A Selection of 14 Tetrameric Microsatellite Markers for Genetic Investigations in Fallow Deer (Dama dama)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Marker Selection and Primer Design
2.2. Sample Collection and DNA Extraction
2.3. Singleplex PCR Amplification, Optimization, and Primer Redesign
2.4. Estimation of the Polymorphism Level of the Functional Markers
2.4.1. Capillary Electrophoresis
2.4.2. Statistical Analyses
2.5. Allele Sequencing of Polymorphic Microsatellites
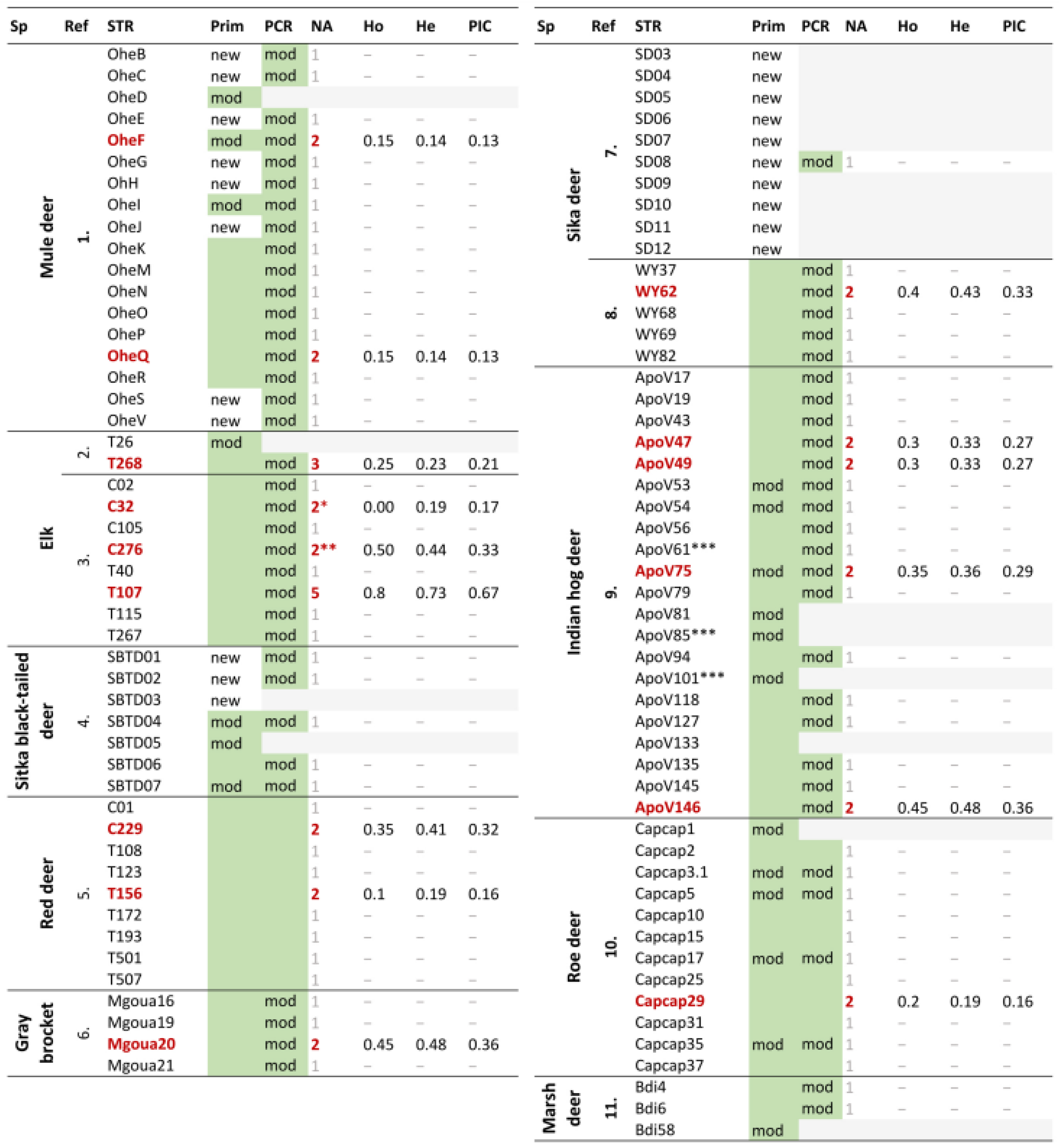
3. Results
3.1. Marker Selection, Primer Design and Redesign, PCR Optimization
3.2. Success Rate and Characteristics of Amplified PCR Product
3.3. Genotyping and Basic Statistical Values
3.4. Allele Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| STR = 14; N = 20 [own study] | STR = 10; N = 52 [20] | STR = 20; N = 262 [55] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NA | Ho | He | NA | Ho | He | NA | Ho | He | |
| average | 2.29 | 0.31 | 0.33 | 2.40 | 0.45 | 0.44 | 3.65 | 0.42 | 0.44 |
| median | 2.00 | 0.30 | 0.33 | 2.00 | 0.49 | 0.45 | 4.00 | 0.41 | 0.44 |
| min | 2.00 | 0.00 | 0.14 | 2.00 | 0.21 | 0.23 | 2.00 | 0.16 | 0.20 |
| max | 5.00 | 0.80 | 0.73 | 4.00 | 0.56 | 0.58 | 7.00 | 0.81 | 0.73 |
| STR = 7; N = 22 [34] | STR = 9; N = 111 [53] | STR = 10; N = 14 (only HU) [14] | |||||||
| NA | Ho | He | NA | Ho | He | NA | Ho | He | |
| average | 2.29 | 0.36 | 3.56 | 0.37 | 0.41 | 2.67 | 0.40 | 0.40 | |
| median | 2.00 | 0.41 | 3.00 | 0.38 | 0.38 | 2.00 | 0.38 | 0.46 | |
| min | 2.00 | 0.09 | 2.00 | 0.06 | 0.06 | 2.00 | 0.07 | 0.07 | |
| max | 3.00 | 0.64 | 5.00 | 0.68 | 0.67 | 4.00 | 0.79 | 0.69 | |
| OheF | OheQ | C229 | T156 | Capcap29 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species [ref] | FD | Elk [23] | Mule [31] | WT [31] | FD | Mule [21] | Mule [31] | WT [31] | FD | Elk [23] | Mule [31] | WT [31] | Red [25] | FD | Elk [23] | Red [25] | FD | Roe [29] | |
| N | 20 | 30 | 556 | 587 | 20 | 602 | 556 | 587 | 20 | 30 | 556 | 587 | 100 | 20 | 43 | 100 | 20 | 513 | |
| n | 2 | 4 | 6 | 10 | 2 | 15 | 5 | 31 | 2 | 4 | 16 | 15 | 5 | 2 | 9 | 15 | 2 | 9 | |
| PIC | 0.13 | 0.36 | 0.75 | 0.13 | 0.85 | 0.93 | 0.32 | 0.64 | 0.79 | 0.45 | 0.16 | 0.89 | 0.16 | 0.72 | |||||
| Ho | 0.15 | 0.87 | 0.32 | 0.65 | 0.15 | 0.53 | 0.84 | 0.35 | 0.50 | 0.61 | 0.68 | 0.49 | 0.10 | 0.44 | 0.85 | 0.20 | 0.74 | ||
| He | 0.14 | 0.69 | 0.38 | 0.78 | 0.14 | 0.86 | 0.93 | 0.41 | 0.69 | 0.68 | 0.81 | 0.50 | 0.19 | 0.68 | 0.90 | 0.19 | 0.76 | ||
| C32 | T107 | ApoV47 | T268 | C276 | ApoV49 | Mgoua20 | ApoV146 | ApoV75 | |||||||||||
