Short-Term Thermal Stress Affects Immune Cell Features in the Sea Urchin Paracentrotus lividus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Collection and Breeding Care
2.3. Experimental Design
2.4. Coelomic Fluid and Cell Sampling
2.5. pH and Osmolarity Evaluation in the Coelomic Fluid (CF)
2.6. Coelomocyte Count and Morphological Typing
2.7. Evaluation of Mitochondrial Membrane Potential (MMP)
2.8. Evaluation of Lipid Peroxidation (LPO)
2.9. Evaluation of Intracellular Hydrogen Peroxide Content (H2O2)
2.10. Respiratory Burst
2.11. Statistical Analysis
3. Results
3.1. Coelomic Fluid Parameters
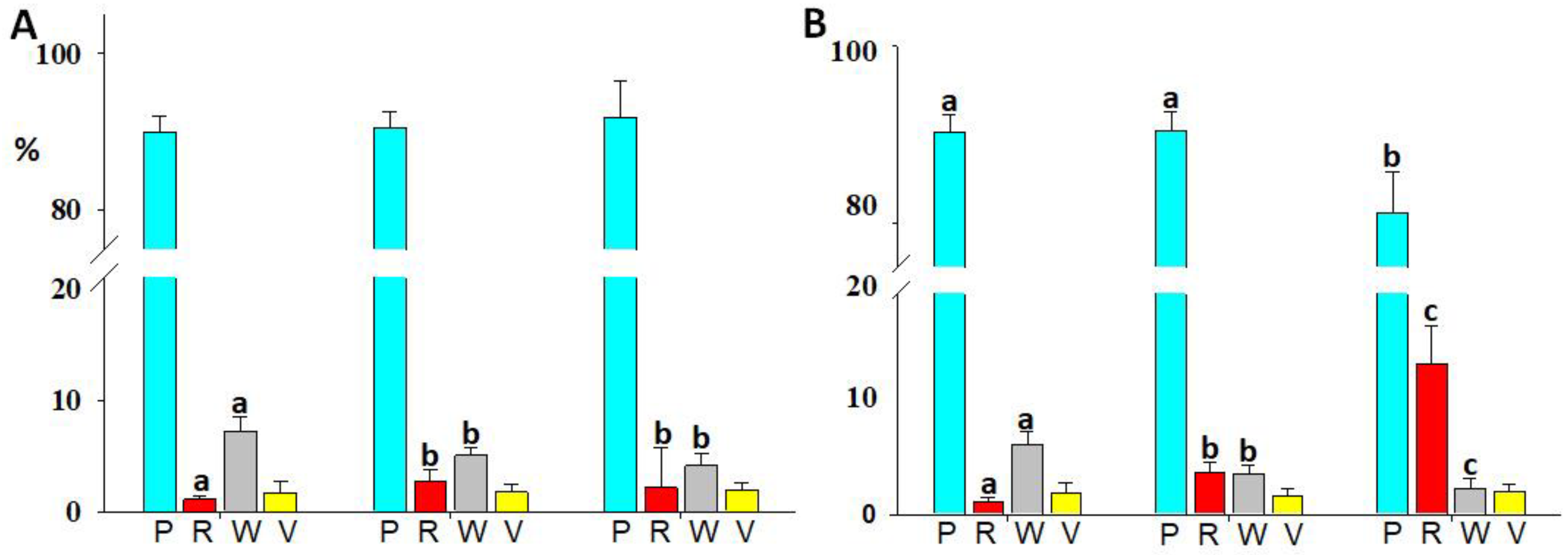
3.2. Immune Cell Number and Cell Type Distribution
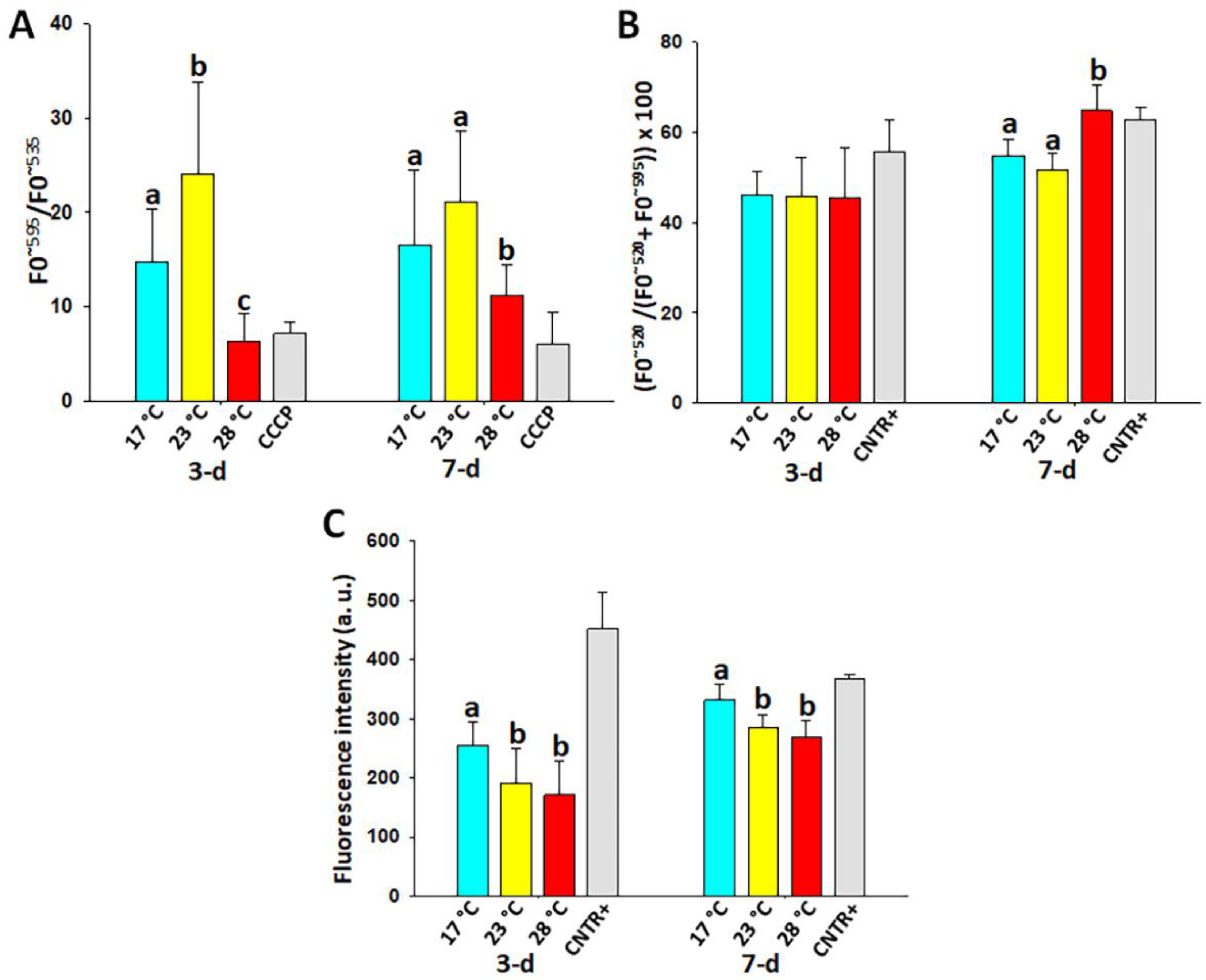
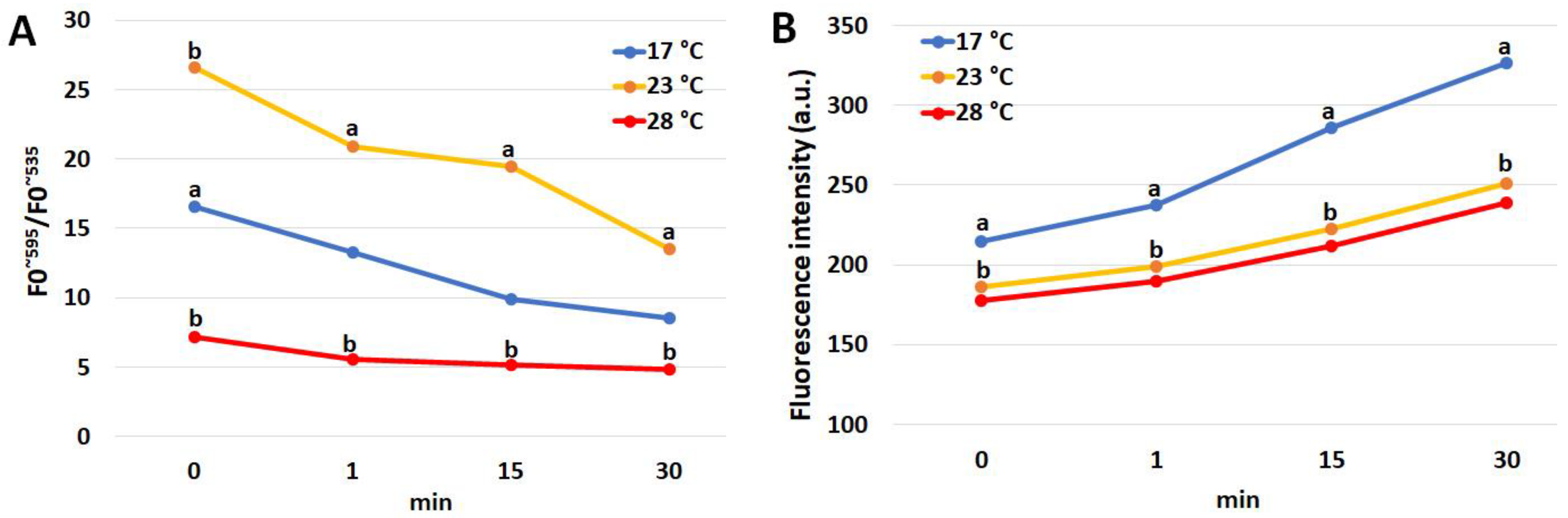
3.3. Mitochondrial Membrane Potential (MMP) in the Coelomocytes
3.4. Lipid Peroxidation (LPO) in the Coelomocytes
3.5. Hydrogen Peroxide (H2O2) Content in the Coelomocytes
3.6. Respiratory Burst in the Coelomocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, D.; Ghorai, P.; Debnath, S.; Roy, D.; Samanta, A.; Maiti, K.S.; Sarkar, S.; Roy, D.; Sarkar, K.; Banerjee, R. Impression of climatic variation on flora, fauna, and human being: A present state of art. In Visualization Techniques for Climate Change with Machine Learning and Artificial Intelligence; Elsevier: Amsterdam, The Netherlands, 2023; pp. 101–122. [Google Scholar]
- Boni, R. Heat stress, a serious threat to reproductive function in animals and humans. Mol. Reprod. Dev. 2019, 86, 1307–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, C.; Fujita, S.; Obara, T.; Ueda, T. Effects of room temperature on reproduction, body and organ weights, food and water intakes, and hematology in mice. Exp. Anim. 1983, 32, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horowitz, M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Ndong, D.; Chen, Y.-Y.; Lin, Y.-H.; Vaseeharan, B.; Chen, J.-C. The immune response of Tilapia oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 2007, 22, 686–694. [Google Scholar] [CrossRef]
- Han, P.; Wang, R.; Yao, T.; Liu, X.; Wang, X. Genome-wide identification of olive flounder (Paralichthys olivaceus) SOCS genes: Involvement in immune response regulation to temperature stress and Edwardsiella tarda infection. Fish Shellfish Immunol. 2023, 133, 108515. [Google Scholar] [CrossRef]
