Pre-Slaughter Stunning of Farmed Atlantic Halibut in CO2-Saturated Seawater: Assessment of Unconsciousness by Electroencephalography (EEG)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Immersion in CO2
2.2. EEG Analyses
2.3. Statistical Analyses
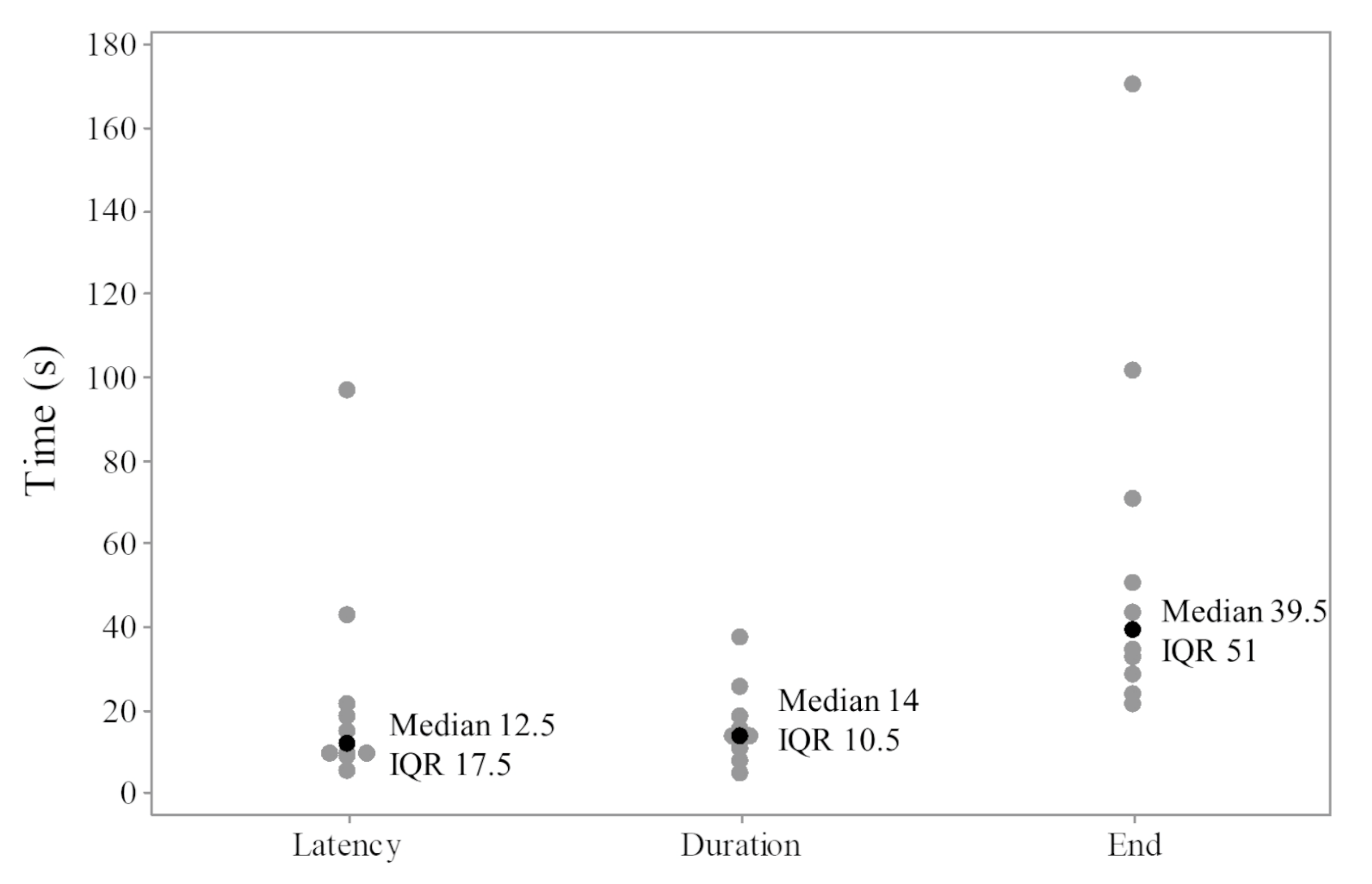
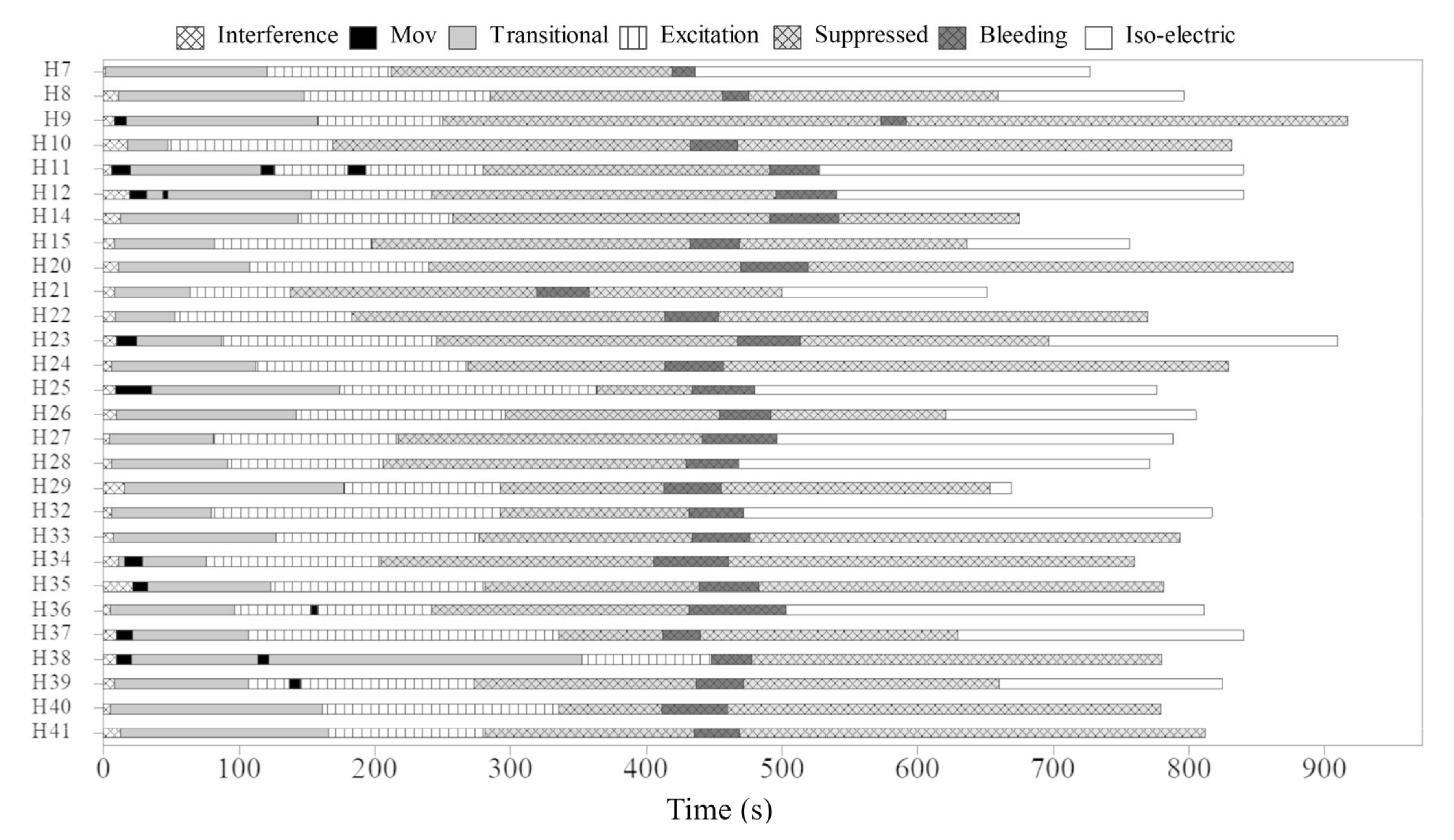
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food & Agriculture Organisation). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- FEAP (Federation of European Aquaculture Producers). European Aquaculture Production Report—2021; FEAP: Biñan, Philippines, 2021. [Google Scholar]
- Imsland, A. The Flatfishes (Order: Pleuronectiformes). In Finfish Aquaculture Diversification; le François, N.R., Jobling, M., Carter, C., Blier, P.U., Eds.; CAB International: Wallingford, UK, 2010; pp. 450–496. ISBN 1845934946. [Google Scholar]
- Ellingsen, K.; Grimsrud, K.; Nielse, H.M.; Mejdell, C.; Olesen, I.; Honkanen, P.; Navrud, S.; Gamborg, C.; Sandøe, P. Who Cares about Fish Welfare?: A Norwegian Study. Br. Food J. 2015, 117, 257–273. [Google Scholar] [CrossRef]
- Norway Animal Welfare Act. Available online: https://www.regjeringen.no/en/dokumenter/animal-welfare-act/id571188/ (accessed on 30 September 2015).
