Relative and Quantitative Characterization of the Bovine Bacterial Ocular Surface Microbiome in the Context of Suspected Ocular Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Confirmation of OSCC
2.3. DNA Extraction
2.4. 16S rRNA Gene Sequencing and Analysis
2.5. RT-PCR Processing and Analysis
2.6. Statistical Analysis
3. Results
3.1. Sampling Demographics
3.2. 16S rRNA Gene Sequencing
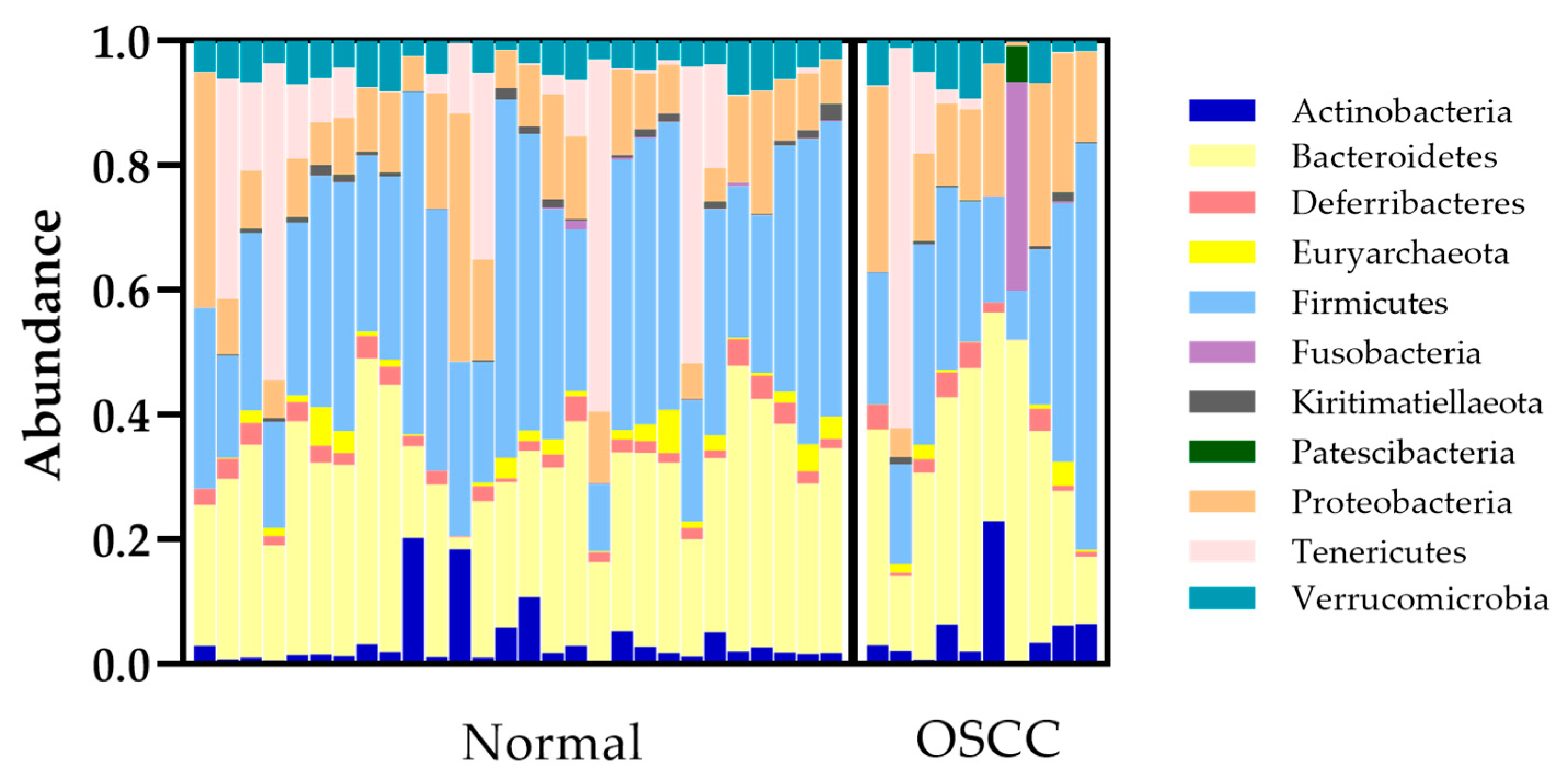
3.2.1. Bacterial Population Composition
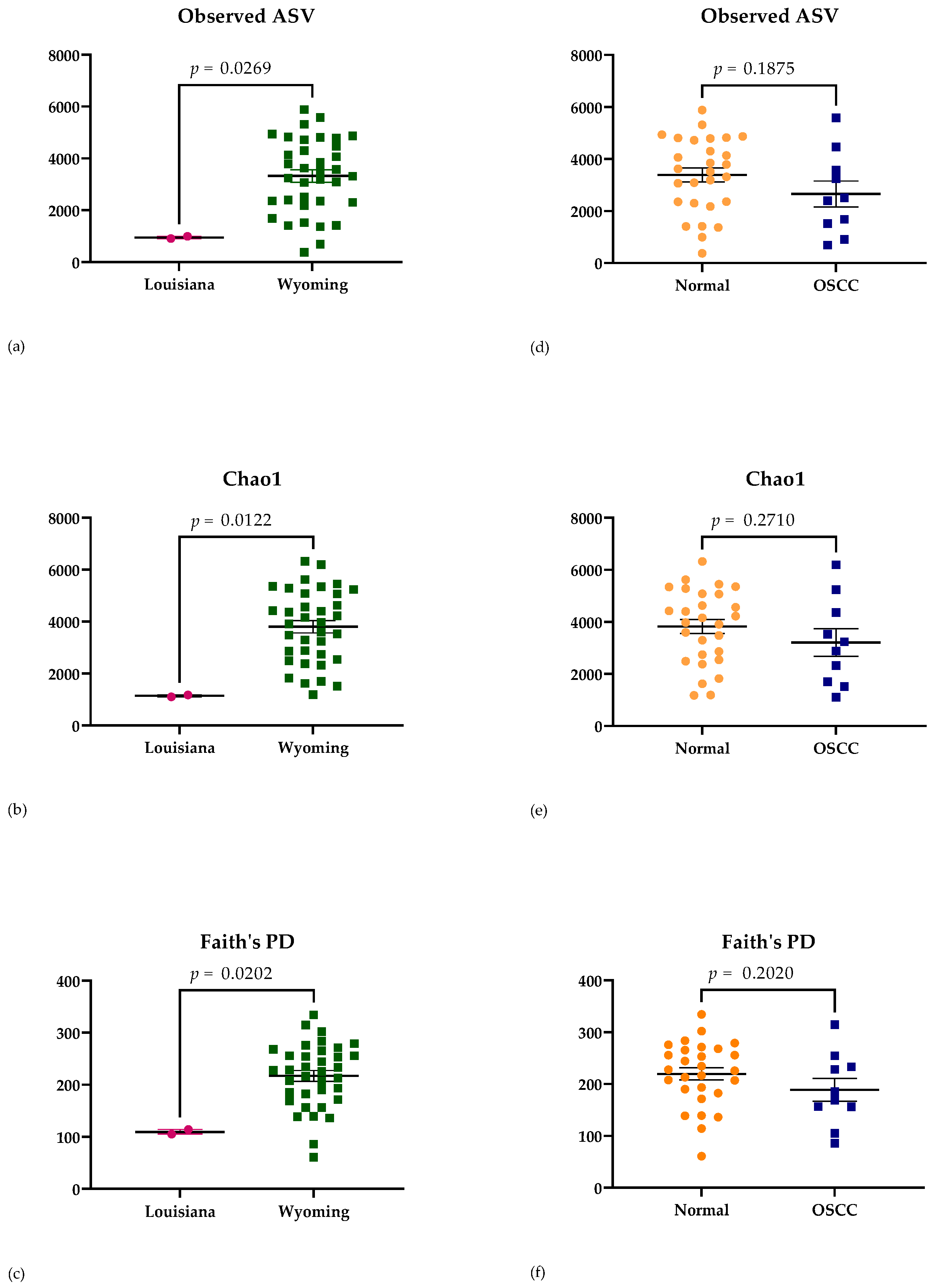
3.2.2. Alpha Diversity Analysis
3.2.3. Beta Diversity Analysis
3.3. RT-PCR Analysis
3.3.1. RT-PCR Results
3.3.2. Quadratic and Linear Discriminant Analyses (QDA/LDA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, B.C. Food and Fiber Animal Ophthalmology. In Veterinary Ophthalmology, 6th ed.; Gelatt, K.N., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2021; p. 1990. [Google Scholar]
- Tsujita, H.; Plummer, C.E. Bovine ocular squamous cell carcinoma. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 511–529. [Google Scholar] [CrossRef]
- Fornazari, G.A.; Kravetz, J.; Kiupel, M.; Sledge, D.; Filho, I.R.B.; Montiani-Ferreira, F. Ocular squamous cell carcinoma in Holstein cows from the South of Brazil. Vet. World 2017, 10, 1413–1420. [Google Scholar] [CrossRef]
- Schulz, K.L.; Anderson, D.E. Bovine enucleation: A retrospective study of 53 cases (1998–2006). Can. Vet. J. 2010, 51, 611–614. [Google Scholar]
- Welker, B.; Modransky, P.D.; Hoffsis, G.F.; Wyman, M.W.; Rings, D.M.; Hull, B.L. Excision of neoplasms of the bovine lower eyelid by H-blepharoplasty. Vet. Surg. 1991, 20, 133–139. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef]
- Curty, G.; de Carvalho, P.S.; Soares, M.A. The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. Int. J. Mol. Sci. 2019, 21, 222. [Google Scholar] [CrossRef]
- Turner, N.D.; Ritchie, L.E.; Bresalier, R.S.; Chapkin, R.S. The microbiome and colorectal neoplasia: Environmental modifiers of dysbiosis. Curr. Gastroenterol. Rep. 2013, 15, 346. [Google Scholar] [CrossRef]
