Pre-Implantation Bovine Embryo Evaluation—From Optics to Omics and Beyond
Abstract
:Simple Summary
Abstract
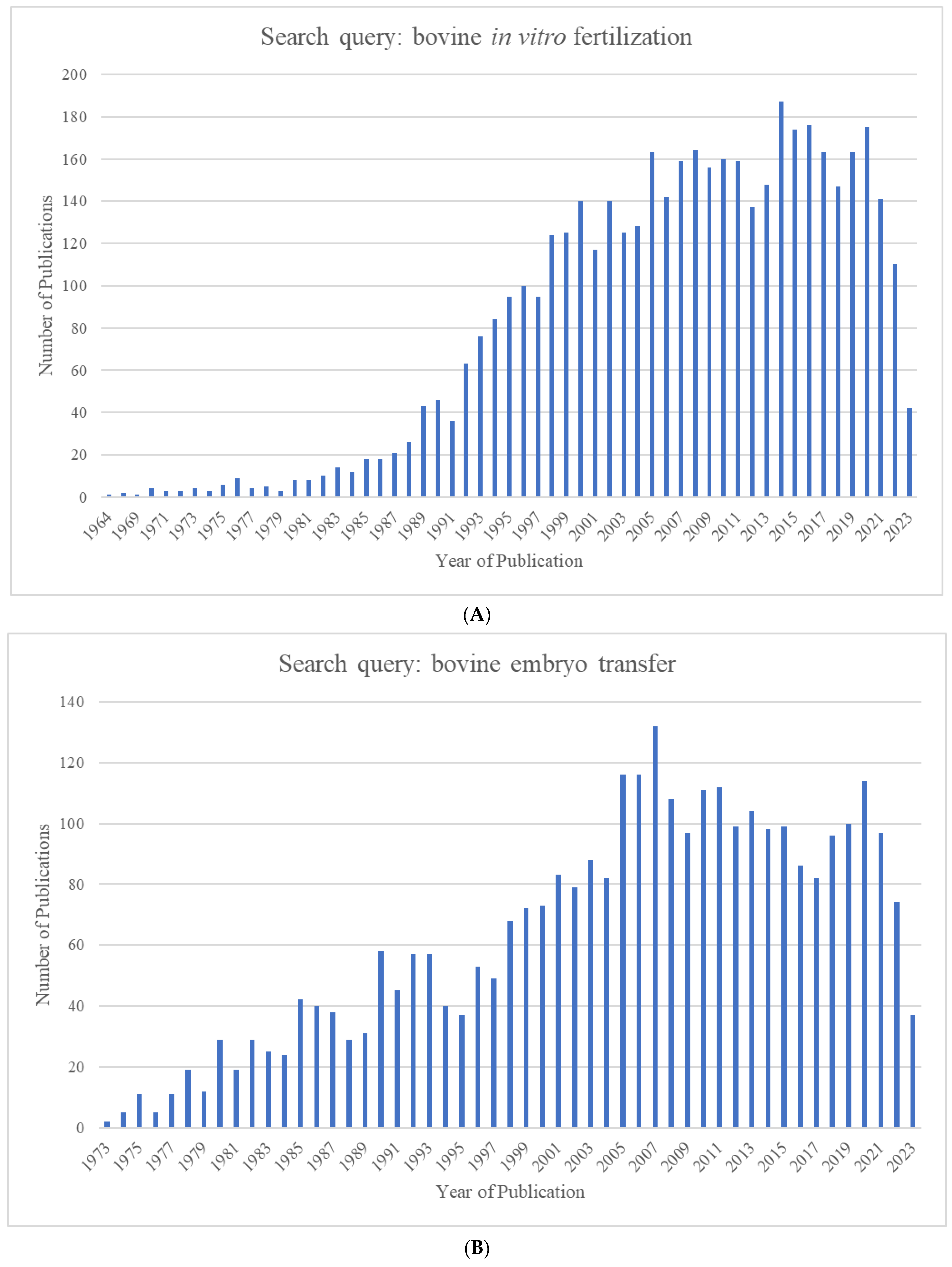
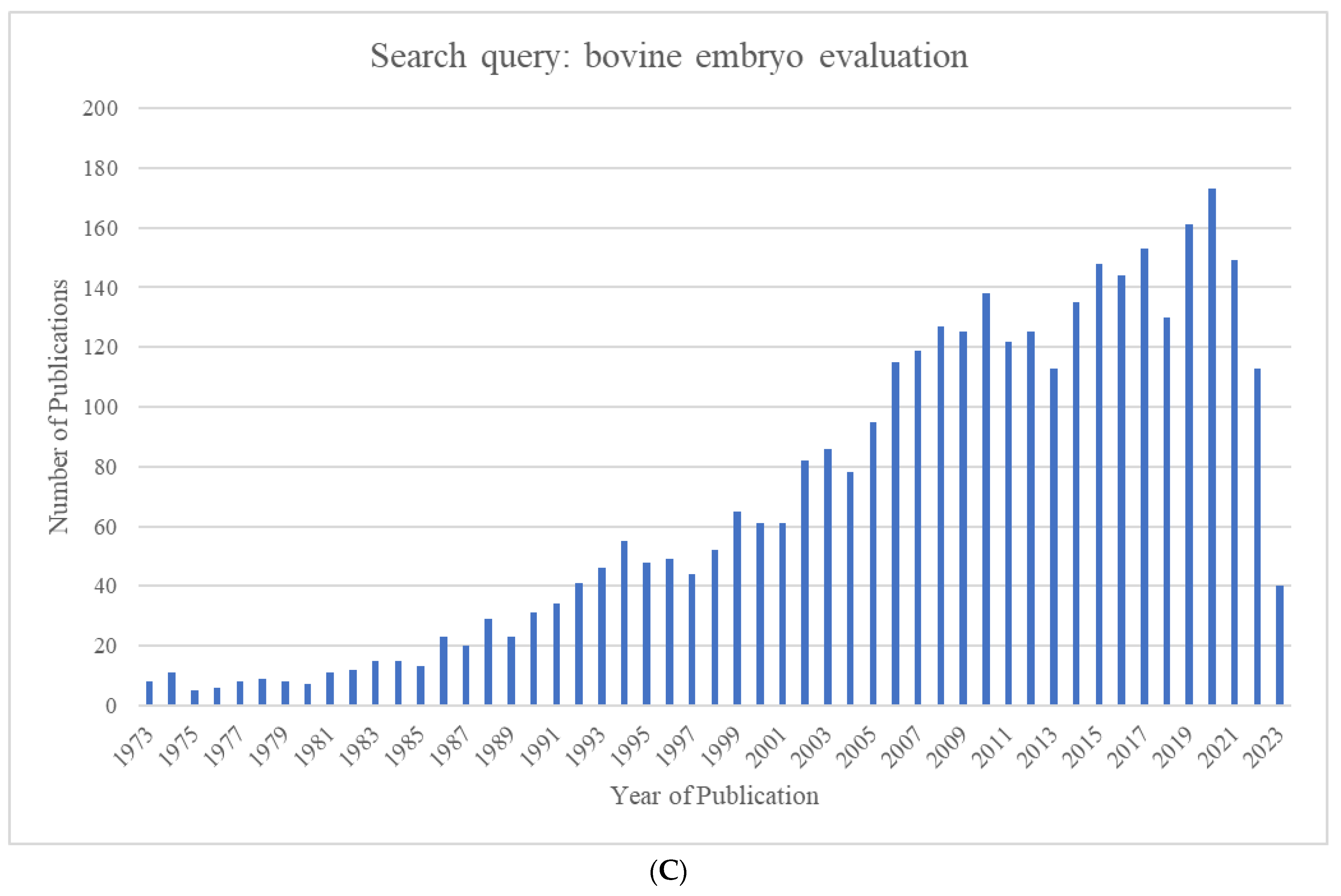
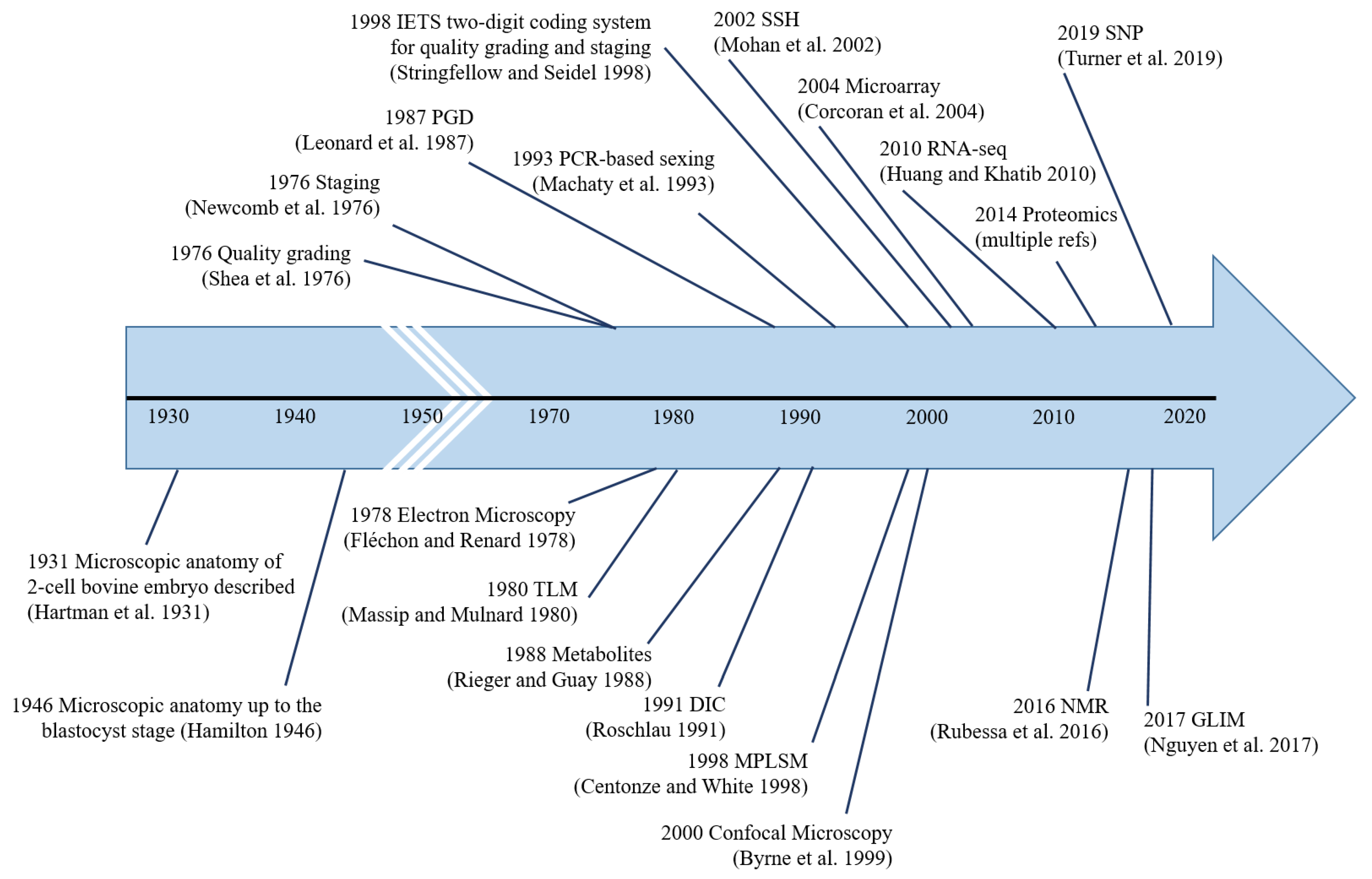
1. Introduction
2. Evaluation of Pre-Implantation Bovine Embryos
2.1. Evaluation for Embryo Quality
| Source | Criteria |
|---|---|
| [16] | “5: excellent appearing 4 3: average appearing 2 1: very poor appearing” rated “on the basis of compactness, symmetry and density of the blastomeres” |
| [17] | “Excellent—judged to be at the normal stage of development at the time of examination; embryos were symmetrical, and blastomeres were polygonal in shape forming a tight mass at the morula stage. Good—similar to excellent embryos but were asymmetrical, contained blastomeres excluded from the main morula mass, or were slightly retarded relative to other embryos recovered from the same donor Fair—embryos were retarded 1 to 2 days in development, had spherical rather than polygonal blastomeres at the morula stage, contained blastomeres of varying sizes, had signs of degeneration such as large vesicles in the cells, and/or were darker or lighter than normal Poor—embryos were retarded 2 or more days in development, had indistinct cell membranes, and/or had more severe faults than the fair embryos” |
| [18] | “4—embryo is above average in appearance (perfectly symmetrical, even granulation, no deformations in the zona pellucida, no blastomeres extruded) 3—embryo is average in appearance 2—embryo is below average in appearance (uneven blastomere size, extensive blastomere extrusion, evidence of membrane rupture)” |
| [7] | “Excellent—an ideal embryo, spherical, symmetrical with cells of uniform size, color and texture Good—trivial imperfections such as a few extruded blastomeres, irregular shape, few vesicles Fair—definite but not severe problems, presence of extruded blastomeres, vesiculation, few degenerated cells Poor—severe problems, numerous extruded blastomeres, degenerated cells, cells of varying sizes, large numerous vesicles but a viable-appearing embryo mass” |
| [20] | “Code 1: Excellent or good Code 2: Fair Code 3: Poor Code 4: Degenerated” |
2.2. Evaluation for Embryo Staging/Grading
3. Embryo Evaluation/Analysis—Tools of the Trade
3.1. Microscopic Analyses
3.1.1. Light Microscopy
3.1.2. Differential Interference Contrast (DIC) Microscopy
3.1.3. Electron Microscopy
3.1.4. Fluorescence Microscopy
3.1.5. Confocal Microscopy
3.1.6. Multiphoton Laser Scanning Microscopy (MPLSM)
3.1.7. Gradient Light Interference Microscopy (GLIM)
3.1.8. Time-Lapse Monitoring (TLM)
| Applications of TLM in Bovine Embryo Analysis | Source |
|---|---|
| To compare cleavage intervals of healthy embryos and degenerate embryos | [97] |
| To study development kinetics of embryos derived from calf oocytes | [98] |
| To study the effects of activin A and follistatin on developmental kinetics | [99] |
| To study effects of glucose on developmental kinetics of male and female embryos | [100] |
| To compare developmental kinetics of IVD and IVP embryos | [101] |
| To study dynamics of the 4th cell cycle coincident with EGA | [102] |
| To compare kinetics of initial cleavage patterns in viable and non-viable embryos | [103] |
| To study embryo development in well-of-the-well (WOW) systems | [104] |
| To identify morphokinetics predictive of blastocyst quality and pregnancy outcome | [94] |
| To study effects of abnormal cleavage patterns on morphokinetics and growth potential | [105] |
| To study development kinetics of IVP embryos fertilized with sex-sorted semen | [106] |
| To study pronuclear morphokinetics | [107] |
| To study effects of first zygotic cleavage dysmorphisms on the metabolomic profile | [108] |
| To correlate embryo morphokinetics with their transcriptomic profiles | [109] |
| To study morphokinetics of embryos derived from vitrified bovine oocytes | [90] |
3.2. Non-Microscopic Analyses
3.2.1. Pre-Implantation Genetic Diagnosis (PGD)
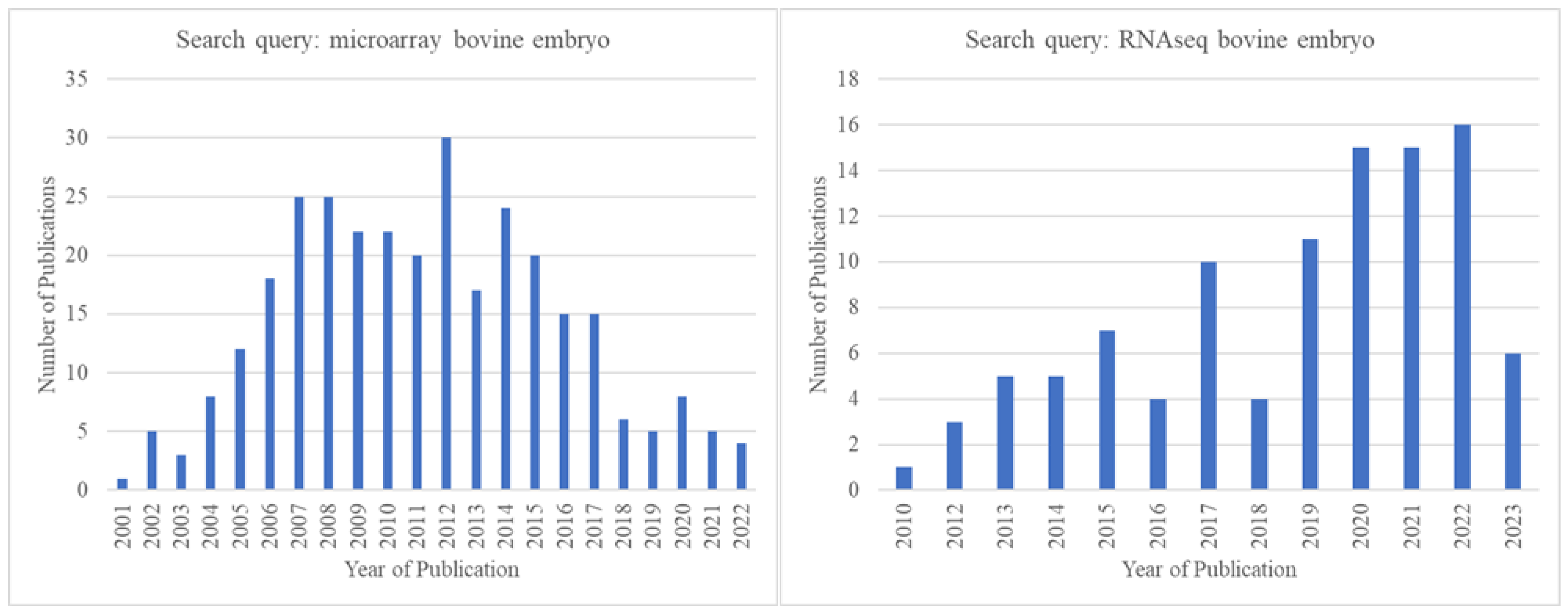
3.2.2. Omics-Based Embryo Analyses
Genomic Studies
Microarray for Transcriptome Profiling
RNA Sequencing (RNA-Seq) for Transcriptome Profiling
Proteomics
Metabolic Profiling
| Analyzed Metabolite or Indicator of Metabolism | Source/s |
|---|---|
| Glucose | [212,215,227,228,229,230] |
| Pyruvate | [212,215,225,228,229,231] |
| Lactate | [215,224,225,229,231] |
| Amino acids | [212,216,220,221,224,225,226,227,228,229,231,232,233] |
| Fatty acids | [221,234,235] |
| Oxygen | [217,218,229,236] |
| Reactive oxygen species (ROS) | [218] |
| Myo-inositol | [224,231] |
| Citrate | [224,231,233] |
| Formate | [224,231] |
| Prostaglandins | [221] |
| Biotin | [220] |
3.2.3. Nuclear Magnetic Resonance (NMR)
3.2.4. Less Commonly Used Tools
4. Discussion
4.1. Microscopic Techniques
4.2. Non-Microscopic Techniques
4.3. The Ideal Embryo Evaluation Tool