- Röcklinsberg, H. Fish Consumption: Choices in the Intersection of Public Concern, Fish Welfare, Food Security, Human Health and Climate Change. J. Agric. Environ. Ethics 2015, 28, 533–551. [Google Scholar] [CrossRef]
- OIE Aquatic Animal Health Code. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-code-online-access/ (accessed on 18 August 2015).
- EU (European Union). COUNCIL REGULATION (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:303:0001:0030:EN:PDF (accessed on 17 April 2016).
- van de Vis, H.; Abbink, W.; Lambooij, B.; Bracke, M. Stunning and Killing of Farmed Fish: How to Put It into Practice? In Encyclopedia of Meat Sciences; Dikeman, M., Devine, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; p. 1712. ISBN 9780123847348. [Google Scholar]
- EFSA (European Food Safety Authority). Guidance on the Assessment Criteria for Applications for New or Modified Stunning Methods Regarding Animal Protection at the Time of Killing. EFSA J. 2018, 16, 5343. [Google Scholar] [CrossRef] [Green Version]
- Robb, D.H.F.; Wotton, S.B.; McKinstry, J.L.; Sørensen, N.K.; Kestin, S.C. Commercial Slaughter Methods Used on Atlantic Salmon: Determination of the Onset of Brain Failure by Electroencephalography. Vet. Rec. 2000, 147, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Kestin, S.; Van de Vis, J.; Robb, D. Protocol for Assessing Brain Function in Fish and the Effectiveness of Methods Used to Stun and Kill Them. Vet. Rec. 2002, 150, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Lambooij, B.; Bracke, M.; Reimert, H.; Foss, A.; Imsland, A.; van de Vis, H. Electrophysiological and Behavioural Responses of Turbot (Scophthalmus Maximus) Cooled in Ice Water. Physiol. Behav. 2015, 149, 23–28. [Google Scholar] [CrossRef]
- Tonner, P.H.; Bein, B. Classic Electroencephalographic Parameters: Median Frequency, Spectral Edge Frequency Etc. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 147–159. [Google Scholar] [CrossRef]
- Martín-Cancho, M.F.; Lima, J.R.; Luis, L.; Crisóstomo, V.; López, M.A.; Ezquerra, L.J.; Carrasco-Jiménez, M.S.; Usón-Gargallo, J. Bispectral Index, Spectral Edge Frequency 95% and Median Frequency Recorded at Varying Desflurane Concentrations in Pigs. Res. Vet. Sci. 2006, 81, 373–381. [Google Scholar] [CrossRef]
- McKeegan, D.E.F.; Reimert, H.G.M.; Hindle, V.A.; Boulcott, P.; Sparrey, J.M.; Wathes, C.M.; Demmers, T.G.M.; Gerritzen, M.A. Physiological and Behavioral Responses of Poultry Exposed to Gas-Filled High Expansion Foam. Poult. Sci. 2013, 92, 1145–1154. [Google Scholar] [CrossRef]
- Sandercock, D.A.; Auckburally, A.; Flaherty, D.; Sandilands, V.; Mckeegan, D.E.F. Avian Reflex and Electroencephalogram Responses in Different States of Consciousness. Physiol. Behav. 2014, 133, 252–259. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ishida, Y.; Ueno, S.; Mitsuda, H. The Use of Sedation Action of CO2 for Long-Term Anesthesia in Carp. Bull. Jpn. Soc. Sci. Fish. 1988, 54, 545–551. [Google Scholar] [CrossRef]