- van Vorstenbosch, R.; Cheng, H.R.; Jonkers, D.; Penders, J.; Schoon, E.; Masclee, A.; van Schooten, F.J.; Smolinska, A.; Mujagic, Z. Systematic Review: Contribution of the Gut Microbiome to the Volatile Metabolic Fingerprint of Colorectal Neoplasia. Metabolites 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, X.; Zeng, R.; Wu, Q.; Sun, H.; Wu, W.; Zhang, X.; Sun, G.; Yan, B.; Wu, L.; et al. Changes of the Gastric Mucosal Microbiome Associated With Histological Stages of Gastric Carcinogenesis. Front. Microbiol. 2020, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Shimizu, D.; Ueda, S.; Miyabe, S.; Oh-Iwa, I.; Nagao, T.; Shimozato, K.; Nomoto, S. Feasibility of oral microbiome profiles associated with oral squamous cell carcinoma. J. Oral Microbiol. 2022, 14, 2105574. [Google Scholar] [CrossRef] [PubMed]
- Su Mun, L.; Wye Lum, S.; Kong Yuiin Sze, G.; Hock Yoong, C.; Ching Yung, K.; Kah Lok, L.; Gopinath, D. Association of Microbiome with Oral Squamous Cell Carcinoma: A Systematic Review of the Metagenomic Studies. Int. J. Environ. Res. Public Health 2021, 18, 7224. [Google Scholar] [CrossRef] [PubMed]
- Sami, A.; Elimairi, I.; Stanton, C.; Ross, R.P.; Ryan, C.A. The Role of the Microbiome in Oral Squamous Cell Carcinoma with Insight into the Microbiome-Treatment Axis. Int. J. Mol. Sci. 2020, 21, 8061. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.Y.; Emiola, A.; Johnson, J.S.; Fleming, E.S.; Nguyen, H.; Zhou, W.; Tsai, K.Y.; Fink, C.; Oh, J. Skin Microbiome Variation with Cancer Progression in Human Cutaneous Squamous Cell Carcinoma. J. Investig. Derm. 2022, 142, 2773–2782.e16. [Google Scholar] [CrossRef]
- Zegans, M.E.; Van Gelder, R.N. Considerations in understanding the ocular surface microbiome. Am. J. Ophthalmol. 2014, 158, 420–422. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Frizon, L.; Demeda, V.F. Ocular Surface Microbiome in Health and Disease. Asia-Pac. J. Ophthalmol. 2020, 9, 505–511. [Google Scholar] [CrossRef]
- Darden, J.E.; Scott, E.M.; Arnold, C.; Scallan, E.M.; Simon, B.T.; Suchodolski, J.S. Evaluation of the bacterial ocular surface microbiome in clinically normal cats before and after treatment with topical erythromycin. PLoS ONE 2019, 14, e0223859. [Google Scholar] [CrossRef]
- Rogers, C.M.; Scott, E.M.; Sarawichitr, B.; Arnold, C.; Suchodolski, J.S. Evaluation of the bacterial ocular surface microbiome in ophthalmologically normal dogs prior to and following treatment with topical neomycin-polymyxin-bacitracin. PLoS ONE 2020, 15, e0234313. [Google Scholar] [CrossRef]