| Species [ref] | FD | Elk [23] | FD | Elk [23] | Roe [29] | FD | Hog [28] | FD | Elk [23] | FD | Elk [23] | FD | Hog [28] | FD | BB [26] | FD | Hog [28] | FD | Hog [28] |
| N | 19 | 24 | 20 | 30 | 513 | 20 | 200 * | 20 | 43 | 18 | 27 | 20 | 213 | 20 | 14 | 20 | 208 | 20 | 200 * |
| n | 2 | 2 | 5 | 4 | 12 | 2 | 1 | 3 | 4 | 2 | 2 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 1 |
| PIC | 0.17 | 0.67 | 0.81 | 0.27 | 0.21 | 0.33 | 0.27 | 0.13 | 0.36 | 0.36 | 0.42 | 0.29 | |||||||
| Ho | 0.00 | 0.37 | 0.80 | 0.37 | 0.80 | 0.30 | 0.00 | 0.25 | 0.53 | 0.50 | 0.12 | 0.30 | 0.00 | 0.45 | 0.54 | 0.45 | 0.13 | 0.35 | 0.00 |
| He | 0.19 | 0.32 | 0.73 | 0.49 | 0.83 | 0.33 | 0.00 | 0.23 | 0.71 | 0.44 | 0.12 | 0.33 | 0.14 | 0.48 | 0.42 | 0.48 | 0.53 | 0.36 | 0.00 |
References
- Chapman, N.; Chapman, D. The Distribution of Fallow Deer: A Worldwide Review. Mammal Rev. 2008, 10, 61–138. [Google Scholar] [CrossRef]
- Esattore, B.; Saggiomo, L.; Sensi, M.; Francia, V.; Cherin, M. Tell Me What You Eat and I’ll Tell You…where You Live: An Updated Review of the Worldwide Distribution and Foraging Ecology of the Fallow Deer (Dama Dama). Mamm. Biol. 2022, 102, 321–338. [Google Scholar] [CrossRef]
- Corlatti, L.; Zachos, F.E. Terrestrial Cetartiodactyla; Springer Nature: Berlin/Heidelberg, Germany, 2022; ISBN 978-3-030-24475-0. [Google Scholar]
- Bijl, H.; Csányi, S. Fallow Deer (Dama Dama) Population and Harvest Changes in Europe since the Early 1980s. Sustainability 2022, 14, 12198. [Google Scholar] [CrossRef]
- Elek, B. The Criminalization of Poaching in Hungary. Zbornik radova Pravnog fakulteta Novi Sad 2019, 53, 639–648. [Google Scholar] [CrossRef]
- Gyurcsó, A.; Kasza, G.; Ózsvári, L. History and Food-Chain Safety Provisions of the Public Consumption of Game Meat in Hungary (A Vadhús Közfogyasztásának Története És Élelmiszerlánc- Biztonsági Előírásai Magyarországon). Magyar Allatorvosok Lapja 2022, 144, 623–639. [Google Scholar]
- Putman, R.; Langbein, J.; Green, P.; Watson, P. Identifying Threshold Densities for Wild Deer in the UK above Which Negative Impacts May Occur. Mammal Rev. 2011, 41, 175–196. [Google Scholar] [CrossRef]
- Gyurcsó, A.; Kasza, G.; Ózsvári, L. The History of Legal Regulation against Illegal Hunting in Hungary. Literature Review with Case Studies (Az Illegális Vadászat Elleni Jogi Szabályozás És a Vadvédelem Története Magyarországon. Irodalmi Áttekintés Esettanulmányokkal). Magyar Allatorvosok Lapja 2023, 145, 297–308. [Google Scholar] [CrossRef]