- The method should provide accurate results;
- It should be non-invasive;
- It should be objective (minimize human subjectivity);
- It should be low-cost (initial and ongoing costs);
- It should be technically simple enough to be carried out at the embryo production facility itself;
- The evaluation should be completed by blastulation so that high-quality blastocysts can be immediately identified for direct transfer, without a lag period like in the case of omics-based techniques;
- The results should be available within hours of sample collection so that a second freeze–thaw cycle can be avoided.
4.4. Application of Omics-Based Tools in Field Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocha, J.C.; Passalia, F.J.; Matos, F.D.; Takahashi, M.B.; Maserati, M.P., Jr.; Alves, M.F.; de Almeida, T.G.; Cardoso, B.L.; Basso, A.C.; Nogueira, M.F.G. Automatized image processing of bovine blastocysts produced in vitro for quantitative variable determination. Sci. Data 2017, 4, 170192. [Google Scholar] [CrossRef] [Green Version]
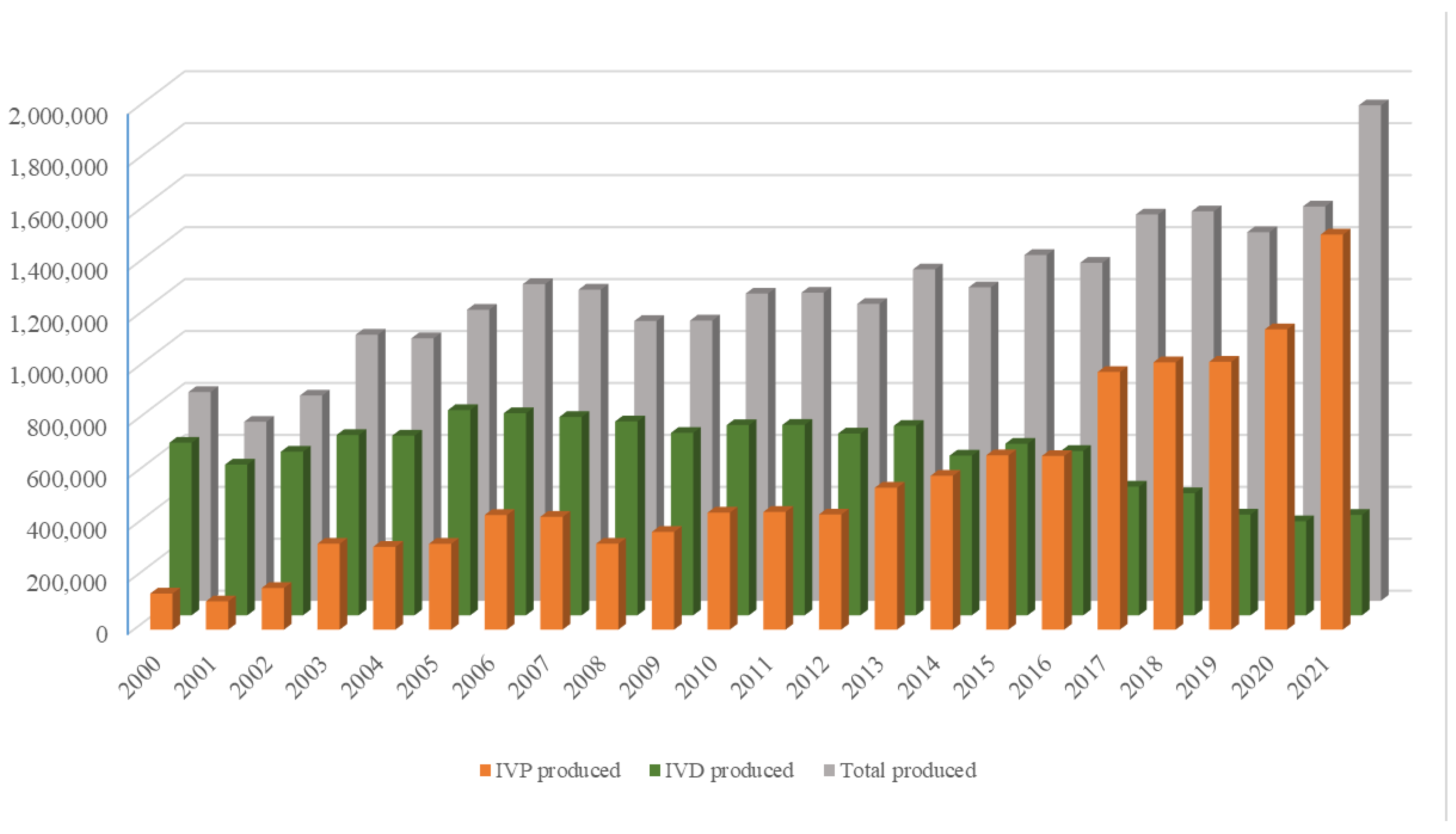
- Viana, J. 2021 Statistics of embryo production and transfer in domestic farm animals. Embryo Technol. Newsl. 2022, 40, 19. [Google Scholar]
- Marsico, T.; De Camargo, J.; Valente, R.S.; Sudano, M.J. Embryo competence and cryosurvival: Molecular and cellular features. Anim. Reprod. 2019, 16, 423–439. [Google Scholar] [CrossRef]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. Board Invited Review: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Farin, P.; Britt, J.; Shaw, D.; Slenning, B. Agreement among evaluators of bovine embryos produced in vivo or in vitro. Theriogenology 1995, 44, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J. The incompletely fulfilled promise of embryo transfer in cattle—Why aren’t pregnancy rates greater and what can we do about it? J. Anim. Sci. 2020, 98, skaa288. [Google Scholar] [CrossRef] [PubMed]
- Lindner, G.M.; Wright, R.W. Bovine embryo morphology and evaluation. Theriogenology 1983, 20, 407–416. [Google Scholar] [CrossRef]
- Schneider, H.; Castleberry, R.; Griffin, J. Commercial aspects of bovine embryo transfer. Theriogenology 1980, 13, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Tervit, H.; Cooper, M.; Goold, P.G.; Haszard, G. Non-surgical embryo transfer in cattle. Theriogenology 1980, 13, 63–71. [Google Scholar] [CrossRef]
- Donnay, I. Metabolic Markers of Embryo Viability. In Assessment of Mammalian Embryo Quality: Invasive and Non-Invasive Techniques; Van Soom, A., Boerjan, M., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 57–94. [Google Scholar]
- Bó, G.; Mapletoft, R. Evaluation and classification of bovine embryos. Anim. Reprod. (AR) 2018, 10, 344–348. [Google Scholar]
- Gomez, E.; Canela, N.; Herrero, P.; Cereto, A.; Gimeno, I.; Carrocera, S.; Martin-Gonzalez, D.; Murillo, A.; Muñoz, M. Metabolites Secreted by Bovine Embryos In Vitro Predict Pregnancies That the Recipient Plasma Metabolome Cannot, and Vice Versa. Metabolites 2021, 11, 162. [Google Scholar] [CrossRef]
- Hartman, C.G.; Lewis, W.H.; Miller, F.W.; Swett, W.W. First findings of tubal ova in the cow, together with notes on oestrus. Anat. Rec. 1931, 48, 267–275. [Google Scholar] [CrossRef]
- Hamilton, W.J.; Laing, J.A. Development of the egg of the cow up to the stage of blastocyst formation. J. Anat. 1946, 80, 194. [Google Scholar] [PubMed]
- Seidel, G.E. Critical Review of Embryo Transfer Procedures with Cattle. In Fertilization and Embryonic Development In Vitro; Mastroianni, L., Biggers, J.D., Eds.; Springer: Boston, MA, USA, 1981; pp. 323–353. [Google Scholar]
- Shea, B.; Hines, D.; Lightfoot, D.; Ollis, G.; Olson, S. The transfer of bovine embryos. In Agriculture Research Seminar; Commission of the European Communities: Brussels, Belgium, 1976; pp. 145–152. [Google Scholar]
- Elsden, R.; Nelson, L.; Seidel, G. Superovulating cows with follicle stimulating hormone and pregnant mare’s serum gonadotrophin. Theriogenology 1978, 9, 17–26. [Google Scholar] [CrossRef]
- Shea, B. Evaluating the bovine embryo. Theriogenology 1981, 15, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, R.; Rowson, L.E.A. Conception rate after uterine transfer of cow eggs, in relation to synchronization of oestrus and age of eggs. Reproduction 1975, 43, 539–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringfellow, D.A.; Seidel, S.M. Manual of the International Embryo Transfer Society; The Society: Burlington, ON, Canada, 1998. [Google Scholar]
- Newcomb, R.; Rowson, L.; Trounson, A. The entry of superovulated eggs into the uterus. In Egg Transfer in Cattle; Commission of the European Communities: Brussels, Belgium, 1976; pp. 1–15. [Google Scholar]
- Rocha, J.C.; Passalia, F.J.; Matos, F.D.; Maserati, M.P., Jr.; Alves, M.F.; De Almeida, T.G.; Cardoso, B.L.; Basso, A.C.; Nogueira, M.F.G. Methods for assessing the quality of mammalian embryos: How far we are from the gold standard? JBRA Assist. Reprod. 2016, 20, 150–158. [Google Scholar] [CrossRef]