- Leis, M.L.; Madruga, G.M.; Costa, M.O. The porcine corneal surface bacterial microbiome: A distinctive niche within the ocular surface. PLoS ONE 2021, 16, e0247392. [Google Scholar] [CrossRef]
- Santibanez, R.; Lara, F.; Barros, T.M.; Mardones, E.; Cuadra, F.; Thomson, P. Ocular Microbiome in a Group of Clinically Healthy Horses. Animals 2022, 12, 943. [Google Scholar] [CrossRef]
- Scott, E.M.; Arnold, C.; Dowell, S.; Suchodolski, J.S. Evaluation of the bacterial ocular surface microbiome in clinically normal horses before and after treatment with topical neomycin-polymyxin-bacitracin. PLoS ONE 2019, 14, e0214877. [Google Scholar] [CrossRef]
- Chiarello, M.; McCauley, M.; Villéger, S.; Jackson, C.R. Ranking the biases: The choice of OTUs vs. ASVs in 16S rRNA amplicon data analysis has stronger effects on diversity measures than rarefaction and OTU identity threshold. PLoS ONE 2022, 17, e0264443. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef] [PubMed]
- Mignard, S.; Flandrois, J.P. 16S rRNA sequencing in routine bacterial identification: A 30-month experiment. J. Microbiol. Methods 2006, 67, 574–581. [Google Scholar] [CrossRef]
- Banks, K.C.; Ericsson, A.C.; Reinero, C.R.; Giuliano, E.A. Veterinary ocular microbiome: Lessons learned beyond the culture. Vet. Ophthalmol. 2019, 22, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet. J. 2016, 215, 30–37. [Google Scholar] [CrossRef]
- Scott, E.M.; Lewin, A.C.; Leis, M.L. Current ocular microbiome investigations limit reproducibility and reliability: Critical review and opportunities. Vet. Ophthalmol. 2021, 24, 4–11. [Google Scholar] [CrossRef]
- Sung, C.H.; Marsilio, S.; Chow, B.; Zornow, K.A.; Slovak, J.E.; Pilla, R.; Lidbury, J.A.; Steiner, J.M.; Park, S.Y.; Hong, M.P.; et al. Dysbiosis index to evaluate the fecal microbiota in healthy cats and cats with chronic enteropathies. J. Feline Med. Surg. 2022, 24, e1–e12. [Google Scholar] [CrossRef]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Bartenslager, A.C.; Althuge, N.D.; Loy, J.D.; Hille, M.M.; Spangler, M.L.; Fernando, S.C. Longitudinal assessment of the bovine ocular bacterial community dynamics in calves. Anim. Microbiome 2021, 3, 16. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2022, 50 (Suppl. 1), 6–17. [Google Scholar] [CrossRef]