- Hoban, S.; Bruford, M.; D’Urban Jackson, J.; Lopes-Fernandes, M.; Heuertz, M.; Hohenlohe, P.A.; Paz-Vinas, I.; Sjögren-Gulve, P.; Segelbacher, G.; Vernesi, C.; et al. Genetic Diversity Targets and Indicators in the CBD Post-2020 Global Biodiversity Framework Must Be Improved. Biol. Conserv. 2020, 248, 108654. [Google Scholar] [CrossRef]
- Nassar, L.R.; Barber, G.P.; Benet-Pagès, A.; Casper, J.; Clawson, H.; Diekhans, M.; Fischer, C.; Gonzalez, J.N.; Hinrichs, A.S.; Lee, B.T.; et al. The UCSC Genome Browser Database: 2023 Update. Nucleic Acids Res. 2023, 51, D1188–D1195. [Google Scholar] [CrossRef]
- Miraldo, A.; Li, S.; Borregaard, M.K.; Flórez-Rodríguez, A.; Gopalakrishnan, S.; Rizvanovic, M.; Wang, Z.; Rahbek, C.; Marske, K.A.; Nogués-Bravo, D. An Anthropocene Map of Genetic Diversity. Science 2016, 353, 1532–1535. [Google Scholar] [CrossRef]
- Ekroth, A.K.E.; Rafaluk-Mohr, C.; King, K.C. Host Genetic Diversity Limits Parasite Success beyond Agricultural Systems: A Meta-Analysis. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191811. [Google Scholar] [CrossRef] [Green Version]
- Ekrami, B.; Tamadon, A.; Jahromi, I.R.; Moghadas, D.; Seno, M.M.G.; Ghaderi-Zefrehei, M. Fertility Reduction in Male Persian Fallow Deer (Dama dama mesopotamica): Inbreeding Detection and Morphometric Parameters Evaluation of Semen. J. Biosci. Med. 2016, 4, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Baker, K.H.; Gray, H.W.I.; Ramovs, V.; Mertzanidou, D.; Akın Pekşen, Ç.; Bilgin, C.C.; Sykes, N.; Hoelzel, A.R. Strong Population Structure in a Species Manipulated by Humans since the Neolithic: The European Fallow Deer (Dama dama dama). Heredity 2017, 119, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Pitarch, J.L.; Raksa, H.C.; Arnal, M.C.; Revilla, M.; Martínez, D.; Fernández de Luco, D.; Badiola, J.J.; Goldmann, W.; Acín, C. Low Sequence Diversity of the Prion Protein Gene (PRNP) in Wild Deer and Goat Species from Spain. Vet. Res. 2018, 49, 33. [Google Scholar] [CrossRef] [Green Version]
- Arifin, M.I.; Hannaoui, S.; Chang, S.C.; Thapa, S.; Schatzl, H.M.; Gilch, S. Cervid Prion Protein Polymorphisms: Role in Chronic Wasting Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 2271. [Google Scholar] [CrossRef]
- Kusza, S.; Ashrafzadeh, M.; Tóth, B.; Jávor, A. Maternal Genetic Variation in the Northeastern Hungarian Fallow Deer (Dama dama) Population. Mamm. Biol. 2018, 93, 21–28. [Google Scholar] [CrossRef]
- Hartl, G.B.; Schleger, A.; Slowak, M. Genetic Variability in Fallow Deer, Dama dama L. Anim. Genet. 1986, 17, 335–341. [Google Scholar] [CrossRef]