- Donnay, I.; Partridge, R.J.; Leese, H.J. Can embryo metabolism be used for selecting bovine embryos before transfer? Reprod. Nutr. Dev. 1999, 39, 523–533. [Google Scholar] [CrossRef] [Green Version]
- de México, A. Assessment of Bos taurus embryos comparing stereoscopic microscopy and transmission electron microscopy. J. Cell Anim. Biol. 2008, 2, 072–078. [Google Scholar]
- Driver, A.M.; Peñagaricano, F.; Huang, W.; Ahmad, K.R.; Hackbart, K.S.; Wiltbank, M.C.; Khatib, H. RNA-Seq analysis uncovers transcriptomic variations between morphologically similar in vivo- and in vitro-derived bovine blastocysts. BMC Genom. 2012, 13, 118. [Google Scholar] [CrossRef] [Green Version]
- Antony, P.P.M.A.; Trefois, C.; Stojanovic, A.; Baumuratov, A.S.; Kozak, K. Light microscopy applications in systems biology: Opportunities and challenges. Cell Commun. Signal. 2013, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, M.M.; Galina, C.S.; Merchant, H.; Montiel, F.; Canseco, R.; Márquez, Y.C. Comparison of stereoscopy, light microscopy and ultrastructural methods for evaluation of bovine embryos. Reprod. Domest. Anim. 2002, 37, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, S.; Narumi, K.; Korenaga, T. Interference phase-contrast imaging technology without beam separation. Sci. Rep. 2019, 9, 1753. [Google Scholar] [CrossRef]
- Rubessa, M.; Wheeler, M.B. Label-free microscopy: A non-invasive new tool to assess gametes and embryo quality. Theriogenology 2020, 150, 241–246. [Google Scholar] [CrossRef]
- Roschlau, K. Gene transfer studies in cattle. J. Reprod. Fertil. 1991, 43, 293–295. [Google Scholar] [CrossRef]
- Bavister, B.D.; Rose-Hellekant, T.A.; Pinyopummintr, T. Development of in vitro matured/in vitro fertilized bovine embryos into morulae and blastocysts in defined culture media. Theriogenology 1992, 37, 127–146. [Google Scholar] [CrossRef]
- Elmileik, A.; Maeda, T.; Terada, T. Higher rates of development into blastocyst following the in vitro fertilization of bovine oocytes matured in a medium supplemented with the fluid from large bovine follicles. Anim. Reprod. Sci. 1995, 38, 85–96. [Google Scholar] [CrossRef]
- Tominaga, K.; Shimizu, M.; Ooyama, S.; Izaike, Y. Effect of lipid polarization by centrifugation at different developmental stages on post-thaw survival of bovine in vitro produced 16-cell embryos. Theriogenology 2000, 53, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2001, 61, 57–66. [Google Scholar] [CrossRef]
- Duszewska, A.; Kozikova, L.; Szydlik, H.; Cybulska, M.; Korwin-Kossakowski, M.; Połoszynowicz, J.; Wicińska, K.; Rosochacki, S.; Waś, B. The use of green fluorescent protein (GFP) to select bovine embryos. J. Anim. Feed. Sci. 2003, 12, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Rosochacki, S.J.; Kozikova, L.V.; Korwin-Kossakowski, M.; Matejczyk, M.; Połoszynowicz, J.; Duszewska, A. Noninvasive fluorescent screening of microinjected bovine embryos to predict transgene integration. Folia Biol. 2003, 51, 97–104. [Google Scholar]
- Neuber, E.; Luetjens, C.; Chan, A.; Schatten, G. Analysis of DNA fragmentation of in vitro cultured bovine blastocysts using TUNEL. Theriogenology 2002, 57, 2193–2202. [Google Scholar] [CrossRef]
- Pereira, R.; Baptista, M.; Vasques, M.; Horta, A.; Portugal, P.; Bessa, R.; e Silva, J.C.; Pereira, M.S.; Marques, C. Cryosurvival of bovine blastocysts is enhanced by culture with trans-10 cis-12 conjugated linoleic acid (10t,12c CLA). Anim. Reprod. Sci. 2007, 98, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Carvalhais, I.; Pimenta, J.; Baptista, M.; Vasques, M.; Horta, A.; Santos, I.; Marques, M.D.R.; Reis, A.; Pereira, M.S.; et al. Biopsied and vitrified bovine embryos viability is improved by trans10, cis12 conjugated linoleic acid supplementation during in vitro embryo culture. Anim. Reprod. Sci. 2007, 106, 322–332. [Google Scholar] [CrossRef]
- Malatesta, M. Transmission Electron Microscopy as a Powerful Tool to Investigate the Interaction of Nanoparticles with Subcellular Structures. Int. J. Mol. Sci. 2021, 22, 12789. [Google Scholar] [CrossRef]
- Flechon, J.-E.; Renard, J.-P. A scanning electron microscope study of the hatching of bovine blastocysts in vitro. Reproduction 1978, 53, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massip, A. Ultrastructure of the cow blastocyst. J. Submicrose. Cytol. 1981, 13, 31–40. [Google Scholar]