- Cullen, J.N.; Lithio, A.; Seetharam, A.S.; Zheng, Y.; Li, G.; Nettleton, D.; O’Connor, A.M. Microbial community sequencing analysis of the calf eye microbiota and relationship to infectious bovine keratoconjunctivitis. Vet. Microbiol. 2017, 207, 267–279. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef]
- Zheng, W.; Porter, E.; Noll, L.; Stoy, C.; Lu, N.; Wang, Y.; Liu, X.; Purvis, T.; Peddireddi, L.; Lubbers, B.; et al. A multiplex real-time PCR assay for the detection and differentiation of five bovine pinkeye pathogens. J. Microbiol. Methods 2019, 160, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Menzies, M.; Ingham, A. Identification and expression of Toll-like receptors 1–10 in selected bovine and ovine tissues. Vet. Immunol. Immunopathol. 2006, 109, 23–30. [Google Scholar] [CrossRef]
- van Kuppeveld, F.J.; van der Logt, J.T.; Angulo, A.F.; van Zoest, M.J.; Quint, W.G.; Niesters, H.G.; Galama, J.M.; Melchers, W.J. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl. Environ. Microbiol. 1992, 58, 2606–2615. [Google Scholar] [CrossRef]
- Bootz, F.; Kirschnek, S.; Nicklas, W.; Wyss, S.K.; Homberger, F.R. Detection of Pasteurellaceae in rodents by polymerase chain reaction analysis. Lab. Anim. Sci. 1998, 48, 542–546. [Google Scholar] [PubMed]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef]
- Morot-Bizot, S.C.; Talon, R.; Leroy, S. Development of a multiplex PCR for the identification of Staphylococcus genus and four staphylococcal species isolated from food. J. Appl. Microbiol. 2004, 97, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Sidjabat, H.E.; Townsend, K.M.; Hanson, N.D.; Bell, J.M.; Stokes, H.W.; Gobius, K.S.; Moss, S.M.; Trott, D.J. Identification of bla(CMY-7) and associated plasmid-mediated resistance genes in multidrug-resistant Escherichia coli isolated from dogs at a veterinary teaching hospital in Australia. J. Antimicrob. Chemother. 2006, 57, 840–848. [Google Scholar] [CrossRef] [PubMed]
- JMP®, Version 16.2.0; SAS Institute, Inc.: Cary, NC, USA, 1989–2021.
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; Wagner, H.; et al. Vegan: Community Ecology Package, R. package version 2.6-2; The Comprehensive R Archive Network. 2022. Available online: https://cran.r-project.org/ (accessed on 31 May 2023).