- Randi, E.; Apollonio, M. Low Biochemical Variability in European Fallow Deer (Dama dama L.): Natural Bottlenecks and the Effects of Domestication. Heredity 1988, 61, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Webley, L.S.; Zenger, K.R.; Hall, G.P.; Cooper, D.W. Genetic Structure of Introduced European Fallow Deer (Dama dama dama) in Tasmania, Australia. Eur. J. Wildl. Res. 2007, 53, 40–46. [Google Scholar] [CrossRef]
- Jones, K.C.; Levine, K.F.; Banks, J.D. DNA-Based Genetic Markers in Black-Tailed Nad Mule Deer for Forensic Applications. Calif. Fish Game 2000, 86, 115–126. [Google Scholar]
- Jones, K.; Levine, K.; Banks, J. Characterization of 11 Polymorphic Tetranucleotide Microsatellites for Forensic Applications in California Elk (Cervus elaphus canadensis). Mol. Ecol. Notes 2002, 2, 425–427. [Google Scholar] [CrossRef]
- Meredith, E.P.; Rodzen, J.A.; Levine, K.F.; Banks, J.D. Characterization of an Additional 14 Microsatellite Loci in California Elk (Cervus elaphus) for Use in Forensic and Population Applications. Conserv. Genet. 2005, 6, 151–153. [Google Scholar] [CrossRef]
- Brinkman, T.J.; Person, D.K.; Schwartz, M.K.; Pilgrim, K.L.; Colson, K.E.; Hundertmark, K.J. Individual Identification of Sitka Black-Tailed Deer (Odocoileus hemionus sitkensis) Using DNA from Fecal Pellets. Conserv. Genet. Resour. 2010, 2, 115–118. [Google Scholar] [CrossRef]
- Szabolcsi, Z.; Egyed, B.; Zenke, P.; Padar, Z.; Borsy, A.; Steger, V.; Pasztor, E.; Csanyi, S.; Buzas, Z.; Orosz, L. Constructing STR Multiplexes for Individual Identification of Hungarian Red Deer. J. Forensic. Sci. 2014, 59, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Caparroz, R.; Mantellatto, A.M.B.; Bertioli, D.J.; Figueiredo, M.G.; Duarte, J.M.B. Characterization of the Complete Mitochondrial Genome and a Set of Polymorphic Microsatellite Markers through Next-Generation Sequencing for the Brown Brocket Deer Mazama Gouazoubira. Genet. Mol. Biol. 2015, 38, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zheng, J.; Jia, B.; Wei, H.; Wang, G.; Yang, F. Isolation of Novel Microsatellite Markers and Their Application for Genetic Diversity and Parentage Analyses in Sika Deer. Gene 2018, 643, 68–73. [Google Scholar] [CrossRef]
- Hill, E. Forensic and Population Genetic Analysis of Introduced and Endangered Hog Deer (Axis porcinus). Master’s Thesis, La Trobe University, Melbourne, VIC, Australia, 2021. [Google Scholar]
- Morf, N.V.; Kopps, A.M.; Nater, A.; Lendvay, B.; Vasiljevic, N.; Webster, L.M.I.; Fautley, R.G.; Ogden, R.; Kratzer, A. STRoe Deer: A Validated Forensic STR Profiling System for the European Roe Deer (Capreolus capreolus). Forensic Sci. Int. Anim. Environ. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Wolfenson, L.I.; McCracken, G.R.; Ruzzante, D.E.; Mirol, P.; Solé-Cava, A. Low STR Variability in the Threatened Marsh Deer, Blastocerus Dichotomus, Detected through Amplicon Sequencing in Non-Invasive Samples. Genet. Mol. Biol. 2022, 45, e20220105. [Google Scholar] [CrossRef]