- Crosier, A.E.; Farin, P.W.; Dykstra, M.J.; Alexander, J.E.; Farin, C.E. Ultrastructural Morphometry of Bovine Blastocysts Produced In Vivo or In Vitro. Biol. Reprod. 2001, 64, 1375–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurincik, J.; Thomsen, P.; Hay-Schmidt, A.; Avery, B.; Greve, T.; Ochs, R.; Hyttel, P. Nucleolar Proteins and Nuclear Ultrastructure in Preimplantation Bovine Embryos Produced In Vitro. Biol. Reprod. 2000, 62, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- King, W.A.; Niar, A.; Chartrain, I.; Betteridge, K.J.; Guay, P. Nucleolus organizer regions and nucleoli in preattachment bovine embryos. Reproduction 1988, 82, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Kopečný, V.; Fléchon, J.E.; Camous, S.; Fulka, J. Nucleologenesis and the onset of transcription in the eight-cell bovine embryo: Fine-structural autoradiographic study. Mol. Reprod. Dev. 1989, 1, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Baran, V.; Fléchon, J.-E.; Pivko, J. Nucleologenesis in the cleaving bovine embryo: Immunocytochemical aspects. Mol. Reprod. Dev. 1996, 44, 63–70. [Google Scholar] [CrossRef]
- Laurincik, J.; Kopecny, V.; Hyttel, P. Detailed analysis of pronucleus development in bovine zygotes in vivo: Ultrastructure and cell cycle chronology. Mol. Reprod. Dev. Inc. Gamete Res. 1996, 43, 62–69. [Google Scholar] [CrossRef]
- Laurincik, J.; Hyttel, P.; Baran, V.; Lucas-Hahn, A.; Eckert, J.; Pivko, J.; Niemann, H.; Brem, G.; Schellander, K. Pronucleus development and intranuclear bodies organization during progress of the first bovine embryonic cell cycle in vitro. Mol. Reprod. Dev. 1998, 50, 192–199. [Google Scholar]
- Crozet, N. Ultrastructural aspects of in vivo fertilization in the cow. Gamete Res. 1984, 10, 241–251. [Google Scholar] [CrossRef]
- Kuwayama, M.; Fujikawa, S.; Nagai, T. Ultrastructure of IVM-IVF Bovine Blastocysts Vitrified after Equilibration in Glycerol 1,2-Propanediol Using 2-Step and 16-Step Procedures. Cryobiology 1994, 31, 415–422. [Google Scholar] [CrossRef]
- Shamsuddin, M.; Rodriguez-Martinez, H. Fine Structure of Bovine Blastocysts Developed Either in Serum-Free Medium or in Conventional Co-Culture With Oviduct Epithelial Cells. J. Vet. Med. Ser. A 1994, 41, 307–316. [Google Scholar] [CrossRef]
- Abe, H.; Hoshi, H. Evaluation of Bovine Embryos Produced in High Performance Serum-Free Media. J. Reprod. Dev. 2003, 49, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Sollecito, N.; Alves, R.; Beletti, M.; Pereira, E.; Miranda, M.; Silva, J.; Borges, A. Morphometry of bovine blastocysts produced in vitro in culture media with antioxidants cysteamine or oily extract of Lippia origanoides. Arq. Bras. Med. Vet. Zootec. 2021, 73, 799–811. [Google Scholar] [CrossRef]
- Fair, T.; Lonergan, P.; Dinnyes, A.; Cottell, D.C.; Hyttel, P.; Ward, F.A.; Boland, M.P. Ultrastructure of bovine blastocysts following cryopreservation: Effect of method of blastocyst production. Mol. Reprod. Dev. 2001, 58, 186–195. [Google Scholar] [CrossRef]
- Abe, H.; Otoi, T.; Tachikawa, S.; Yamashita, S.; Satoh, T.; Hoshi, H. Fine structure of bovine morulae and blastocysts in vivo and in vitro. Anat. Embryol. 1999, 199, 519–527. [Google Scholar] [CrossRef]
- Plante, L.; King, W.A. Light and electron microscopic analysis of bovine embryos derived byin Vitro andin Vivo fertilization. J. Assist. Reprod. Genet. 1994, 11, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Kandel, M.E.; Rubessa, M.; Wheeler, M.B.; Popescu, G. Gradient light interference microscopy for 3D imaging of unlabeled specimens. Nat. Commun. 2017, 8, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, M.J.; Smith, I.; Parker, I.; Bootman, M.D. Fluorescence Microscopy. Cold Spring Harb. Protoc. 2014, 2014, pdb.top071795. [Google Scholar] [CrossRef] [Green Version]
- Specht, E.A.; Braselmann, E.; Palmer, A.E. A Critical and Comparative Review of Fluorescent Tools for Live-Cell Imaging. Annu. Rev. Physiol. 2017, 79, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, M.; Kobayashi, S.-I.; Takahashi, M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol. Reprod. Dev. 2004, 67, 77–82. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhang, H.; Wang, Y.-S.; Xu, W.-B.; Xiong, X.-R.; Li, Y.-Y.; Su, J.-M.; Hua, S.; Zhang, Y. Scriptaid improves in vitro development and nuclear reprogramming of somatic cell nuclear transfer bovine embryos. Cell. Reprogram. 2011, 13, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Slimane, W.; Heyman, Y.; Lavergne, Y.; Humblot, P.; Renard, J.P. Assessing Chromosomal Abnormalities in Two-Cell Bovine In Vitro-Fertilized Embryos by Using Fluorescent In Situ Hybridization with Three Different Cloned Probes. Biol. Reprod. 2000, 62, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Daigneault, B.W.; Rajput, S.; Smith, G.W.; Ross, P.J. Embryonic POU5F1 is Required for Expanded Bovine Blastocyst Formation. Sci. Rep. 2018, 8, 7753. [Google Scholar] [CrossRef] [Green Version]
- Amos, W.B.; White, J.G.; Fordham, M. Use of confocal imaging in the study of biological structures. Appl. Opt. 1987, 26, 3239–3243. [Google Scholar] [CrossRef]
- White, J.G.; Amos, W.B.; Fordham, M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J. Cell Biol. 1987, 105, 41–48. [Google Scholar] [CrossRef]