- Chao, A.; Chiu, C.-H.; Hsieh, T.C.; Davis, T.; Nipperess, D.A.; Faith, D.P. Rarefaction and extrapolation of phylogenetic diversity. Methods Ecol. Evol. 2015, 6, 380–388. [Google Scholar] [CrossRef]
- Cattle; National Agricultural Statistics Service: Washington, DC, USA, 22 July 2022.
- Thomas, C.M.; Desmond-Le Quemener, E.; Gribaldo, S.; Borrel, G. Factors shaping the abundance and diversity of the gut archaeome across the animal kingdom. Nat. Commun. 2022, 13, 3358. [Google Scholar] [CrossRef]
- Horz, H.P.; Conrads, G. The discussion goes on: What is the role of Euryarchaeota in humans? Archaea 2010, 2010, 967271. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, T.; Wu, Z.; Zheng, W.; Zhang, T.; Howe, S.; Chai, J.; Deng, F.; Li, Y.; Zhao, J. Archaea: An under-estimated kingdom in livestock animals. Front. Vet. Sci. 2022, 9, 973508. [Google Scholar] [CrossRef] [PubMed]
- Chaitman, J.; Ziese, A.L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs With Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Gaschen, F.P.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; AlShawaqfeh, M.K.; et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Gichuhi, S.; Sagoo, M.S. Squamous cell carcinoma of the conjunctiva. Community Eye Health 2016, 29, 52–53. [Google Scholar]
- Garcia, R.L.B.; Jimenez, J.; Gubbay, C.; Castaneda, J.F.; Granados, A. Squamous cell carcinoma of the conjunctiva. Case report. Int. J. Surg. Case Rep. 2022, 91, 106785. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K. Ocular Surface Squamous Neoplasia; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]

| Target | Forward Primer | Reverse Primer | Reference |
|---|---|---|---|
| Bovine GAPDH | CCTGGAGAAACCTGCCAAGT | GCCAAATTCATTGTCGTACCA | [39] |
| Moraxella bovis | GGTGACGACCGCTTGTTT | ATCATCGCCTTCATCTCCAG | [38] |
| Moraxella bovoculi | GGTGATATTTATCATGAAGTTGTGAAA | TYTCAATTCATAATCACGATACTCAAG | [38] |
| Mycoplasma | TGCACCATCTGTCACTCTGTTAACCTC | ACTCCTACGGGAGGCAGCAGTA | [40] |
| Staphylococcus | GGCCGTGTTGAACGTGGTCAAATCA | TIACCATTTCAGTACCTTCTGGTAA | [43] |
| Pasteurellaceae | CATAAGATGAGCCCAAG | GTCAGTACATTCCCAAGG | [41] |
| Prevotellaceae | GGTTCTGAGAGGAAGGTCCCC | TCCTGCACGCTACTTGGCTG | [42] |
| Weeksellaceae | ATCCAGCCATCCCGCGT | CTGCTGGCACGGAGTTAGC | None; novel |