- Hamlin, B.C.; Meredith, E.P.; Rodzen, J.; Strand, J.M. OdoPlex: An STR Multiplex Panel Optimized and Validated for Forensic Identification and Sex Determination of North American Mule Deer (Odocoileus hemionus) and White-Tailed Deer (Odocoileus virginianus). Forensic Sci. Int. Anim. Environ. 2021, 1, 100026. [Google Scholar] [CrossRef]
- Reiner, G.; Lang, M.; Willems, H. Impact of Different Panels of Microsatellite Loci, Different Numbers of Loci, Sample Sizes, and Gender Ratios on Population Genetic Results in Red Deer. Eur. J. Wildl. Res. 2019, 65, 25. [Google Scholar] [CrossRef]
- Rębała, K.; Nedzvetskaya, D.E.; Kotova, S.A.; Zabavskaya, T.V.; Rybakova, V.I.; Kholodova, M.V.; Tsybovsky, I.S. STR Typing of European Elk (Moose) and European Roe Deer with Novel Forensic Assays Reveals Contrasting Patterns of Genetic Structure of the Two Cervids in Belarus. Russ. J. Genet. 2022, 58, 1493–1503. [Google Scholar] [CrossRef]
- Poetsch, M.; Seefeldt, S.; Maschke, M.; Lignitz, E. Analysis of Microsatellite Polymorphism in Red Deer, Roe Deer, and Fallow Deer—Possible Employment in Forensic Applications. Forensic Sci. Int. 2001, 116, 1–8. [Google Scholar] [CrossRef]
- Butler, J.M. Advanced Topics in Forensic DNA Typing: Methodology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Tagliaro, F.; Manetto, G.; Crivellente, F.; Smith, F.P. A Brief Introduction to Capillary Electrophoresis. Forensic Sci. Int. 1998, 92, 75–88. [Google Scholar] [CrossRef]
- Zianni, M.; Tessanne, K.; Merighi, M.; Laguna, R.; Tabita, F.R. Identification of the DNA Bases of a DNase I Footprint by the Use of Dye Primer Sequencing on an Automated Capillary DNA Analysis Instrument. J. Biomol. Tech. 2006, 17, 103–113. [Google Scholar]
- Blacket, M.; Robin, C.; Good, R.; Lee, S.F.; Miller, A.D. Universal Primers for Fluorescent Labelling of PCR Fragments-an Efficient and Cost-Effective Approach to Genotyping by Fluorescence. Mol. Ecol. Resour. 2012, 12, 456–463. [Google Scholar] [CrossRef]
- Sim, Z.; Monderman, L.; Hildebrand, D.; Packer, T.; Jobin, R.M. Development and Implementation of a STR Based Forensic Typing System for Moose (Alces Alces). Forensic Sci. Int. Genet. 2021, 53, 102536. [Google Scholar] [CrossRef]