- Somero, G. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Bonacina, L.; Fasano, F.; Mezzanotte, V.; Fornaroli, R. Effects of water temperature on freshwater macroinvertebrates: A systematic review. Biol. Rev. 2023, 98, 191–221. [Google Scholar] [CrossRef]
- Seuront, L.; Nicastro, K.R.; Zardi, G.I.; Goberville, E. Decreased thermal tolerance under recurrent heat stress conditions explains summer mass mortality of the blue mussel Mytilus edulis. Sci. Rep. 2019, 9, 17498. [Google Scholar] [CrossRef] [Green Version]
- Boni, R.; Gallo, A.; Montanino, M.; Macina, A.; Tosti, E. Dynamic changes in the sperm quality of Mytilus galloprovincialis under continuous thermal stress. Mol. Reprod. Dev. 2016, 83, 162–173. [Google Scholar] [CrossRef]
- Johnstone, J.; Nash, S.; Hernandez, E.; Rahman, M.S. Effects of elevated temperature on gonadal functions, cellular apoptosis, and oxidative stress in Atlantic sea urchin Arbacia punculata. Mar. Environ. Res. 2019, 149, 40–49. [Google Scholar] [CrossRef]
- Lester, S.E.; Tobin, E.D.; Behrens, M.D. Disease dynamics and the potential role of thermal stress in the sea urchin, Strongylocentrotus purpuratus. Can. J. Fish. Aquat. 2007, 64, 314–323. [Google Scholar] [CrossRef]
- Morales-Lange, B.; González-Aravena, M.; Font, A.; Guzmán, F.; Mercado, L. Detection of peroxiredoxin-like protein in Antarctic sea urchin (Sterechinus neumayeri) under heat stress and induced with pathogen-associated molecular pattern from Vibrio anguillarum. Polar Biol. 2018, 41, 2065–2073. [Google Scholar] [CrossRef]
- Pinsino, A.; Matranga, V. Sea urchin immune cells as sentinels of environmental stress. Dev. Comp. Immunol. 2015, 49, 198–205. [Google Scholar] [CrossRef]
- Chia, F.-S.; Xing, J. Echinoderm coelomocytes. Zool. Stud. 1996, 35, 231–254. [Google Scholar]
- Ito, T.; Matsutani, T.; Mori, K.; Nomura, T. Phagocytosis and hydrogen peroxide production by phagocytes of the sea urchin Strongylocentrotus nudus. Dev. Comp. Immunol. 1992, 16, 287–294. [Google Scholar] [CrossRef]
- D’Agostino, A.; Di Palma, T.; Cecchini Gualandi, S.; Boni, R. Fluorescence Spectroscopy for the Diagnosis of Endometritis in the Mare. Animals 2022, 12, 1157. [Google Scholar] [CrossRef]
- Knaus, U.G.; Heyworth, P.G.; Evans, T.; Curnutte, J.T.; Bokoch, G.M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science 1991, 254, 1512–1515. [Google Scholar] [CrossRef]
