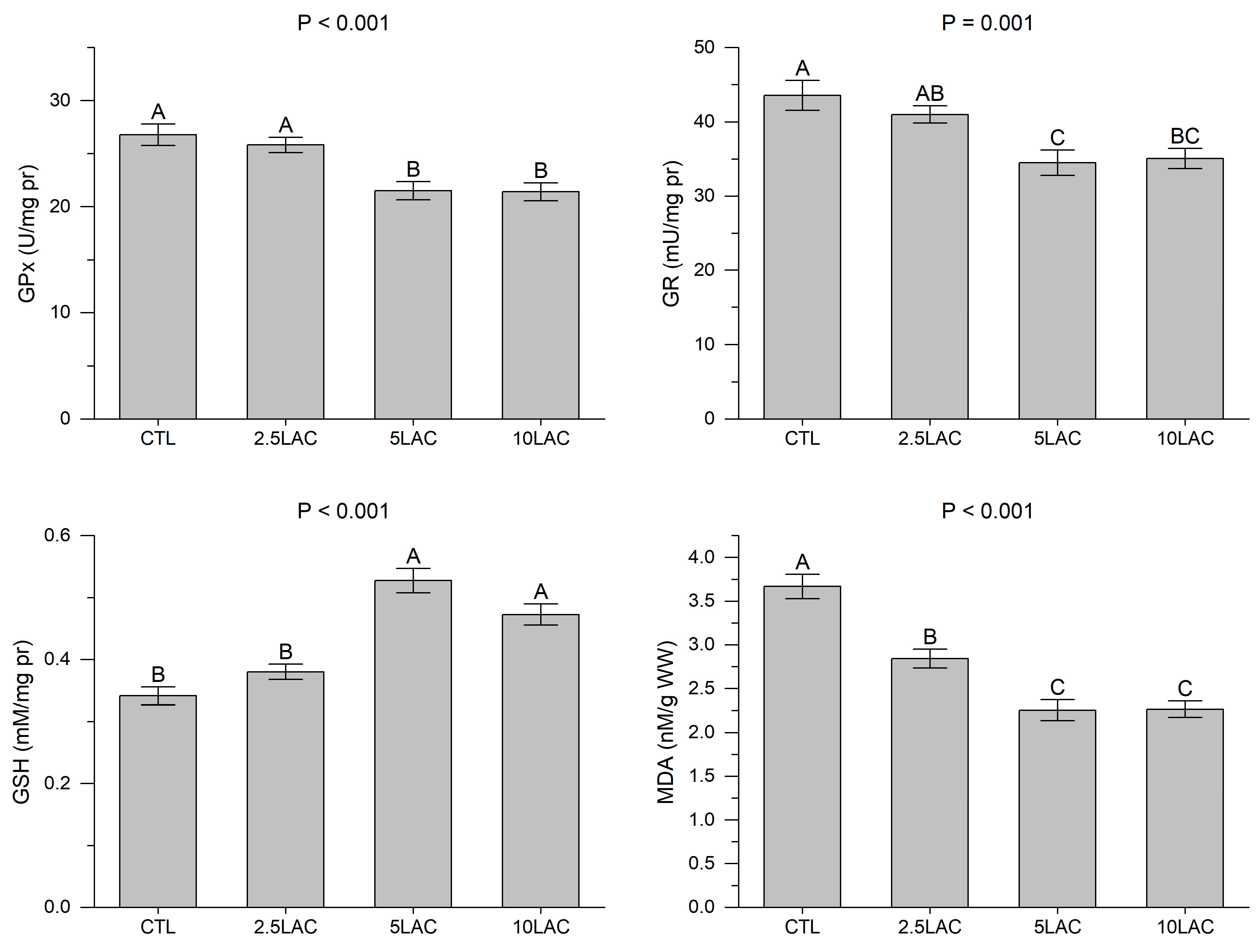
2.3.1. Hepatic Antioxidant Assays
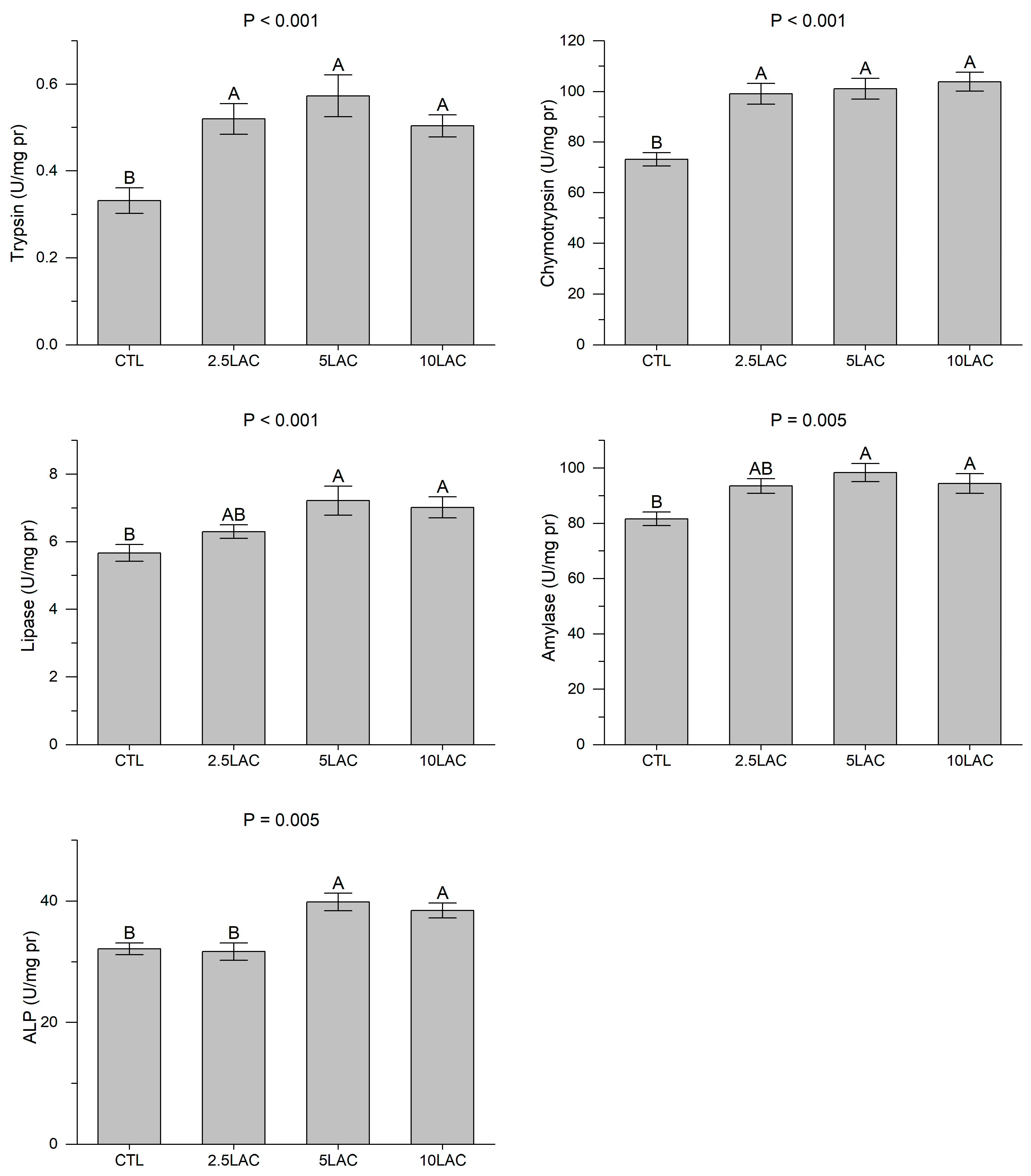
2.3.2. Digestive Enzymes’ Assay
2.3.3. Intestinal Gene Expression
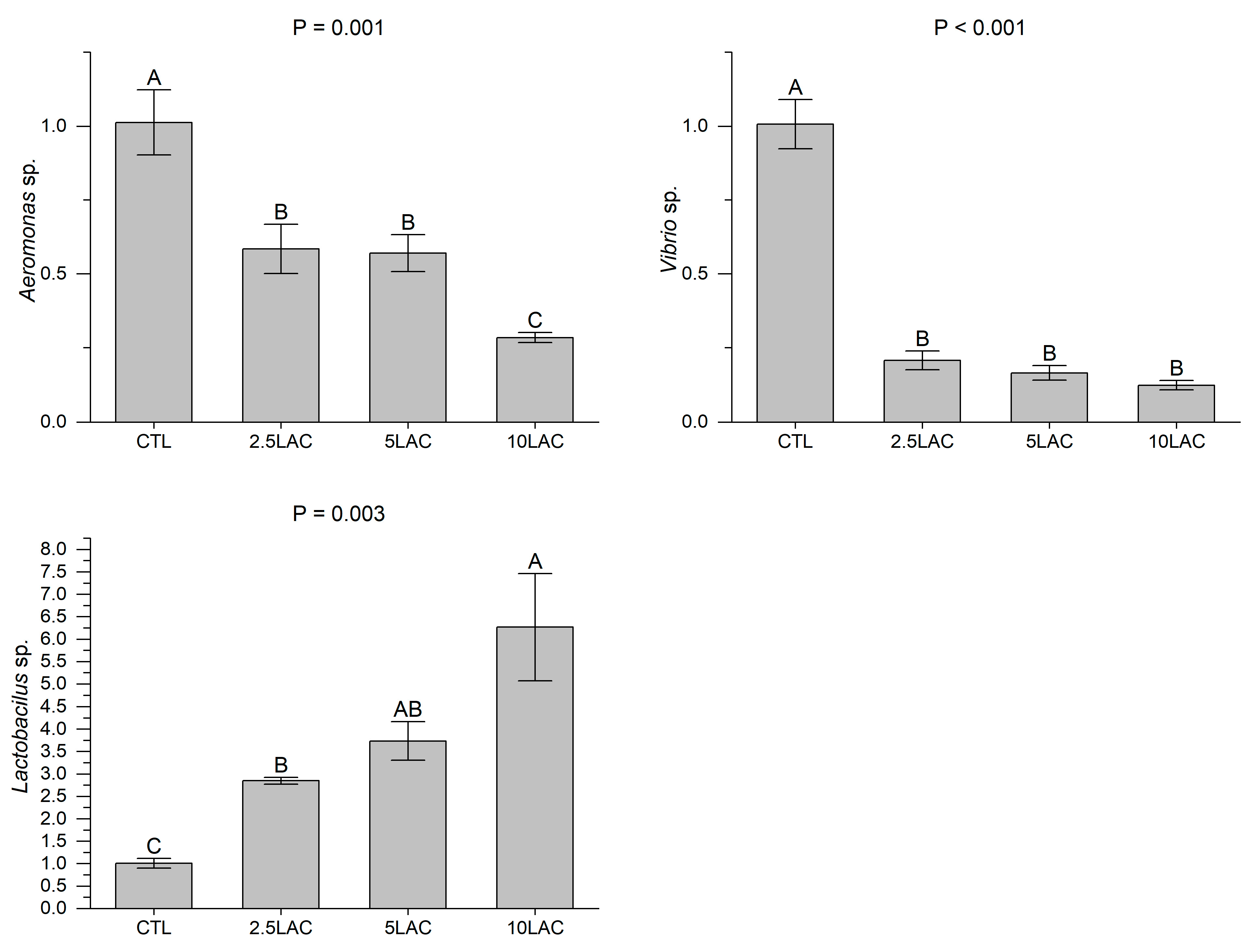
2.3.4. Intestinal Bacterial Genus Populations
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wassef, E.A.; Abdel-Momen, S.A.-G.; Saleh, N.E.-S.; Al-Zayat, A.M.; Ashry, A.M. Is sodium diformate a beneficial feed supplement for European seabass (Dicentrarchus labrax)? Effect on growth performance and health status. Egypt. J. Aquat. Res. 2017, 43, 229–234. [Google Scholar] [CrossRef]
- Ringø, E.; Olsen, R.; Gifstad, T.; Dalmo, R.; Amlund, H.; Hemre, G.I.; Bakke, A. Prebiotics in aquaculture: A review. Aquac. Nutr. 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Ringø, E.; Song, S.K. Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquac. Nutr. 2016, 22, 4–24. [Google Scholar] [CrossRef]
- Luckstadt, C. The use of acidifiers in fish nutrition. CABI Rev. 2008, 3, 8pp. [Google Scholar] [CrossRef] [Green Version]
- Reda, R.M.; Mahmoud, R.; Selim, K.M.; El-Araby, I.E. Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 50, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, X.; Zhai, S. Effect of dietary compound acidifiers supplementation on growth performance, serum biochemical parameters, and body composition of juvenile American eel (Anguilla rostrata). Fishes 2022, 7, 203. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Zhang, J.; Gatlin, D.M.; Ringø, E.; Zhou, Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014, 112, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, V.; Schmidt, J.; Slater, M.; Zentek, J.; Buck, B.; Steinhagen, D. The effect of supplementation with polysaccharides, nucleotides, acidifiers and Bacillus strains in fish meal and soy bean based diets on growth performance in juvenile turbot (Scophthalmus maximus). Aquaculture 2015, 437, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Assan, D.; Kuebutornye, F.K.A.; Hlordzi, V.; Chen, H.; Mraz, J.; Mustapha, U.F.; Abarike, E.D. Effects of probiotics on digestive enzymes of fish (finfish and shellfish); status and prospects: A mini review. Com. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110653. [Google Scholar] [CrossRef]
- Karataş, S.; Turgay, E.; Yıldız, M.; Kaiza, V.E.; Yardımcı, R.E.; Steinum, T.M. Mucosal bacteriomes of rainbow trout (Oncorhynchus mykiss) intestines are modified in response to dietary phytase. Aquaculture 2023, 574, 739672. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, S.; Liu, Y.; Yang, P.; Ai, Q.; Zhang, W.; Xu, W.; Zhang, Y.; Zhang, Y.; Mai, K. Dietary citric acid supplementation alleviates soybean meal-induced intestinal oxidative damage and micro-ecological imbalance in juvenile turbot, Scophthalmus maximus L. Aquac. Res. 2018, 49, 3804–3816. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Sinha, R.; Fazel, A.; Khosraviani, K.; Delavar, F.H.; Arghideh, M.; Sedaghat, M.; Paolucci, M.; Hoseinifar, S.H.; Van Doan, H. Histopathological damage and stress- and immune-related genes’ expression in the intestine of common carp, Cyprinus carpio exposed to copper and polyvinyl chloride microparticle. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Anuta, J.D.; Buentello, A.; Patnaik, S.; Lawrence, A.L.; Mustafa, A.; Hume, M.E.; Gatlin, D.M., III; Kemp, M.C. Effect of dietary supplementation of acidic calcium sulfate (Vitoxal) on growth, survival, immune response and gut microbiota of the Pacific white shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2011, 42, 834–844. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Rajabiesterabadi, H.; Abbasi, M.; Khosraviani, K.; Hoseinifar, S.H.; Van Doan, H. Modulation of humoral immunological and antioxidant responses and gut bacterial community and gene expression in rainbow trout, Oncorhynchus mykiss, by dietary lactic acid supplementation. Fish Shellfish Immunol. 2022, 125, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Shang, Z.-H.; Wu, M.-X.; Zhang, L.-J.; Zhang, Y.-L. Regulation of Rhesus glycoprotein-related genes in large-scale loach Paramisgurnus dabryanus during ammonia loading. Ecotoxicol. Environ. Saf. 2022, 244, 114077. [Google Scholar] [CrossRef] [PubMed]
- Safari, O.; Paolucci, M.; Ahmadniaye Motlagh, H. Effect of dietary encapsulated organic salts (Na-acetate, Na-butyrate, Na-lactate and Na-propionate) on growth performance, haemolymph, antioxidant and digestive enzyme activities and gut microbiota of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz, 1823. Aquac. Nutr. 2021, 27, 91–104. [Google Scholar]
- Matani Bour, H.A.; Esmaeili, M.; Abedian Kenari, A. Growth performance, muscle and liver composition, blood traits, digestibility and gut bacteria of beluga (Huso huso) juvenile fed different levels of soybean meal and lactic acid. Aquac. Nutr. 2018, 24, 1361–1368. [Google Scholar] [CrossRef]
- Ringø, E. Effects of dietary lactate and propionate on growth and digesta in Arctic charr, Salvelinus alpinus (L.). Aquaculture 1991, 96, 321–333. [Google Scholar] [CrossRef]
- Gislason, G.; Olsen, R.E.; Hinge, E. Comparative effects of dietary Na+-lactate on Arctic char, Salvelinus alpinus L., and Atlantic salmon, Salmo salar L. Aquac. Res. 1996, 27, 429–435. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020–Sustainability in Action; FAO: Rome, Italy, 2022. [Google Scholar]
- Weber, M.J.; Brown, M.L.; Willis, D.W. Spatial variability of common carp populations in relation to lake morphology and physicochemical parameters in the upper Midwest United States. Ecol. Freshw. Fish 2010, 19, 555–565. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Hoseini, S.M.; Mirghaed, A.T.; Iri, Y.; Ghelichpour, M. Effects of dietary cineole administration on growth performance, hematological and biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 766–772. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Seegers, S.; Cao, W.; Wanasundara, J.; Chen, J.; da Silva, A.E.; Ross, K.; Franco, A.L.; Vrijenhoek, T.; Bhowmik, P.; et al. An international collaborative study on trypsin inhibitor assay for legumes, cereals, and related products. J. Am. Oil Chem. Soc. 2021, 98, 375–390. [Google Scholar] [CrossRef]
- González-Félix, M.L.; De La Reé-Rodríguez, C.; Perez-Velazquez, M. Optimum activity and partial characterization of chymotrypsin from the sciaenids Cynoscion othonopterus, Cynoscion parvipinnis, and Cynoscion xanthulus. J. Aquat. Food Prod. Technol. 2021, 30, 670–682. [Google Scholar] [CrossRef]
- Ferreira, A.; Cahú, T.; Xu, J.; Blennow, A.; Bezerra, R. A highly stable raw starch digesting α-amylase from Nile tilapia (Oreochromis niloticus) viscera. Food Chem. 2021, 354, 129513. [Google Scholar] [CrossRef]