- Inoué, S. Handbook of Biological Confocal Microscopy; Pawley, J.B., Ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Jonkman, J.; Brown, C.M.; Wright, G.D.; Anderson, K.I.; North, A.J. Tutorial: Guidance for quantitative confocal microscopy. Nat. Protoc. 2020, 15, 1585–1611. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.J.; Perez, G.I.; Ko, T.; Yoo, M.S.; Cibelli, J.B. Full developmental potential of mammalian preimplantation embryos is maintained after imaging using a spinning-disk confocal microscope. Biotechniques 2006, 41, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, E.; Babbey, C.M.; Dunn, K.W. Performance comparison between the high-speed Yokogawa spinning disc confocal system and single-point scanning confocal systems. J. Microsc. 2005, 218, 148–159. [Google Scholar] [CrossRef]
- Byrne, A.; Southgate, J.; Brison, D.R.; Leese, H.J. Analysis of apoptosis in the preimplantation bovine embryo using TUNEL. Reproduction 1999, 117, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Matwee, C.; Betts, D.H.; King, W.A. Apoptosis in the early bovine embryo. Zygote 1999, 8, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Vanroose, G.; Nauwynck, H.; Van Soom, A.; Ysebaert, M.-T.; Charlier, G.; Van Oostveldt, P.; de Kruif, A. Structural Aspects of the Zona Pellucida of In Vitro-Produced Bovine Embryos: A Scanning Electron and Confocal Laser Scanning Microscopic Study. Biol. Reprod. 2000, 62, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Pennetier, S.; Perreau, C.; Uzbekova, S.; Thélie, A.; Delaleu, B.; Mermillod, P.; Dalbiès-Tran, R. MATER protein expression and intracellular localization throughout folliculogenesis and preimplantation embryo development in the bovine. BMC Dev. Biol. 2006, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Liu, L.; Lei, T.; Cui, X.; An, X.; Chen, Y. Genomic DNA methylation patterns in bovine preimplantation embryos derived from in vitro fertilization. Sci. China Life Sci. 2007, 50, 56–61. [Google Scholar] [CrossRef]
- Wang, L.; Feng, H.; Ma, Y.; Cang, M.; Li, H.; Yan, Z.; Zhou, P.; Wen, J.; Bou, S.; Liu, D. Expression of IGF receptors and its ligands in bovine oocytes and preimplantation embryos. Anim. Reprod. Sci. 2009, 114, 99–108. [Google Scholar] [CrossRef]
- Kuijk, E.W.; van Tol, L.T.A.; Van de Velde, H.; Wubbolts, R.; Welling, M.; Geijsen, N.; Roelen, B.A.J. The roles of FGF and MAP kinase signaling in the segregation of the epiblast and hypoblast cell lineages in bovine and human embryos. Development 2012, 139, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeja, Z.E.; Sosnowski, J.; Hryniewicz, K.; Warzych, E.; Pawlak, P.; Rozwadowska, N.; Plusa, B.; Lechniak, D. Changes in sub-cellular localisation of trophoblast and inner cell mass specific transcription factors during bovine preimplantation development. BMC Dev. Biol. 2013, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Simmet, K.; Zakhartchenko, V.; Philippou-Massier, J.; Blum, H.; Klymiuk, N.; Wolf, E. OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2770–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, D.R.; Dubé, D.; Gall, L.; Peynot, N.; Ruffini, S.; Laffont, L.; Le Bourhis, D.; Degrelle, S.; Jouneau, A.; Duranthon, V. Expression of pluripotency master regulators during two key developmental transitions: Ega and early lineage specification in the bovine embryo. PLoS ONE 2012, 7, e34110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, T.; Suzuki, R.; Furuta, N.; Suzuki, Y.; Kabe, K.; Tokoro, M.; Sugawara, A.; Yajima, A.; Nagasawa, T.; Matoba, S.; et al. Live-cell imaging of nuclear–chromosomal dynamics in bovine in vitro fertilised embryos. Sci. Rep. 2018, 8, 7460. [Google Scholar] [CrossRef] [PubMed]
- Mesalam, A.; Lee, K.-L.; Khan, I.; Chowdhury, M.M.R.; Zhang, S.; Song, S.-H.; Joo, M.-D.; Lee, J.-H.; Jin, J.-I.; Kong, I.-K. A combination of bovine serum albumin with insulin–transferrin–sodium selenite and/or epidermal growth factor as alternatives to fetal bovine serum in culture medium improves bovine embryo quality and trophoblast invasion by induction of matrix metalloproteinases. Reprod. Fertil. Dev. 2019, 31, 333–346. [Google Scholar] [CrossRef]
- Monteiro, C.A.S.; Chow, D.J.; Leal, G.R.; Tan, T.C.; Ferreira, A.M.R.; Thompson, J.G.; Dunning, K.R. Optical imaging of cleavage stage bovine embryos using hyperspectral and confocal approaches reveals metabolic differences between on-time and fast-developing embryos. Theriogenology 2020, 159, 60–68. [Google Scholar] [CrossRef]
- Squirrell, J.M.; Wokosin, D.L.; White, J.G.; Bavister, B.D. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat. Biotechnol. 1999, 17, 763–767. [Google Scholar] [CrossRef]
- Centonze, V.E.; White, J.G. Multiphoton Excitation Provides Optical Sections from Deeper within Scattering Specimens than Confocal Imaging. Biophys. J. 1998, 75, 2015–2024. [Google Scholar] [CrossRef] [Green Version]