- Loukovitis, D.; Szabó, M.; Chatziplis, D.; Monori, I.; Kusza, S. Genetic Diversity and Substructuring of the Hungarian Merino Sheep Breed Using Microsatellite Markers. Anim. Biotechnol. 2022, 0, 1–9. [Google Scholar] [CrossRef]
- Roche Applied Science GS Guide to Amplicon Sequencing 2006.
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a Genetic Linkage Map in Man Using Restriction Fragment Length Polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Peakall, R.; Smouse, P.E. Genalex 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Waits, L.P.; Luikart, G.; Taberlet, P. Estimating the Probability of Identity among Genotypes in Natural Populations: Cautions and Guidelines. Mol. Ecol. 2001, 10, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peery, M.Z.; Beissinger, S.R.; House, R.F.; Bérubé, M.; Hall, L.A.; Sellas, A.; Palsbøll, P.J. Characterizing Source–Sink Dynamics with Genetic Parentage Assignments. Ecology 2008, 89, 2746–2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linacre, A.; Gusmão, L.; Hecht, W.; Hellmann, A.P.; Mayr, W.R.; Parson, W.; Prinz, M.; Schneider, P.M.; Morling, N. ISFG: Recommendations Regarding the Use of Non-Human (Animal) DNA in Forensic Genetic Investigations. Forensic Sci. Int. Genet. 2011, 5, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Leibelt, C.; Budowle, B.; Collins, P.; Daoudi, Y.; Moretti, T.; Nunn, G.; Reeder, D.; Roby, R. Identification of a D8S1179 Primer Binding Site Mutation and the Validation of a Primer Designed to Recover Null Alleles. Forensic Sci. Int. 2003, 133, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Takada, N.; Suzuki, R.; Nagaoka, S.; Watanabe, Y. Identification of a Rare Mutation in a TH01 Primer Binding Site. Leg. Med. 2007, 9, 289–292. [Google Scholar] [CrossRef]
- Luttman, A.M.; Komine, M.; Thaiwong, T.; Carpenter, T.; Ewart, S.L.; Kiupel, M.; Langohr, I.M.; Venta, P.J. Development of a 17-Plex of Penta- and Tetra-Nucleotide Microsatellites for DNA Profiling and Paternity Testing in Horses. Front. Vet. Sci. 2022, 9, 861623. [Google Scholar] [CrossRef]
- Scandura, M. The Use of Microsatellites in the Study of Social Structure in Large Mammals: Italian Wolf and Fallow Deer as Case Studies; Bielefeld University: Bielefeld, Germany, 2004. [Google Scholar]
- Turi, O.; Wagenhoffer, Z.; Battay, M.; Lehotzky, P.; Zorkóczy, O. Cross-species testing of microsatellite markers for individual identification in fallow deer (Dama dama) (Mikroszatellita-markerek tesztelése dámszarvasok egyedi azonosítása céljából). Magyar Allatorvosok Lapja 2023, 145, 183–192. [Google Scholar] [CrossRef]