| Universal bacteria | CCTACGGGAGGCAGCAGT | ATTACCGCGGCTGCTGG | [29] |
| Escherichia coli | CCGATACGCTGCCAATCAGT | ACGCAGACCGTAGGCCAGAT | [44] |
| Median (Min–Max) Abundance Composition (%) | ||||
|---|---|---|---|---|
| Phylum | Disease Status | Location | ||
| Normal (n = 28) | OSCC (n = 10) | Louisiana (n = 2) | Wyoming (n = 36) | |
| Actinobacteria | 1.75 (0.49–20.17) | 3.19 (0.25–22.85) | 2.92 (2.86–2.98) | 1.90 (0.25–22.85) |
| Bacteroidetes | 29.27 (1.88–45.95) | 33.69 (10.70–51.72) | 28.62 (22.61–34.62) | 30.23 (1.88–51.72) |
| Deferribacteres | 2.08 (0.16–4.29) | 1.87 (0.06–4.23) | 3.25 (2.55–3.95) | 2.10 (0.06–4.29) |
| Euryarchaeota | 1.26 (0.02–6.90) * | 0.40 (0.00–3.81) * | 0.01 (0.00–0.02) * | 1.15 (0.01–6.90) * |
| Firmicutes | 32.88 (10.76–57.51) | 23.69 (7.89–65.20) | 25.21 (21.27–29.15) | 29.35 (7.89–65.20) |
| Fusobacteria | 0.04 (0.00–1.46) | 0.01 (0.00–33.53) | 0.00 (0.00–0.00) | 0.03 (0.00–33.53) |
| Kiritimatiellaeota | 0.60 (0.00–2.61) | 0.23 (0.00–1.51) | 0.00 (0.00–0.00) * | 0.52 (0.00–2.61) * |
| Patescibacteria | 0.00 (0.00–0.06) | 0.00 (0.00–5.70) | 0.00 (0.00–0.00) | 0.00 (0.00–5.70) |
| Proteobacteria | 9.56 (5.39–39.85) | 14.58 (0.59–29.80) | 33.75 (29.80–37.71) * | 10.07 (0.59–39.85) * |
| Tenericutes | 1.97 (0.06–56.49) | 0.20 (0.02–60.99) | 0.16 (0.16–0.17) | 1.36 (0.02–60.99) |
| Verrucomicrobia | 4.85 (0.38–8.59) | 4.38 (0.15–9.26) | 6.08 (4.94–7.21) | 4.62 (0.15–9.26) |
| Median (Min–Max) Log DNA (ag) per 10 ng Isolated DNA | ||||
|---|---|---|---|---|
| Target Primer | Disease Status | Location | ||
| Normal (n = 28) | OSCC (n = 10) | Louisiana (n = 2) | Wyoming (n = 36) | |
| Bovine GAPDH | 7.39 (7.11–7.59) | 7.46 (6.96–7.57) | 7.44 (7.42–7.47) | 7.39 (6.96–7.59) |
| Moraxella bovis | 3.45 (0.00–4.87) | 3.25 (2.42–4.26) | 2.71 (2.42–3.00) * | 3.38 (0.00–4.87) * |
| Moraxella bovoculi | 3.11 (0.00–5.75) | 3.50 (0.00–6.43) | 3.83 (2.27–5.38) | 3.11 (0.00–6.43) |
| Mycoplasma | 6.07 (3.54–8.06) | 5.08 (0,00–8.42) | 1.79 (0.00–3.58) * | 5.98 (3.46–8.42) * |
| Staphylococcus | 4.13 (0.00–7.74) | 5.02 (0.00–7.46) | 5.62 (5.24–6.00) | 4.19 (0.00–7.74) |
| Pasteurellaceae | 4.27 (3.72–7.22) * | 5.63 (3.69–7.10) * | 5.09 (4.51–5.66) | 4.34 (3.69–7.22) |
| Prevotellaceae | 7.00 (5.55–7.85) | 8.82 (4.97–8.98) | 5.26 (4.97–5.55) * | 7.00 (5.85–8.99) * |