- Branco, P.C.; Borges, J.C.S.; Santos, M.F.; Junior, B.E.J.; da Silva, J.R.M.C. The impact of rising sea temperature on innate immune parameters in the tropical subtidal sea urchin Lytechinus variegatus and the intertidal sea urchin Echinometra lucunter. Mar. Environ. Res. 2013, 92, 95–101. [Google Scholar] [CrossRef]
- Dahlgren, C.; Karlsson, A. Respiratory burst in human neutrophils. J. Immunol. Methods 1999, 232, 3–14. [Google Scholar] [CrossRef]
- Brundu, G.; Cannavacciuolo, A.; Nannini, M.; Somma, E.; Munari, M.; Zupo, V.; Farina, S. Development of an efficient, noninvasive method for identifying gender year-round in the sea urchin Paracentrotus lividus. Aquaculture 2023, 564, 739082. [Google Scholar] [CrossRef]
- Pérez-Portela, R.; Leiva, C. Sex-specific transcriptomic differences in the immune cells of a key Atlantic-Mediterranean sea urchin. Front. Mar. Sci. 2022, 9, 1166. [Google Scholar] [CrossRef]
- Murano, C.; Bergami, E.; Liberatori, G.; Palumbo, A.; Corsi, I. Interplay between nanoplastics and the immune system of the mediterranean sea urchin Paracentrotus lividus. Front. Mar. Sci. 2021, 8, 647394. [Google Scholar] [CrossRef]
- Murano, C.; Nonnis, S.; Scalvini, F.G.; Maffioli, E.; Corsi, I.; Tedeschi, G.; Palumbo, A. Response to microplastic exposure: An exploration into the sea urchin immune cell proteome. Environ. Pollut. 2023, 320, 121062. [Google Scholar] [CrossRef]
- Matranga, V.; Pinsino, A.; Celi, M.; Bella, G.D.; Natoli, A. Impacts of UV-B radiation on short-term cultures of sea urchin coelomocytes. Mar. Biol. 2006, 149, 25–34. [Google Scholar] [CrossRef]
- Smith, L.C.; Ghosh, J.; Buckley, K.M.; Clow, L.A.; Dheilly, N.M.; Haug, T.; Henson, J.H.; Li, C.; Lun, C.M.; Majeske, A.J.; et al. Echinoderm immunity. Adv. Exp. Med. Biol. 2010, 708, 260–301. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Boni, R.; Buttino, I.; Tosti, E. Spermiotoxicity of nickel nanoparticles in the marine invertebrate Ciona intestinalis (ascidians). Nanotoxicology 2016, 10, 1096–1104. [Google Scholar] [CrossRef] [Green Version]
- Ordás, M.C.; Novoa, B.; Figueras, A. Modulation of the chemiluminescence response of Mediterranean mussel (Mytilus galloprovincialis) haemocytes. Fish Shellfish Immunol. 2000, 10, 611–622. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Slein, M.A.; Bernhardt, J.R.; O’Connor, M.I.; Fey, S.B. Effects of thermal fluctuations on biological processes: A meta-analysis of experiments manipulating thermal variability. Proc. Royal Soc. B 2023, 290, 20222225. [Google Scholar] [CrossRef]