- Najm, T.A.; Walsh, M.K. Characterization of lipases from Geobacillus stearothermophilus and Anoxybacillus flavithermuscell lysates. Food. Nutr. Sci. 2022, 13, 238–251. [Google Scholar]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Uysal, E. Levels of transaminases, alkaline phosphatase, and protein in tissues of Clarias gariepienus fingerlings exposed to sublethal concentrations of cadmium chloride. Environ. Toxicol. 2008, 23, 672–678. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Tall, A.; Teillon, A.; Boisset, C.; Delesmont, R.; Touron-Bodilis, A.; Hervio-Heath, D. Real-time PCR optimization to identify environmental Vibrio spp. strains. J. Appl. Microbiol. 2012, 113, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-P.; Farrell, S.K.; Robinson, B.; Chu, K.-H. Development and application of real-time PCR assays for quantifying total and aerolysin gene-containing Aeromonas in source, intermediate, and finished drinking water. Environ. Sci. Technol. 2008, 42, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.C.; Xing, S.J.; Chen, P.; Wu, X.F.; Gu, X.; Luo, L.; Liang, X.F.; Xue, M. Plant protein diet-induced hypoimmunity by affecting the spiral valve intestinal microbiota and bile acid enterohepatic circulation in Amur sturgeon (Acipenser schrenckii). Fish Shellfish Immunol. 2020, 106, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Lückstädt, C.; Webster, C.D.; Kesius, P. Organic acids and their salts. In Dietary Nutrients, Additives, and Fish Health; Lee, C.-S., Lim, C., Webster, C.D., Eds.; Willey-Blackwell: Hoboken, NJ, USA; Blackwell-Publishing: Hoboken, NJ, USA, 2015; pp. 305–320. [Google Scholar]
- Lin, Y.-H.; Cheng, M.-Y. Effects of dietary organic acid supplementation on the growth, nutrient digestibility and intestinal histology of the giant grouper Epinephelus lanceolatus fed a diet with soybean meal. Aquaculture 2017, 469, 106–111. [Google Scholar] [CrossRef]
- Castillo, S.; Rosales, M.; Pohlenz, C.; Gatlin, D.M., III. Effects of organic acids on growth performance and digestive enzyme activities of juvenile red drum Sciaenops ocellatus. Aquaculture 2014, 433, 6–12. [Google Scholar] [CrossRef]
- Sotoudeh, E.; Sangari, M.; Bagheri, D.; Morammazi, S.; Torfi Mozanzadeh, M. Dietary organic acid salts mitigate plant protein induced inflammatory response and improve humoral immunity, antioxidative status and digestive enzyme activities in yellowfin seabream, Acanthopagrus latus. Aquac. Nutr. 2020, 26, 1669–1680. [Google Scholar] [CrossRef]
- Safari, O.; Sarkheil, M.; Shahsavani, D.; Paolucci, M. Effects of single or combined administration of dietary synbiotic and sodium propionate on humoral immunity and oxidative defense, digestive enzymes and growth performances of African cichlid (Labidochromis lividus) challenged with Aeromonas hydrophila. Fishes 2021, 4, 63. [Google Scholar] [CrossRef]
- Sotoudeh, E.; Saghaei, S.; Dehghani, M. Effects of dietary sodium citrate on growth performance, body composition and digestive enzymes activity of yellowfin seabream (Acanthopagrus latus) fingerling. Aquac. Sci. 2020, 8, 32–42. [Google Scholar]
- Barlaya, G.; Ananda Kumar, B.S.; Huchchappa, R.C.; Basumatary, P.; Kannur, H. Effect of fish meal replacement with toasted guar meal on growth, food conversion, digestive enzyme activity and final carcass composition of rohu, Labeo rohita. Aquac. Res. 2021, 52, 5551–5557. [Google Scholar] [CrossRef]
- Huan, D.; Li, X.; Yao, W.; Yang, H.; Liang, G.; Leng, X. Effects of organic acids (salt) supplementation in low fish meal diet on growth performance, digestive enzyme activities and nutrient apparent digestibility of Litopenaeus vannamei. Chin. J. Anim. Nutr. 2018, 30, 4526–4537. [Google Scholar]