| Weeksellaceae | 6.09 (5.09–7.72) | 6.22 (5.19–7.10) | 5.56 (5.19–5.93) | 6.20 (5.09–7.72) |
| Disease Status (n = 30) | Location (n = 38) | ||
|---|---|---|---|
| Training Set #1 | Training Set #2 | ||
| Canonical Standardized Coefficients | |||
| Bovine GAPDH | 0.1824 | 0.3792 | 0.3741 |
| Moraxella bovis | −0.4893 | 0.1722 | 0.2181 |
| Moraxella bovoculi | 0.1398 | 0.2992 | 0.2206 |
| Mycoplasma | 0.4204 | 0.2334 | −0.7565 |
| Staphylococcus | 0.5929 | −0.3859 | −0.6343 |
| Pasteurellaceae | −0.9643 | 0.9771 | 0.1322 |
| Prevotellaceae | 0.0860 | 0.3149 | −0.5426 |
| Weeksellaceae | 0.3759 | 0.7158 | 0.2818 |
| Sensitivity (%) | 100 a, 100 b | 100 a, 100 b | |
| Specificity (%) | 95.5 a, 83.3 b | 100 a, 100 b | |
| Misclassified (%) | 2.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gafen, H.B.; Liu, C.-C.; Ineck, N.E.; Scully, C.M.; Mironovich, M.A.; Guarneri, L.; Taylor, C.M.; Luo, M.; Leis, M.L.; Scott, E.M.; et al. Relative and Quantitative Characterization of the Bovine Bacterial Ocular Surface Microbiome in the Context of Suspected Ocular Squamous Cell Carcinoma. Animals 2023, 13, 1976. https://doi.org/10.3390/ani13121976
Gafen HB, Liu C-C, Ineck NE, Scully CM, Mironovich MA, Guarneri L, Taylor CM, Luo M, Leis ML, Scott EM, et al. Relative and Quantitative Characterization of the Bovine Bacterial Ocular Surface Microbiome in the Context of Suspected Ocular Squamous Cell Carcinoma. Animals. 2023; 13(12):1976. https://doi.org/10.3390/ani13121976
Chicago/Turabian StyleGafen, Hannah B., Chin-Chi Liu, Nikole E. Ineck, Clare M. Scully, Melanie A. Mironovich, Lauren Guarneri, Christopher M. Taylor, Meng Luo, Marina L. Leis, Erin M. Scott, and et al. 2023. "Relative and Quantitative Characterization of the Bovine Bacterial Ocular Surface Microbiome in the Context of Suspected Ocular Squamous Cell Carcinoma" Animals 13, no. 12: 1976. https://doi.org/10.3390/ani13121976
APA StyleGafen, H. B., Liu, C.-C., Ineck, N. E., Scully, C. M., Mironovich, M. A., Guarneri, L., Taylor, C. M., Luo, M., Leis, M. L., Scott, E. M., Carter, R. T., & Lewin, A. C. (2023). Relative and Quantitative Characterization of the Bovine Bacterial Ocular Surface Microbiome in the Context of Suspected Ocular Squamous Cell Carcinoma. Animals, 13(12), 1976. https://doi.org/10.3390/ani13121976