- Erikson, U. Live Chilling and Carbon Dioxide Sedation at Slaughter of Farmed Atlantic Salmon: A Description of a Number of Commercial Case Studies. J. Appl. Aquac. 2008, 20, 38–61. [Google Scholar] [CrossRef]
- Erikson, U. Assessment of Different Stunning Methods and Recovery of Farmed Atlantic Salmon (Salmo Salar): Isoeugenol, Nitrogen and Three Levels of Carbon Dioxide. Anim. Welf. 2011, 20, 365–375. [Google Scholar] [CrossRef]
- Bernier, N.; Randall, D. Carbon Dioxide Anaesthesia in Rainbow Trout: Effects of Hypercapnic Level and Stress on Induction and Recovery from Anaesthetic Treatment. J. Fish Biol. 1998, 52, 621–637. [Google Scholar] [CrossRef]
- Robb, D.H.F.; Kestin, S.C. Methods Used to Kill Fish: Field Observations and Literature Reviewed. Anim. Welf. 2002, 11, 269–282. [Google Scholar] [CrossRef]
- Lambooij, E.; Grimsbø, E.; van de Vis, J.W.; Reimert, H.G.M.; Nortvedt, R.; Roth, B. Percussion and Electrical Stunning of Atlantic Salmon (Salmo Salar) after Dewatering and Subsequent Effect on Brain and Heart Activities. Aquaculture 2010, 300, 107–112. [Google Scholar] [CrossRef]
- McKeegan, D.E.F.; Sparks, N.H.C.; Sandilands, V.; Demmers, T.G.M.; Boulcott, P.; Wathes, C.M. Physiological Responses of Laying Hens during Whole-House Killing with Carbon Dioxide. Br. Poult. Sci. 2011, 52, 645–657. [Google Scholar] [CrossRef] [PubMed]
- McKeegan, D.E.F.; Sandercock, D.A.; Gerritzen, M.A. Physiological Responses to Low Atmospheric Pressure Stunning and Their Implications for Welfare. Poult. Sci. 2013, 92, 858–868. [Google Scholar] [CrossRef]
- Velarde, A.; Ruiz-de-la-Torre, J.L.; Roselló, C.; Fàbrega, E.; Diestre, A.; Manteca, X. Assessment of Return to Consciousness after Electrical Stunning in Lambs. Anim. Welf. 2002, 11, 333–341. [Google Scholar] [CrossRef]
- Gibson, T.J.; Johnson, C.B.; Murrell, J.C.; Mitchinson, S.L.; Stafford, K.J.; Mellor, D.J. Electroencephalographic Responses to Concussive Non-Penetrative Captive-Bolt Stunning in Halothane-Anaesthetised Calves. N. Z. Vet. J. 2009, 57, 90–95. [Google Scholar] [CrossRef]
- Gibson, T.J.; Johnson, C.B.; Murrell, J.C.; Mitchinson, S.L.; Stafford, K.J.; Mellor, D.J. Amelioration of Electroencephalographic Responses to Slaughter by Non-Penetrative Captive-Bolt Stunning after Ventral-Neck Incision in Halothane-Anaesthetised Calves. N. Z. Vet. J. 2009, 57, 96–101. [Google Scholar] [CrossRef]
- Bowman, J.; Hjelmstedt, P.; Gräns, A. Non-invasive Recording of Brain Function in Rainbow Trout: Evaluations of the Effects of MS-222 Anaesthesia Induction. Aquac. Res. 2019, 50, 3420–3428. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 25 April 2022).