- Scandura, M.; Tiedemann, R.; Apollonio, M.; Hartl, G.B. Genetic Variation in an Isolated Italian Population of Fallow Deer Dama dama as Revealed by RAPD-PCR. Acta Theriologica Suppl. 1998, 43, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Luikart, G.; Allendorf, F.W.; Cornuet, J.M.; Sherwin, W.B. Distortion of Allele Frequency Distributions Provides a Test for Recent Population Bottlenecks. J. Hered. 1998, 89, 238–247. [Google Scholar] [CrossRef]
- Say, L.; Naulty, F.; Hayden, T.J. Genetic and Behavioural Estimates of Reproductive Skew in Male Fallow Deer. Mol. Ecol. 2003, 12, 2793–2800. [Google Scholar] [CrossRef]
- Chapman, D. Fallow Deer: Their History, Distribution, and Biology; Coch-y-bonddu Books: Machynlleth, United Kingdom, 1997; ISBN 978-0-9528510-5-9. [Google Scholar]
- Masseti, M.; Pecchioli, E.; Vernesi, C. Phylogeography of the Last Surviving Populations of Rhodian and Anatolian Fallow Deer (Dama dama dama L., 1758). Biol. J. Linn. Soc. 2008, 93, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Yannouli, E.; Trantalidou, K. The Fallow deer (Dama dama L. 1758) in Greece. Archaeological Presence and Representation. In The Holocene History of the European Vertebrate Fauna: Modern Aspects of Research; Verlag Marie Leidorf GmbH: Rahden, Germany, 1999; pp. 247–281. [Google Scholar]
- Davis, S.; MacKinnon, M. Did the Romans Bring Fallow Deer to Portugal? Environ. Archaeol. 2009, 14, 15–26. [Google Scholar] [CrossRef]
- Valenzuela, A.; Baker, K.; Carden, R.; Evans, J.; Higham, T.; Hoelzel, R.; Lamb, A.; Madgwick, R.; Miller, H.; Alcover, J.; et al. Both Introduced and Extinct: The Fallow Deer of Roman Mallorca. J. Archaeol. Sci. Rep. 2016, 9, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Madgwick, R.; Sykes, N.; Miller, H.; Symmons, R.; Morris, J.; Lamb, A. Fallow Deer (Dama dama dama) Management in Roman South-East Britain. Archaeol. Anthropol. Sci. 2013, 5, 111–122. [Google Scholar] [CrossRef]
- Perdikaris, S.; Bain, A.; Grouard, S.; Baker, K.; Gonzalez, E.; Hoelzel, A.R.; Miller, H.; Persaud, R.; Sykes, N. From Icon of Empire to National Emblem: New Evidence for the Fallow Deer of Barbuda. Environ. Archaeol. 2018, 23, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Lu, G.; Clover, G. Novel Approaches to Mitigate Primer Interaction and Eliminate Inhibitors in Multiplex PCR, Demonstrated Using an Assay for Detection of Three Strawberry Viruses. J. Virol. Methods 2008, 151, 132–139. [Google Scholar] [CrossRef]
| Marker | Tail | Size (bp) | Forward Primer | Reverse Primer | PCR Protocol |
|---|---|---|---|---|---|
| OheF | B | 199–211 | CAGGCGATCAAGAAATGTGG | GTGGCTTCTGGATGGAGAAC | 32 × (94 °C 30 s, 56 °C 30 s, 72 °C 30 s) + 72 °C 20 m |