- Yao, W.; Li, X.; Kabir Chowdhury, M.A.; Wang, J.; Leng, X. Dietary protease, carbohydrase and micro-encapsulated organic acid salts individually or in combination improved growth, feed utilization and intestinal histology of Pacific white shrimp. Aquaculture 2019, 503, 88–95. [Google Scholar] [CrossRef]
- Santigosa, E.; Sánchez, J.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J.; Gallardo, M.A. Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 2008, 282, 68–74. [Google Scholar] [CrossRef]
- Tibaldi, E.; Hakim, Y.; Uni, Z.; Tulli, F.; de Francesco, M.; Luzzana, U.; Harpaz, S. Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax). Aquaculture 2006, 261, 182–193. [Google Scholar] [CrossRef]
- Konietzny, U.; Greiner, R. Phytic acid|Nutritional impact. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4555–4563. [Google Scholar]
- Abbasi, A.; Oujifard, A.; Mozanzadeh, M.T.; Habibi, H.; Nafisi Bahabadi, M. Dietary simultaneous replacement of fish meal and fish oil with blends of plant proteins and vegetable oils in yellowfin seabream (Acanthopagrus latus) fry: Growth, digestive enzymes, antioxidant status and skin mucosal immunity. Aquac. Nutr. 2020, 26, 1131–1142. [Google Scholar] [CrossRef]
- Estensoro, I.; Ballester-Lozano, G.; Benedito-Palos, L.; Grammes, F.; Martos-Sitcha, J.A.; Mydland, L.-T.; Calduch-Giner, J.A.; Fuentes, J.; Karalazos, V.; Ortiz, Á. Dietary butyrate helps to restore the intestinal status of a marine teleost (Sparus aurata) fed extreme diets low in fish meal and fish oil. PLoS ONE 2016, 11, e0166564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Zhou, X.-Q.; Jiang, W.-D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 66, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Daeman, N.H.; Chong, C.M.; Karami, A.; Kumar, V.; Hoseinifar, S.H.; Romano, N. Comparing the effects of different dietary organic acids on the growth, intestinal short-chain fatty acids, and liver histopathology of red hybrid tilapia (Oreochromis sp.) and potential use of these as preservatives. Fish Physiol. Biochem. 2017, 43, 1195–1207. [Google Scholar] [CrossRef]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adineh, H.; Naderi, M.; Hamidi, M.K.; Harsij, M. Biofloc technology improves growth, innate immune responses, oxidative status, and resistance to acute stress in common carp (Cyprinus carpio) under high stocking density. Fish Shellfish Immunol. 2019, 95, 440–448. [Google Scholar] [CrossRef]
- Yusefi, M.; Mohammadiazarm, H.; Salati, A.P. Effects of dietary sodium diformate on growth performance, immunological and biochemical blood indices, antioxidant capacity, and thermal stress tolerance of juvenile common carp (Cprinus carpio). Aquac. Rep. 2022, 22, 100963. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. 4.13–Glutathione peroxidases. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 229–242. [Google Scholar]
- Cherian, D.A.; Peter, T.; Narayanan, A.; Madhavan, S.S.; Achammada, S.; Vynat, G.P. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J. Pharm. Bioallied Sci. 2019, 11, S297. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chang, K.; Chen, J.; Zhao, X.; Gao, S. Dietary sodium butyrate supplementation attenuates intestinal inflammatory response and improves gut microbiota composition in largemouth bass (Micropterus salmoides) fed with a high soybean meal diet. Fish Physiol. Biochem. 2021, 47, 1805–1819. [Google Scholar] [CrossRef]