- Bates, D.; Maechler, M.; Bolker, B.M.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Woodbury, D.M.; Karler, R. The Role of Carbon Dioxide in the Nervous System. Anesthesiology 1960, 21, 686–703. [Google Scholar]
- Lambooij, E.; Pilarczyk, M.; Bialowas, H.; van den Boogaart, J.G.M.; van de Vis, J.W. Electrical and Percussive Stunning of the Common Carp (Cyprinus carpio L.): Neurological and Behavioural Assessment. Aquac. Eng. 2007, 37, 171–179. [Google Scholar] [CrossRef]
- Bowman, J.; van Nuland, N.; Hjelmstedt, P.; Berg, C.; Gräns, A. Evaluation of the Reliability of Indicators of Consciousness during CO2 Stunning of Rainbow Trout and the Effects of Temperature. Aquac. Res. 2020, 51, 5194–5202. [Google Scholar] [CrossRef]
- Verhoeven, M.; Gerritzen, M.; Velarde, A.; Hellebrekers, L.; Kemp, B. Time to Loss of Consciousness and Its Relation to Behavior in Slaughter Pigs during Stunning with 80 or 95% Carbon Dioxide. Front. Vet. Sci. 2016, 3, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeegan, D.E.F.; McIntryre, J.A.; Demmers, T.G.M.; Lowe, J.C.; Wathes, C.M.; van den Broek, P.L.C.; Coenen, A.M.L.; Gentle, M.J. Physiological and Behavioural Responses of Broilers to Controlled Atmosphere Stunning: Implications for Welfare. Anim. Welf. 2007, 16, 409–426. [Google Scholar] [CrossRef]
- Gerritzen, M.A.; Reimert, H.G.M.; Hindle, V.A.; Verhoeven, M.T.W.; Veerkamp, W.B. Multistage Carbon Dioxide Gas Stunning of Broilers. Poult. Sci. 2013, 92, 41–50. [Google Scholar] [CrossRef]
- OSHA (Occupational Safety and Health Administration). Carbon Dioxide In Workplace Atmospheres. Available online: https://www.osha.gov/dts/sltc/methods/inorganic/id172/id172.html (accessed on 1 October 2018).
- Berenbrink, M. Evolution of the Bohr Effect. In Encyclopedia of Fish Physiology from Genome to Enviroment; Farrell, A.P., Ed.; Elsevier Inc.: San Diego, CA, USA, 2011; pp. 921–928. ISBN 9780080923239. [Google Scholar]
- Nikinmaa, M. Hemoglobin. In Encyclopedia of Fish Physiology from Genome to Enviroment; Farrell, A.P., Ed.; Elsevier Inc.: San Diego, CA, USA, 2011; pp. 887–892. Available online: https://www.elsevier.com/books/encyclopedia-of-fish-physiology/farrell/978-0-12-374545-3 (accessed on 1 October 2018).