- You, S.; Tu, H.; Chaney, E.J.; Sun, Y.; Zhao, Y.; Bower, A.J.; Liu, Y.-Z.; Marjanovic, M.; Sinha, S.; Pu, Y.; et al. Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nat. Commun. 2018, 9, 2125. [Google Scholar] [CrossRef] [Green Version]
- Yadlowsky, M.J.; Schmitt, J.M.; Bonner, R.F. Multiple scattering in optical coherence microscopy. Appl. Opt. 1995, 34, 5699–5707. [Google Scholar] [CrossRef] [PubMed]
- Rivenson, Y.; Ozcan, A. Deep learning accelerates whole slide imaging for next-generation digital pathology applications. Light. Sci. Appl. 2022, 11, 300. [Google Scholar] [CrossRef]
- Welte, M.A. As the fat flies: The dynamic lipid droplets of Drosophila embryos. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 1156–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angel-Velez, D.; De Coster, T.; Azari-Dolatabad, N.; Fernández-Montoro, A.; Benedetti, C.; Pavani, K.; Van Soom, A.; Pascottini, O.B.; Smits, K. Embryo morphokinetics derived from fresh and vitrified bovine oocytes predict blastocyst development and nuclear abnormalities. Sci. Rep. 2023, 13, 4765. [Google Scholar] [CrossRef] [PubMed]
- Magata, F. Time-lapse monitoring technologies for the selection of bovine in vitro fertilized embryos with high implantation potential. J. Reprod. Dev. 2023, 69, 57–64. [Google Scholar] [CrossRef]
- Massip, A.; Mulnard, J. Time-lapse cinematographic analysis of hatching of normal and frozen--thawed cow blastocysts. Reproduction 1980, 58, 475–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massip, A.; Mulnard, J.; Vanderzwalmen, P.; Hanzen, C.; Ectors, F. The behaviour of cow blastocyst in vitro: Cinematographic and morphometric analysis. J. Anat. 1982, 134, 399–405. [Google Scholar]
- Sugimura, S.; Akai, T.; Hashiyada, Y.; Somfai, T.; Inaba, Y.; Hirayama, M.; Yamanouchi, T.; Matsuda, H.; Kobayashi, S.; Aikawa, Y.; et al. Promising System for Selecting Healthy In Vitro–Fertilized Embryos in Cattle. PLoS ONE 2012, 7, e36627. [Google Scholar] [CrossRef] [Green Version]
- Sugimura, S.; Akai, T.; Imai, K. Selection of viable in vitro-fertilized bovine embryos using time-lapse monitoring in microwell culture dishes. J. Reprod. Dev. 2017, 63, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Hardin, J. Confocal and Multi-Photon Imaging of Living Embryos. In Handbook Of Biological Confocal Microscopy; Pawley, J.B., Ed.; Springer: Boston, MA, USA, 2006; pp. 746–768. [Google Scholar]
- Holm, P.; Shukri, N.; Vajta, G.; Booth, P.; Bendixen, C.; Callesen, H. Developmental kinetics of the first cell cycles of bovine in vitro produced embryos in relation to their in vitro viability and sex. Theriogenology 1998, 50, 1285–1299. [Google Scholar] [CrossRef]
- Majerus, V.; Lequarré, A.S.; Ferguson, E.M.; Kaidi, S.; Massip, A.; Dessy, F.; Donnay, I. Characterization of embryos derived from calf oocytes: Kinetics of cleavage, cell allocation to inner cell mass, and trophectoderm and lipid metabolism. Mol. Reprod. Dev. 2000, 57, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Suzuki, C.; Iwamura, S. Effects of activin A and follistatin on developmental kinetics of bovine embryos: Cinematographic analysis in a chemically defined medium. J. Reprod. Fertil. 2000, 118, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Peippo, J.; Kurkilahti, M.; Bredbacka, P. Developmental kinetics of in vitro produced bovine embryos: The effect of sex, glucose and exposure to time-lapse environment. Zygote 2001, 9, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.; Booth, P.J.; Callesen, H. Kinetics of early in vitro development of bovine in vivo- and in vitro-derived zygotes produced and/or cultured in chemically defined or serum-containing media. Reproduction 2002, 123, 553–565. [Google Scholar] [CrossRef]
- Lequarre, A.; Marchandise, J.; Moreau, B.; Massip, A.; Donnay, I. Cell Cycle Duration at the Time of Maternal Zygotic Transition for In Vitro Produced Bovine Embryos: Effect of Oxygen Tension and Transcription Inhibition. Biol. Reprod. 2003, 69, 1707–1713. [Google Scholar] [CrossRef] [Green Version]
- Somfai, T.; Inaba, Y.; Aikawa, Y.; Ohtake, M.; Kobayashi, S.; Konishi, K.; Imai, K. Relationship between the length of cell cycles, cleavage pattern and developmental competence in bovine embryos generated by in vitro fertilization or parthenogenesis. J. Reprod. Dev. 2010, 56, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Sugimura, S.; Akai, T.; Somfai, T.; Hirayama, M.; Aikawa, Y.; Ohtake, M.; Hattori, H.; Kobayashi, S.; Hashiyada, Y.; Konishi, K.; et al. Time-lapse cinematography-compatible polystyrene-based microwell culture system: A novel tool for tracking the development of individual bovine embryos. Biol. Reprod. 2010, 83, 970–978. [Google Scholar] [CrossRef] [Green Version]