- Ashouri, G.; Soofiani, N.M.; Hoseinifar, S.H.; Jalali, S.A.H.; Morshedi, V.; Valinassab, T.; Bagheri, D.; Van Doan, H.; Mozanzadeh, M.T.; Carnevali, O. Influence of dietary sodium alginate and Pediococcus acidilactici on liver antioxidant status, intestinal lysozyme gene expression, histomorphology, microbiota, and digestive enzymes activity, in Asian sea bass (Lates calcarifer) juveniles. Aquaculture 2020, 518, 734638. [Google Scholar] [CrossRef]
- Kong, Y.; Li, M.; Chu, G.; Liu, H.; Shan, X.; Wang, G.; Han, G. The positive effects of single or conjoint administration of lactic acid bacteria on Channa argus: Digestive enzyme activity, antioxidant capacity, intestinal microbiota and morphology. Aquaculture 2021, 531, 735852. [Google Scholar] [CrossRef]
- Peran, L.; Camuesco, D.; Comalada, M.; Nieto, A.; Concha, A.; Adrio, J.L.; Olivares, M.; Xaus, J.; Zarzuelo, A.; Galvez, J. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int. J. Colorectal Dis. 2006, 21, 737–746. [Google Scholar] [CrossRef]
- Pophaly, S.D.; Poonam, S.; Pophaly, S.D.; Kapila, S.; Nanda, D.K.; Tomar, S.K.; Singh, R. Glutathione biosynthesis and activity of dependent enzymes in food-grade lactic acid bacteria harbouring multidomain bifunctional fusion gene (gshF). J. Appl. Microbiol. 2017, 123, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Tomar, S.K.; De, S. Lactic acid bacteria as a cell factory for riboflavin production. Microb. Biotechnol. 2016, 9, 441–451. [Google Scholar] [CrossRef]
- Kumar, N. Dietary riboflavin enhances immunity and anti-oxidative status against arsenic and high temperature in Pangasianodon hypophthalmus. Aquaculture 2021, 533, 736209. [Google Scholar] [CrossRef]
- Olfat, N.; Ashoori, M.; Saedisomeolia, A. Riboflavin is an antioxidant: A review update. Br. J. Nutr. 2022, 128, 1887–1895. [Google Scholar]
- Hoseinifar, S.H.; Sun, Y.-Z.; Caipang, C.M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquac. Res. 2017, 48, 1380–1391. [Google Scholar]
- Alves Jesus, G.F.; Owatari, M.S.; Pereira, S.A.; Silva, B.C.; Syracuse, N.M.; Lopes, G.R.; Addam, K.; Cardoso, L.; Mouriño, J.L.P.; Martins, M.L. Effects of sodium butyrate and Lippia origanoides essential oil blend on growth, intestinal microbiota, histology, and haemato-immunological response of Nile tilapia. Fish Shellfish Immunol. 2021, 117, 62–69. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Yarahmadi, P.; Soltani, M.; Paknejad, H.; Hoseini, S.M. Dietary sodium butyrate (Butirex® C4) supplementation modulates intestinal transcriptomic responses and augments disease resistance of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 92, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ding, Q.; Wang, A.; Liu, Y.; Teame, T.; Ran, C.; Yang, Y.; He, S.; Zhou, W.; Olsen, R.E.; et al. Effects of dietary sodium acetate on food intake, weight gain, intestinal digestive enzyme activities, energy metabolism and gut microbiota in cultured fish: Zebrafish as a model. Aquaculture 2020, 523, 735188. [Google Scholar] [CrossRef]
- Addam, K.G.S.; Pereira, S.A.; Jesus, G.F.A.; Cardoso, L.; Syracuse, N.; Lopes, G.R.; Lehmann, N.B.; da Silva, B.C.; de Sá, L.S.; Chaves, F.C.M.; et al. Dietary organic acids blend alone or in combination with an essential oil on the survival, growth, gut/liver structure and de hemato-immunological in Nile tilapia Oreochromis niloticus. Aquac. Res. 2019, 50, 2960–2971. [Google Scholar] [CrossRef]
- Katya, K.; Park, G.; Bharadwaj, A.S.; Browdy, C.L.; Vazquez-Anon, M.; Bai, S.C. Organic acids blend as dietary antibiotic replacer in marine fish olive flounder, Paralichthys olivaceus. Aquac. Res. 2018, 49, 2861–2868. [Google Scholar] [CrossRef]