| OheQ | D | 248–264 | AATGTGTCAGTGAAGGTCTTC | ATCCAGGCAACCATCTAG | |
| C229 | A | 117–125 | TTATTCATCCACCCATCCATCACCA | GGCACATGCTCATAAGTGAAGGGA | 29 × (94 °C 40 s, 61 °C 40 s, 72 °C 60 s) + 60 °C 60 m |
| T156 | C | 135–148 | CCTGGCCTGTGTCTTGAATTGAAC | GGCGATGAATACCCAGTCTTGTCT | 29 × (94 °C 40 s, 63 °C 40 s, 72 °C 60 s) + 60 °C 60 m |
| Capcap29 | C | 199–203 | AAGCCCATGACCTGAAACCAA | GCTTCCAGCAGGAGGGTATAT | 5 × (94 °C 30 s, 62 °C 90 s, 72 °C 90 s) |
| 5 × (94 °C 30 s, 58 °C 90 s, 72 °C 90 s) | |||||
| 5 × (94 °C 30 s, 55 °C 90 s, 72 °C 90 s) | |||||
| 20 × (94 °C 30 s, 50 °C 90 s, 72 °C 90 s) + 72 °C 10 m | |||||
| ApoV47 | A | 329–333 | TGCTCATTCTAGGGTCAGGC | AGGTCTTCTGCATTGTAGGC | 32 × (94 °C 30 s, 64 °C 30 s, 72 °C 60 s) + 60 °C 30 m |
| ApoV49 | B | 408–412 | ACTATGGGATGTGACCGTGG | ACAGGAATCTTGTTGACTCTGC | 32 × (94 °C 30 s, 56 °C 30 s, 72 °C 60 s) + 60 °C 30 m |
| ApoV146 | D | 143–148 | GGGCCCTCAATTCTCTTCC | GGAGACATCACATTCCCTGAC | 32 × (94 °C 30 s, 58 °C 30 s, 72 °C 60 s) + 60 °C 30 m |
| Mgoua20 | B | 193–197 | ACAACTGGAGAAAACCCTTGTG | AGCCTTTAGAGATGTTCTGTTTGG | 15 × (94 °C 30 s, 55 °C 60 s, 72 °C 40 s) 20 × (94 °C 30 s, 50 °C 60 s, 72 °C 40 s) + 72 °C 20 m |
| T107 | D | 283–308 | ACATCCGTTCAGGTGTGA | CCAGAGGTAAGATAAATGGTGA | |
| T268 | B | 224–240 | ATTCCCTTCTCCAGTGTATG | GATGATAACAGCTCAACAGATC | |
| C32 | A | 285–289 | ACAACTGTGTGAGCCAATAC | AGCAAGTGAAGAAGAATGTTC | 15 × (94 °C 30 s, 60 °C 60 s, 72 °C 40 s) |
| 20 × (94 °C 30 s, 55 °C 60 s, 72 °C 40 s) + 72 °C 20 m | |||||
| C276 | A | 376–380 | AAACAGAACATTCACCAGAAAC | TCCCAGACACACAGAACAA | 32 × (94 °C 30 s, 63.3 °C 60 s, 72 °C 40 s) + 72 °C 20 m |
| ApoV75 | A | 328–336 | TCGTTTTACATTCCTATCAGCAACG | GTTTCTTTACTGAGATGCCGACTCCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorkóczy, O.K.; Turi, O.; Wagenhoffer, Z.; Ózsvári, L.; Lehotzky, P.; Pádár, Z.; Zenke, P. A Selection of 14 Tetrameric Microsatellite Markers for Genetic Investigations in Fallow Deer (Dama dama). Animals 2023, 13, 2083. https://doi.org/10.3390/ani13132083
Zorkóczy OK, Turi O, Wagenhoffer Z, Ózsvári L, Lehotzky P, Pádár Z, Zenke P. A Selection of 14 Tetrameric Microsatellite Markers for Genetic Investigations in Fallow Deer (Dama dama). Animals. 2023; 13(13):2083. https://doi.org/10.3390/ani13132083
Chicago/Turabian StyleZorkóczy, Orsolya Krisztina, Orsolya Turi, Zsombor Wagenhoffer, László Ózsvári, Pál Lehotzky, Zsolt Pádár, and Petra Zenke. 2023. "A Selection of 14 Tetrameric Microsatellite Markers for Genetic Investigations in Fallow Deer (Dama dama)" Animals 13, no. 13: 2083. https://doi.org/10.3390/ani13132083