- Magata, F.; Ideta, A.; Okubo, H.; Matsuda, F.; Urakawa, M.; Oono, Y. Growth potential of bovine embryos presenting abnormal cleavage observed through time lapse cinematography. Theriogenology 2019, 133, 119–124. [Google Scholar] [CrossRef]
- Magata, F.; Urakawa, M.; Matsuda, F.; Oono, Y. Developmental kinetics and viability of bovine embryos produced in vitro with sex-sorted semen. Theriogenology 2020, 161, 243–251. [Google Scholar] [CrossRef]
- Suzuki, R.; Okada, M.; Nagai, H.; Kobayashi, J.; Sugimura, S. Morphokinetic analysis of pronuclei using time-lapse cinematography in bovine zygotes. Theriogenology 2021, 166, 55–63. [Google Scholar] [CrossRef]
- Lechniak, D.; Sell-Kubiak, E.; Warzych, E. The metabolic profile of bovine blastocysts is affected by in vitro culture system and the pattern of first zygotic cleavage. Theriogenology 2022, 188, 43–51. [Google Scholar] [CrossRef]
- Yaacobi-Artzi, S.; Kalo, D.; Roth, Z. Association between the morphokinetics of in-vitro-derived bovine embryos and the transcriptomic profile of the derived blastocysts. PLoS ONE 2022, 17, e0276642. [Google Scholar] [CrossRef] [PubMed]
- Verlinsky, Y.; Pergament, E.; Strom, C. The preimplantation genetic diagnosis of genetic diseases. J. Assist. Reprod. Genet. 1990, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.L.; Edwards, R.G. Control of the Sex Ratio at Full Term in the Rabbit by transferring Sexed Blastocysts. Nature 1968, 218, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.; Reed, K. Micronanipulation of bovine embryos for sex determination. Theriogenology 1991, 35, 45–54. [Google Scholar] [CrossRef]
- Leonard, M.; Kirszenbaum, M.; Cotinot, C.; Chesne, P.; Heyman, Y.; Stinnakre, M.; Bishop, C.; Delouis, C.; Vaiman, M.; Fellous, M. Sexing bovine embryos using Y chromosome specific DNA probe. Theriogenology 1987, 27, 248. [Google Scholar] [CrossRef]
- Bondioli, K.; Ellis, S.; Pryor, J.; Williams, M.; Harpold, M. The use of male-specific chromosomal DNA fragments to determine the sex of bovine preimplantation embryos. Theriogenology 1989, 31, 95–104. [Google Scholar] [CrossRef]
- Mullis, K.B. The unusual origin of the polymerase chain reaction. Sci. Am. 1990, 262, 56–65. [Google Scholar] [CrossRef]
- Machaty, Z.; Paldi, A.; Csaki, T.; Varga, Z.; Kiss, I.; Bárándi, Z.; Vajta, G. Biopsy and sex determination by PCR of IVF bovine embryos. Reproduction 1993, 98, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, J.; Choi, K.; Joung, S.; Kim, J.; Chung, G.; Jin, D.; Im, K. Rapid sexing of preimplantation bovine embryo using consecutive and multiplex polymerase chain reaction (PCR) with biopsied single blastomere. Theriogenology 2001, 55, 1843–1853. [Google Scholar] [CrossRef]
- Chrenek, P.; Boulanger, L.; Heyman, Y.; Uhrin, P.; Laurincik, J.; Bulla, J.; Renard, J.-P. Sexing and multiple genotype analysis from a single cell of bovine embryo. Theriogenology 2001, 55, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Hu, C.L.; Wang, C.H.; Hung, C.M.; Wu, H.K.; Choo, K.B.; Cheng, W.T.K. Gender determination in single bovine blastomeres by polymerase chain reaction amplification of sex-specific polymorphic fragments in the amelogenin gene. Mol. Reprod. Dev. 1999, 54, 209–214. [Google Scholar] [CrossRef]
- Wrenzycki, C.; Herrmann, D.; Carnwath, J.W.; Niemann, H. Expression of the gap junction gene connexin43 (Cx43) in preimplantation bovine embryos derived in vitro or in vivo. Reproduction 1996, 108, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneault, C.; McGraw, S.; Massicotte, L.; Sirard, M.-A. Transcription Factor Expression Patterns in Bovine In Vitro-Derived Embryos Prior to Maternal-Zygotic Transition. Biol. Reprod. 2004, 70, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.M.N.; Zhang, Y.; Hansen, P.J. Single-cell gene expression of the bovine blastocyst. Reproduction 2017, 154, 627–644. [Google Scholar] [CrossRef]
- Mundim, T.; Ramos, A.; Sartori, R.; Dode, M.; Melo, E.; Gomes, L.; Rumpf, R.; Franco, M. Changes in gene expression profiles of bovine embryos produced in vitro, by natural ovulation, or hormonal superstimulation. Genet. Mol. Res. 2009, 8, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Ponsart, C.; Le Bourhis, D.; Knijn, H.; Fritz, S.; Guyader-Joly, C.; Otter, T.; Lacaze, S.; Charreaux, F.; Schibler, L.; Dupassieux, D.; et al. Reproductive technologies and genomic selection in dairy cattle. Reprod. Fertil. Dev. 2014, 26, 12–21. [Google Scholar] [CrossRef]
- Mullaart, E.; Wells, D. Embryo biopsies for genomic selection. In Animal Biotechnology 2: Emerging Breeding Technologies; Springer: Berlin, Germany, 2018; pp. 81–94. [Google Scholar]
- de Sousa, R.V.; Cardoso, C.R.D.S.; Butzke, G.; Dode, M.A.N.; Rumpf, R.; Franco, M.M. Biopsy of bovine embryos produced in vivo and in vitro does not affect pregnancy rates. Theriogenology 2017, 90, 25–31. [Google Scholar] [CrossRef]