- Scanes, C.G.; Dridi, S. Sturkie’s Avian Physiology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Raj, A.B.M.; Wotton, S.B.; McKinstry, J.L.; Hillebrand, S.J.W.; Pieterse, G. Changes in the Somatosensory Evoked Potentials and Spontaneous Electroencephalogram of Broiler Chickens during Exposure to Gas Mixtures. Br. Poult. Sci. 1998, 39, 686–695. [Google Scholar] [CrossRef]
- Gibson, T.J.; Johnson, C.B.; Stafford, K.J.; Mitchinson, S.L.; Mellor, D.J. Validation of the Acute Electroencephalographic Responses of Calves to Noxious Stimulus with Scoop Dehorning. N. Z. Vet. J. 2007, 55, 152–157. [Google Scholar] [CrossRef]
- Hernandez, E.; James, F.; Torrey, S.; Widowski, T.; Schwean-Lardner, K.; Monteith, G.; Turner, P.V. Electroencephalographic, Physiologic and Behavioural Responses during Cervical Dislocation Euthanasia in Turkeys. BMC Vet. Res. 2019, 15, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, E.; James, F.; Torrey, S.; Widowski, T.; Schwean-Lardner, K.; Monteith, G.; Turner, P.V. Evaluation of Brain Death in Laying Hens During On-Farm Killing by Cervical Dislocation Methods or Pentobarbital Sodium Injection. Front. Vet. Sci. 2019, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Lambooij, E.; Van de Vis, J.W.; Kloosterboer, R.J.; Pieterse, C. Evaluation of Captive Needle Stunning of Farmed Eel (Anguilla anguilla L.): Suitability for Humane Slaughter. Aquaculture 2002, 212, 141–148. [Google Scholar] [CrossRef]
- Velarde, A.; Raj, M. Animal Welfare at Slaughter, 1st ed.; Velarde, A., Raj, M., Eds.; 5m Publishing: Sheffield, UK, 2016. [Google Scholar]