- Soualili, D.; Dubois, P.; Gosselin, P.; Pernet, P.; Guillou, M. Assessment of seawater pollution by heavy metals in the neighbourhood of Algiers: Use of the sea urchin, Paracentrotus lividus, as a bioindicator. ICES J. Mar. Sci. 2008, 65, 132–139. [Google Scholar] [CrossRef]
- Yeruham, E.; Rilov, G.; Shpigel, M.; Abelson, A. Collapse of the echinoid Paracentrotus lividus populations in the Eastern Mediterranean—Result of climate change? Sci. Rep. 2015, 5, 13479. [Google Scholar] [CrossRef] [Green Version]
- Migliaccio, O.; Pinsino, A.; Maffioli, E.; Smith, A.M.; Agnisola, C.; Matranga, V.; Nonnis, S.; Tedeschi, G.; Byrne, M.; Gambi, M.C. Living in future ocean acidification, physiological adaptive responses of the immune system of sea urchins resident at a CO2 vent system. Sci. Total Environ. 2019, 672, 938–950. [Google Scholar] [CrossRef]
- Murano, C.; Vaccari, L.; Casotti, R.; Corsi, I.; Palumbo, A. Occurrence of microfibres in wild specimens of adult sea urchin Paracentrotus lividus (Lamarck, 1816) from a coastal area of the central Mediterranean Sea. Mar. Pollut. Bull. 2022, 176, 113448. [Google Scholar] [CrossRef]
- Stickle, W.; Diehl, W. Effects of salinity on echinoderms. In Echinoderm Studies 2; Jaungoux, M., Lawrence, J.M., Eds.; Balkema Rotterdam: Rotterdam, The Netherlands, 1987; pp. 235–285. [Google Scholar]
- Shick, J.M. Respiratory gas exchange in echinoderms. In Echinoderm Studies 1; Jaungoux, M., Lawrence, J.M., Eds.; CRC Press: Rotterdam, The Netherlands, 2020; pp. 67–110. [Google Scholar]
- Catarino, A.I.; Bauwens, M.; Dubois, P. Acid–base balance and metabolic response of the sea urchin Paracentrotus lividus to different seawater pH and temperatures. Environ. Sci. Pollut. Res. 2012, 19, 2344–2353. [Google Scholar] [CrossRef]
- Hudgell, M.A.B.; Grayfer, L.; Smith, L.C. Coelomocyte populations in the sea urchin, Strongylocentrotus purpuratus, undergo dynamic changes in response to immune challenge. Front. Immunol. 2022, 13, 940852. [Google Scholar] [CrossRef]
- Matranga, V.; Toia, G.; Bonaventura, R.; Müller, W.E. Cellular and biochemical responses to environmental and experimentally induced stress in sea urchin coelomocytes. Cell Stress Chaperones 2000, 5, 113–120. [Google Scholar] [CrossRef]