- Medina-Félix, D.; Garibay-Valdez, E.; Vargas-Albores, F.; Martínez-Porchas, M. Fish disease and intestinal microbiota: A close and indivisible relationship. Rev. Aquac. 2023, 15, 820–839. [Google Scholar] [CrossRef]
- Guo, M.; Wei, J.; Huang, X.; Huang, Y.; Qin, Q. Antiviral effects of β-defensin derived from orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 2012, 32, 828–838. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, M.-Y.; Jiang, Q.; Yin, L.; Zhou, X.-Q.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, J.; et al. Isoleucine improved growth performance, and intestinal immunological and physical barrier function of hybrid catfish Pelteobagrus vachelli × Leiocassis longirostris. Fish Shellfish Immunol. 2021, 109, 20–33. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, W.-D.; Jiang, J.; Zhao, J.; Liu, Y.; Zhang, Y.-A.; Zhou, X.-Q.; Feng, L. Dietary choline deficiency and excess induced intestinal inflammation and alteration of intestinal tight junction protein transcription potentially by modulating NF-κB, STAT and p38 MAPK signaling molecules in juvenile Jian carp. Fish Shellfish Immunol. 2016, 58, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Yin, L.; Li, J.-Y.; Li, Q.; Shi, D.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, Y.; et al. Glutamate attenuates lipopolysaccharide-induced oxidative damage and mRNA expression changes of tight junction and defensin proteins, inflammatory and apoptosis response signaling molecules in the intestine of fish. Fish Shellfish Immunol. 2017, 70, 473–484. [Google Scholar] [CrossRef]
- Niu, C.J.; Rummer, J.L.; Brauner, C.J.; Schulte, P.M. Heat shock protein (Hsp70) induced by a mild heat shock slightly moderates plasma osmolarity increases upon salinity transfer in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 437–444. [Google Scholar] [CrossRef]
- Vijayan, M.M.; Pereira, C.; Forsyth, R.B.; Kennedy, C.J.; Iwama, G.K. Handling stress does not affect the expression of hepatic heat shock protein 70 and conjugation enzymes in rainbow trout treated with β-naphthoflavone. Life Sci. 1997, 61, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Wang, H.-P.; Yao, H.; Shen, Z.-G.; Shaheen, A.A.; Abou-ElGheit, E.N. Expression of Hsp70, Igf1, and three oxidative stress biomarkers in response to handling and salt treatment at different water temperatures in yellow perch, Perca flavescens. Front. Physiol. 2017, 8, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefi, M.; Ghafarifarsani, H.; Raissy, M.; Yilmaz, S.; Vatnikov, Y.A.; Kulikov, E.V. Effects of dietary malic acid supplementation on growth performance, antioxidant and immunological parameters, and intestinal gene expressions in rainbow trout, Oncorhynchus mykiss. Aquaculture 2023, 563, 738864. [Google Scholar] [CrossRef]
- Willemsen, L.; Koetsier, M.; Van Deventer, S.; Van Tol, E. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [Green Version]
- Onrust, L.; Ducatelle, R.; Van Driessche, K.; De Maesschalck, C.; Vermeulen, K.; Haesebrouck, F.; Eeckhaut, V.; Van Immerseel, F. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front. Vet. Sci. 2015, 2, 75. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Calduch-Giner, J.A.; Fouz, B.; Estensoro, I.; Simó-Mirabet, P.; Puyalto, M.; Karalazos, V.; Palenzuela, O.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Under control: How a dietary additive can restore the gut microbiome and proteomic profile, and improve disease resilience in a marine teleostean fish fed vegetable diets. Microbiome 2017, 5, 164. [Google Scholar] [CrossRef] [Green Version]