- Gerritzen, M.A.; Lambooij, B.; Reimert, H.; Stegeman, A.; Spruijt, B. On-Farm Euthanasia of Broiler Chickens: Effects of Different Gas Mixtures on Behavior and Brain Activity. Poult. Sci. 2004, 83, 1294–1301. [Google Scholar] [CrossRef]
- Lambooij, E.; Gerritzen, M.A.; Reimert, H.; Burggraaf, D.; van de Vis, J.W. A Humane Protocol for Electro-Stunning and Killing of Nile Tilapia in Fresh Water. Aquaculture 2008, 275, 88–95. [Google Scholar] [CrossRef]
- Raj, M.; O’Callaghan, M.; Thompson, K.; Beckett, D.; Morrish, I.; Love, A.; Hickman, G.; Howson, S. Large Scale Killing of Poultry Species on Farm during Outbreaks of Diseases: Evaluation and Development of a Humane Containerised Gas Killing System. Worlds Poult. Sci. J. 2008, 64, 227–243. [Google Scholar] [CrossRef]
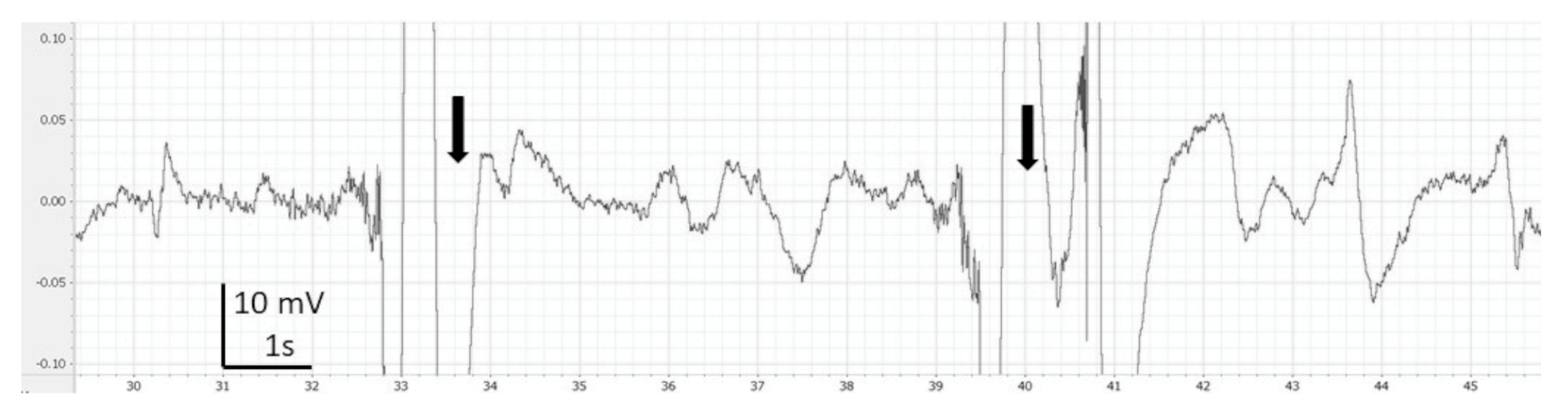
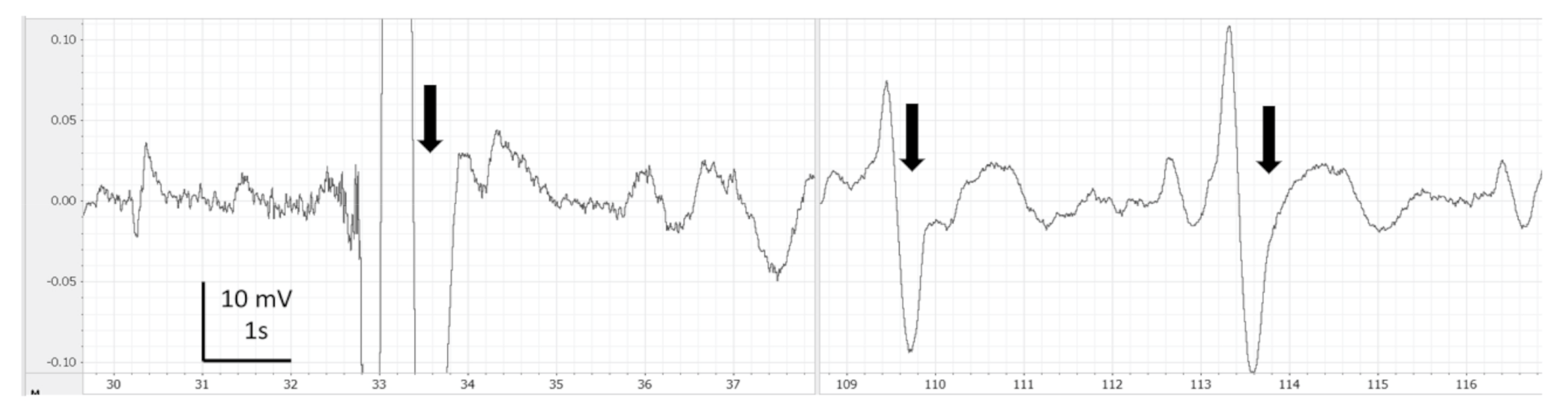
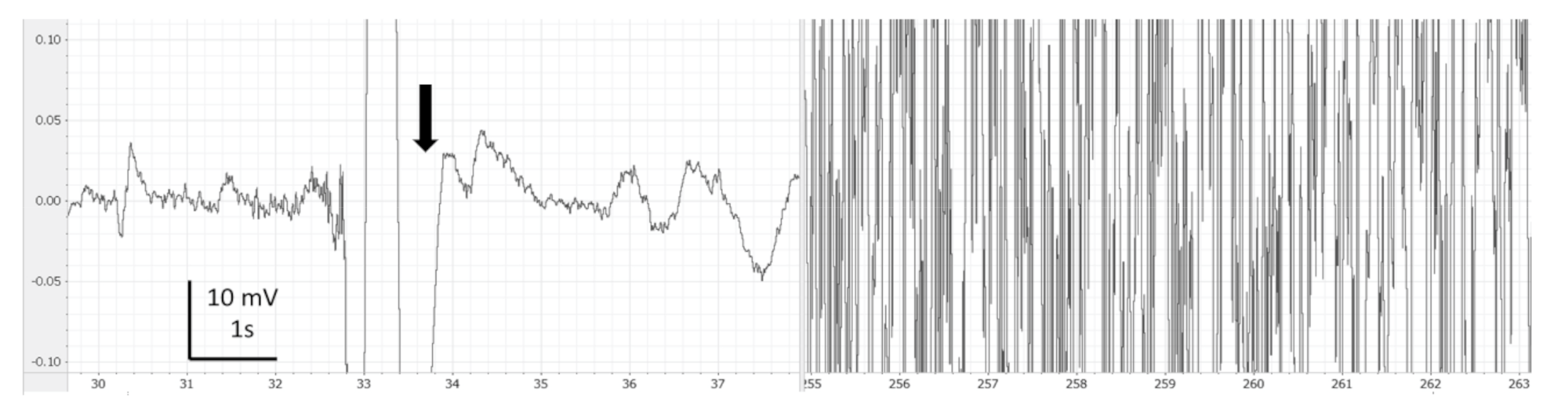


| Transitional | Excitation Phase | Suppressed | Suppressed * | Iso-Electric * | |
|---|---|---|---|---|---|
| Onset | 13.7 ± 10.3 | 126.2 ± 55.0 | 258.8 ± 46.2 | 482.6 ± 32.4 | 567.4 ± 89.1 |
| Duration | 107.9 ± 54.3 | 134.8 ± 36.5 | 180.2 ± 65.6 | 251.4 ± 83.7 | 237.5 ± 94.1 |
| n | 29 | 29 | 28 | 19 | 17 |
| Phase | Ptot (µV2) | F50 (Hz) | SEF (Hz) | LF (%) | HF (%) |
|---|---|---|---|---|---|
| Baseline | 21.4 (11.7; 39.0) B | 10.5 (9.2; 11.7) B | 31.6 (29.5; 33.7) A | 38.9 (31.9; 45.8) B | 55.4 (49.3; 61.6) B |
| Transitional | 32.98 (18.08; 60.2) B | 10.0 (8.7; 11.2) BC | 32.3 (30.2; 34.4) A | 41.9 (34.9; 48.8) B | 51.9 (45.7; 58.1) BC |
| Excitation | 5729.9 (3141.22; 10,452.1) A | 12.1 (10.9; 13.4) A | 32.7 (30.6; 34.8) A | 25.5 (18.5; 32.5) C | 68.4 (62.2; 74.5) A |