- Brothers, C.; Harianto, J.; McClintock, J.; Byrne, M. Sea urchins in a high-CO2 world: The influence of acclimation on the immune response to ocean warming and acidification. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161501. [Google Scholar] [CrossRef] [Green Version]
- Matranga, V.; Pinsino, A.; Celi, M.; Natoli, A.; Bonaventura, R.; Schröder, H.; Müller, W. Monitoring chemical and physical stress using sea urchin immune cells. Echinodermata 2005, 39, 85–110. [Google Scholar] [CrossRef]
- Canesi, L.; Procházková, P. The invertebrate immune system as a model for investigating the environmental impact of nanoparticles. In Nanoparticles and the Immune System; Elsevier: Amsterdam, The Netherlands, 2014; pp. 91–112. [Google Scholar]
- Eliachar, S.; Snyder, G.A.; Barkan, S.K.; Talice, S.; Otolenghi, A.; Jaimes-Becerra, A.; Sharoni, T.; Sultan, E.; Hadad, U.; Levy, O. Heat stress increases immune cell function in Hexacorallia. Front. Immunol. 2022, 13, 1016097. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Yin, J.; Lin, W. Organic fluorescent probes for detecting mitochondrial membrane potential. Coord. Chem. Rev. 2020, 420, 213419. [Google Scholar] [CrossRef]
- Chen, L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell Biol. 1988, 4, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Gallo, A.; Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 2017, 5, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, A.; Faruolo, M.P.; Sileo, C.; Petillo, A.; Boni, R. Effect of follicle size and atresia grade on mitochondrial membrane potential and steroidogenic acute regulatory protein expression in bovine granulosa cells. Zygote 2018, 26, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Hüttemann, M.; Lee, I.; Pecinova, A.; Pecina, P.; Przyklenk, K.; Doan, J.W. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J. Bioenerg. Biomembr. 2008, 40, 445–456. [Google Scholar] [CrossRef]
- Delorme, N.J.; Venter, L.; Rolton, A.; Ericson, J.A. Integrating animal health and stress assessment tools using the green-lipped mussel Perna canaliculus as a case study. J. Shellfish Res. 2021, 40, 93–112. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: Oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Yu, Z.-X.; Yao, C.-L. The impact of acute thermal stress on green mussel Perna viridis: Oxidative damage and responses. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 222, 7–15. [Google Scholar] [CrossRef]