- Jung-Schroers, V.; Adamek, M.; Harris, S.; Syakuri, H.; Jung, A.; Irnazarow, I.; Steinhagen, D. Response of the intestinal mucosal barrier of carp (Cyprinus carpio) to a bacterial challenge by Aeromonas hydrophila intubation after feeding with β-1,3/1,6-glucan. J. Fish Dis. 2018, 41, 1077–1092. [Google Scholar] [CrossRef]
- Neuhaus, H.; Van der Marel, M.; Caspari, N.; Meyer, W.; Enss, M.; Steinhagen, D. Biochemical and histochemical effects of perorally applied endotoxin on intestinal mucin glycoproteins of the common carp Cyprinus carpio. Dis. Aquat. Organ. 2007, 77, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sánchez, J.; Estensoro, I.; Redondo, M.J.; Calduch-Giner, J.A.; Kaushik, S.; Sitjà-Bobadilla, A. Mucins as diagnostic and prognostic biomarkers in a fish-parasite model: Transcriptional and functional analysis. PLoS ONE 2013, 8, e65457. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Dietary chitosan nanoparticles: Potential role in modulation of rainbow trout (Oncorhynchus mykiss) antibacterial defense and intestinal immunity against enteric redmouth disease. Mar. Drugs. 2021, 2, 72. [Google Scholar] [CrossRef]
- Adamek, M.; Hazerli, D.; Matras, M.; Teitge, F.; Reichert, M.; Steinhagen, D. Viral infections in common carp lead to a disturbance of mucin expression in mucosal tissues. Fish Shellfish Immunol. 2017, 71, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Taheri Mirghaed, A.; Yarahmadi, P.; Soltani, M.; Paknejad, H.; Kheirabadi, E.P. Beneficial effects of a sodium butyrate source on growth performance, intestinal bacterial communities, digestive enzymes, immune responses and disease resistance in rainbow trout (Oncorhynchus mykiss). Surv. Fish. Sci. 2022, 8, 1–15. [Google Scholar] [CrossRef]
- Gao, C.; Fu, Q.; Zhou, S.; Song, L.; Ren, Y.; Dong, X.; Su, B.; Li, C. The mucosal expression signatures of g-type lysozyme in turbot (Scophthalmus maximus) following bacterial challenge. Fish Shellfish Immunol. 2016, 54, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Sahoo, P. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Li, S.; Heng, X.; Guo, L.; Lessing, D.J.; Chu, W. SCFAs improve disease resistance via modulate gut microbiota, enhance immune response and increase antioxidative capacity in the host. Fish Shellfish Immunol. 2022, 120, 560–568. [Google Scholar] [CrossRef]

| Dietary Lactic Acid Levels (g/kg) | ||||
|---|---|---|---|---|
| 0 (CTL) | 2.5 (2.5LAC) | 5 (5LAC) | 10 (10LAC) | |
| Corn meal 1 | 100.0 | 100.0 | 100.0 | 100.0 |
| Wheat flour 2 | 280.0 | 277.5 | 275.0 | 270.0 |
| Soybean oilcake 3 | 200.0 | 200.0 | 200.0 | 200.0 |
| Poultry by-product 4 | 380.0 | 380.0 | 380.0 | 380.0 |
| Plant oil (corn oil + sunflower oil; 1:1 ratio) | 20.0 | 20.0 | 20.0 | 20.0 |
| Vitamin + Mineral premix 5 | 15.0 | 15.0 | 15.0 | 15.0 |
| Methionine 6 | 3.0 | 3.0 | 3.0 | 3.0 |
| Lysine 7 | 2.0 | 2.0 | 2.0 | 2.0 |
| Lactic acid 8 | 0.0 | 2.50 | 5.0 | 10.0 |
| Proximate composition (g/kg) | ||||
| Moisture | 88.6 | 87.4 | 87.3 | 87.9 |
| Crude protein | 381 | 384 | 386 | 379 |
| Crude fat | 105 | 108 | 105 | 107 |
| Crude ash | 53.4 | 54.0 | 53.4 | 55.0 |
| Crude fiber | 42.1 | 42.0 | 41.0 | 42.3 |
| Crude energy (kcal/g) | 3789 | 3806 | 3798 | 3794 |
| Primer | Sequence (5-3) | Length | Tm | Amplicon (bp) | Accession No. | Amplification Efficiency | |