| Suppressed | 28.42 (15.58; 51.8) B | 8.1 (6.8; 9.3) D | 24.2 (22.0; 26.3) B | 52.7 (45.7; 59.7) A | 45.0 (38.8; 51.2) C |
| Iso-electric | 5.11 (2.39; 10.9) C | 8.3 (6.9; 9.8) CD | 26.3 (23.7; 28.9) B | 53.2 (44.9; 61.5) A | 42.7 (35.4; 50.0) C |
| Random effect | 21.4 (11.7; 39.0) B | 10.5 (9.2; 11.7) B | 31.6 (29.5; 33.7) A | 38.9 (31.9; 45.8) B | 55.4 (49.3; 61.6) B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rucinque, D.S.; van de Vis, H.; Reimert, H.; Roth, B.; Foss, A.; Taconeli, C.A.; Gerritzen, M. Pre-Slaughter Stunning of Farmed Atlantic Halibut in CO2-Saturated Seawater: Assessment of Unconsciousness by Electroencephalography (EEG). Animals 2023, 13, 1993. https://doi.org/10.3390/ani13121993
Rucinque DS, van de Vis H, Reimert H, Roth B, Foss A, Taconeli CA, Gerritzen M. Pre-Slaughter Stunning of Farmed Atlantic Halibut in CO2-Saturated Seawater: Assessment of Unconsciousness by Electroencephalography (EEG). Animals. 2023; 13(12):1993. https://doi.org/10.3390/ani13121993
Chicago/Turabian StyleRucinque, Daniel Santiago, Hans van de Vis, Henny Reimert, Bjørn Roth, Atle Foss, Cesar Augusto Taconeli, and Marien Gerritzen. 2023. "Pre-Slaughter Stunning of Farmed Atlantic Halibut in CO2-Saturated Seawater: Assessment of Unconsciousness by Electroencephalography (EEG)" Animals 13, no. 12: 1993. https://doi.org/10.3390/ani13121993
APA StyleRucinque, D. S., van de Vis, H., Reimert, H., Roth, B., Foss, A., Taconeli, C. A., & Gerritzen, M. (2023). Pre-Slaughter Stunning of Farmed Atlantic Halibut in CO2-Saturated Seawater: Assessment of Unconsciousness by Electroencephalography (EEG). Animals, 13(12), 1993. https://doi.org/10.3390/ani13121993