- Clément, M.-V.; Pervaiz, S. Intracellular superoxide and hydrogen peroxide concentrations: A critical balance that determines survival or death. Redox Rep. 2001, 6, 211–214. [Google Scholar] [CrossRef]
- Rothe, G.; Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′, 7′-dichlorofluorescin. J. Leukoc. Biol. 1990, 47, 440–448. [Google Scholar] [CrossRef]
- Fossati, G.; Moulding, D.A.; Spiller, D.G.; Moots, R.J.; White, M.R.; Edwards, S.W. The mitochondrial network of human neutrophils: Role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J. Immunol. 2003, 170, 1964–1972. [Google Scholar] [CrossRef] [Green Version]
- Prado, P.; Tomas, F.; Pinna, S.; Farina, S.; Roca, G.; Ceccherelli, G.; Romero, J.; Alcoverro, T. Habitat and scale shape the demographic fate of the keystone sea urchin Paracentrotus lividus in Mediterranean macrophyte communities. PLoS ONE 2012, 7, e35170. [Google Scholar] [CrossRef]
| Time of Exposure (Days) | Animals n | Coelomocyte Concentration (×106/mL) | pHCF | CF Osmolarity (mOsm) | |
|---|---|---|---|---|---|
| 17 °C | 3 7 | 6 6 | 10.0 ± 5.6 6.9 ± 3.3 | 7.87 ± 0.03 7.83 ± 0.09 | 1143 ± 2 1144 ± 3 |
| 23 °C | 3 7 | 6 6 | 11.6 ± 2.6 7.5 ± 2.9 | 7.84 ± 0.09 7.86 ± 0.10 | 1149 ± 7 1139 ± 9 |
| 28 °C | 3 7 | 6 6 | 8.8 ± 5.5 7.6 ± 3.2 | 7.82 ± 0.07 7.82 ± 0.18 | 1144 ± 2 1138 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murano, C.; Gallo, A.; Nocerino, A.; Macina, A.; Cecchini Gualandi, S.; Boni, R. Short-Term Thermal Stress Affects Immune Cell Features in the Sea Urchin Paracentrotus lividus. Animals 2023, 13, 1954. https://doi.org/10.3390/ani13121954
Murano C, Gallo A, Nocerino A, Macina A, Cecchini Gualandi S, Boni R. Short-Term Thermal Stress Affects Immune Cell Features in the Sea Urchin Paracentrotus lividus. Animals. 2023; 13(12):1954. https://doi.org/10.3390/ani13121954
Chicago/Turabian StyleMurano, Carola, Alessandra Gallo, Aurora Nocerino, Alberto Macina, Stefano Cecchini Gualandi, and Raffaele Boni. 2023. "Short-Term Thermal Stress Affects Immune Cell Features in the Sea Urchin Paracentrotus lividus" Animals 13, no. 12: 1954. https://doi.org/10.3390/ani13121954
APA StyleMurano, C., Gallo, A., Nocerino, A., Macina, A., Cecchini Gualandi, S., & Boni, R. (2023). Short-Term Thermal Stress Affects Immune Cell Features in the Sea Urchin Paracentrotus lividus. Animals, 13(12), 1954. https://doi.org/10.3390/ani13121954