|---|---|---|---|---|---|---|---|
| hsp | F | ATGTTGCCTTCACAGACACTG | 21 | 60 | 120 | XM_042720446.1 | 1.67 |
| R | GGTCATCAAACTTTCTGCCGA | 21 | 60 | ||||
| muc2 | F | ATTGGCATTGAGTTCACCGAG | 21 | 60 | 135 | XM_042752573.1 | 1.86 |
| R | GACAGTGATGCCCATTTTGGA | 21 | 60 | ||||
| muc5 | F | TGTGTGAGCATGGGGTGTATA | 21 | 60 | 141 | XM_052598245.1 | 1.85 |
| R | CTGTTGAACTTGCTCTCCAGG | 21 | 60 | ||||
| lys | F | CAGGTGGAAAGAACAAGTGCA | 21 | 60 | 150 | XM_019104788.1 | 1.78 |
| R | ACATCTTACGCCCCTTACAGT | 21 | 60 | ||||
| def | F | GCAAAGAGAATGAGGCTGTGT | 21 | 60 | 132 | JF343439.1 | 1.84 |
| R | CACAGCACAAAAATCCCTTGC | 21 | 60 | ||||
| tnfa | F | GAACAATCAGGAAGGCGGAAA | 21 | 60 | 128 | XM_019088899.1 | 1.69 |
| R | GGGTTTCTGTGGACACTTCAG | 20 | 60 | ||||
| il1b | F | CATTGCTTGTACCCAGTCTGG | 21 | 60 | 121 | XM_042733144.1 | 1.82 |
| R | TCTGAAGAAGAGGAGGCTGTC | 21 | 60 | ||||
| beta-actin | F | TCTGCTATGTGGCTCTTGACT | 21 | 60 | 118 | XM_042721308.1 | 1.94 |
| R | AACCTCTCATTGCCAATGGTG | 21 | 60 | ||||
| Bacterium | Name | Sequences | Reference |
|---|---|---|---|
| Lactobacillus sp. | Lacto-F | TGGAAACAGRTGCTAATACCG | [31] |
| Lacto-R | GTCCATTGTGGAAGATTCCC | ||
| Vibrio sp. | Vibrio-F | GGCGTAAAGCGCATGCAGGT | [32] |
| Vibrio-R | GAAATTCTACCCCCCTCTACAG | ||
| Aeromonas sp. | Aeromonas-F | GAGAAGGTGACCACCAAGAACA | [33] |
| Aeromonas-R | CTGACATCGGCCTTGAACTC | ||
| All bacteria | 338F | ACTCCTACGGGAGGCAGCAG | [34] |
| 518R | ATTACCGCGGCTGCTGG |
| CTL | 2.5LAC | 5LAC | 10LAC | P | |
|---|---|---|---|---|---|
| Initial weight (g) | 25.8 ± 0.09 | 25.8 ± 0.11 | 25.7 ± 0.04 | 25.8 ± 0.09 | 0.987 |
| Final weight (G) | 45.8 ± 0.65 B | 47.2 ± 0.69 AB | 49.7 ± 0.96 A | 46.0 ± 0.83 B | 0.029 |
| SGR (%/d) | 1.03 ± 0.02 B | 1.07 ± 0.03 AB | 1.17 ± 0.04 A | 1.03 ± 0.03 B | 0.031 |
| Weight gain (%) | 77.7 ± 2.26 B | 83.1 ± 3.50 AB | 93.6 ± 4.08 A | 78.2 ± 3.07 B | 0.031 |
| Feed efficiency (%) | 0.58 ± 0.02 B | 0.60 ± 0.01 AB | 0.65 ± 0.02 A | 0.58 ± 0.01 B | 0.028 |
| Survival (%) | 100 | 100 | 100 | 100 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoseini, S.M.; Yousefi, M.; Afzali-Kordmahalleh, A.; Pagheh, E.; Taheri Mirghaed, A. Effects of Dietary Lactic Acid Supplementation on the Activity of Digestive and Antioxidant Enzymes, Gene Expressions, and Bacterial Communities in the Intestine of Common Carp, Cyprinus carpio. Animals 2023, 13, 1934. https://doi.org/10.3390/ani13121934
Hoseini SM, Yousefi M, Afzali-Kordmahalleh A, Pagheh E, Taheri Mirghaed A. Effects of Dietary Lactic Acid Supplementation on the Activity of Digestive and Antioxidant Enzymes, Gene Expressions, and Bacterial Communities in the Intestine of Common Carp, Cyprinus carpio. Animals. 2023; 13(12):1934. https://doi.org/10.3390/ani13121934
Chicago/Turabian StyleHoseini, Seyyed Morteza, Morteza Yousefi, Alireza Afzali-Kordmahalleh, Esmaeil Pagheh, and Ali Taheri Mirghaed. 2023. "Effects of Dietary Lactic Acid Supplementation on the Activity of Digestive and Antioxidant Enzymes, Gene Expressions, and Bacterial Communities in the Intestine of Common Carp, Cyprinus carpio" Animals 13, no. 12: 1934. https://doi.org/10.3390/ani13121934
APA StyleHoseini, S. M., Yousefi, M., Afzali-Kordmahalleh, A., Pagheh, E., & Taheri Mirghaed, A. (2023). Effects of Dietary Lactic Acid Supplementation on the Activity of Digestive and Antioxidant Enzymes, Gene Expressions, and Bacterial Communities in the Intestine of Common Carp, Cyprinus carpio. Animals, 13(12), 1934. https://doi.org/10.3390/ani13121934