- Fisher, P.J.; Hyndman, D.L.; Bixley, M.J.; Oback, F.C.; Popovic, L.; McGowan, L.T.; Berg, M.C. Potential for genomic selection of bovine embryos. In Proceedings of the New Zealand Society of Animal Production; New Zealand Society of Animal Production: Christchurch, New Zealand, 2012; pp. 156–158. [Google Scholar]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Pas, M.F.W.T.; van Hemert, S.; Hulsegge, B.; Hoekman, A.J.W.; Pool, M.H.; Rebel, J.M.J.; Smits, M.A. A pathway analysis tool for analyzing microarray data of species with low physiological information. Adv. Bioinform. 2008, 2008, 719468. [Google Scholar] [CrossRef] [Green Version]
- Scott, B.; Haile-Mariam, M.; Cocks, B.; Pryce, J. How genomic selection has increased rates of genetic gain and inbreeding in the Australian national herd, genomic information nucleus, and bulls. J. Dairy Sci. 2021, 104, 11832–11849. [Google Scholar] [CrossRef]
- Retallick, K.J.; Lu, D.; Garcia, A.; Miller, S.P. Genomic selection in the US: Where it has been and where it is going? In Proceedings of the 12th World Congress on Genetics Applied to Livestock Production (WCGALP), Rotterdam, The Netherland, 3–8 July 2022; pp. 1795–1798. [Google Scholar]
- Silvestri, G.; Canedo-Ribeiro, C.; Serrano-Albal, M.; Labrecque, R.; Blondin, P.; Larmer, S.G.; Marras, G.; Tutt, D.A.; Handyside, A.H.; Farré, M.; et al. Preimplantation Genetic Testing for Aneuploidy Improves Live Birth Rates with In Vitro Produced Bovine Embryos: A Blind Retrospective Study. Cells 2021, 10, 2284. [Google Scholar] [CrossRef]
- Herrera, C. Clinical Applications of Preimplantation Genetic Testing in Equine, Bovine, and Human Embryos. J. Equine Veter. Sci. 2016, 41, 29–34. [Google Scholar] [CrossRef]
- Saadi, H.A.S.; Vigneault, C.; Sargolzaei, M.; Gagné, D.; Fournier, É.; de Montera, B.; Chesnais, J.; Blondin, P.; Robert, C. Impact of whole-genome amplification on the reliability of pre-transfer cattle embryo breeding value estimates. BMC Genom. 2014, 15, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, K.J.; Silvestri, G.; Black, D.H.; Dobson, G.; Smith, C.; Handyside, A.H.; Sinclair, K.D.; Griffin, D.K. Karyomapping for simultaneous genomic evaluation and aneuploidy screening of preimplantation bovine embryos: The first live-born calves. Theriogenology 2018, 125, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Tutt, D.; Silvestri, G.; Serrano-Albal, M.; Simmons, R.; Kwong, W.; Guven-Ates, G.; Canedo-Ribeiro, C.; Labrecque, R.; Blondin, P.; Handyside, A.; et al. Analysis of bovine blastocysts indicates ovarian stimulation does not induce chromosome errors, nor discordance between inner-cell mass and trophectoderm lineages. Theriogenology 2020, 161, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.C.; Mullaart, E. Screening of in vitro-produced cattle embryos to assess incidence and characteristics of unbalanced chromosomal aberrations. JDS Commun. 2023, 4, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Masset, H.; Ding, J.; Dimitriadou, E.; Ardeshirdavani, A.; Debrock, S.; Tšuiko, O.; Smits, K.; Peeraer, K.; Moreau, Y.; Voet, T.; et al. Single-cell genome-wide concurrent haplotyping and copy-number profiling through genotyping-by-sequencing. Nucleic Acids Res. 2022, 50, e63. [Google Scholar] [CrossRef]
- Griffin, D.K.; Jturner, K.; Silvestri, G.; Smith, C.; Dobson, G.; Black, D.H.; Sinclair, K.D.; Handyside, A.H. The use of Karyomapping for genomic evaluation and PGT-A of preimplantation cattle embryos: The first live-born calves. Reprod. Biomed. Online 2019, 38, e54–e55. [Google Scholar] [CrossRef]
- Mohan, M.; Ryder, S.; Claypool, P.; Geisert, R.; Malayer, J. Analysis of Gene Expression in the Bovine Blastocyst Produced In Vitro Using Suppression-Subtractive Hybridization. Biol. Reprod. 2002, 67, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Mohan, M.; Hurst, A.; Malayer, J. Global gene expression analysis comparing bovine blastocysts flushed on day 7 or produced in vitro. Mol. Reprod. Dev. 2004, 68, 288–298. [Google Scholar] [CrossRef]
- Corcoran, D.; Fair, T.; Rizos, D.; Smith, G.; Coussens, P.; Patel, O.; Ireland, J.; Boland, M.; Evans, A.; Lonergan, P. 216 Identification of Differentially Expressed Genes in Bovine Embryos Cultured In Vivo or In Vitro. Reprod. Fertil. Dev. 2005, 17, 259. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, D.; Fair, T.; Park, S.; Rizos, D.; Patel, O.V.; Smith, G.W.; Coussens, P.M.; Ireland, J.J.; Boland, M.P.; Evans, A.C.O.; et al. Suppressed expression of genes involved in transcription and translation in in vitro compared with in vivo cultured bovine embryos. Reproduction 2006, 131, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M.; Dufort, I.; Desrosiers, S.; Labbe, A.; Gravel, C.; Gilbert, I.; Robert, C.; Sirard, M.-A. Revealing the bovine embryo transcript profiles during early in vivo embryonic development. Reproduction 2009, 138, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.; Hoelker, M.; Rings, F.; Salilew, D.; Jennen, D.; Tholen, E.; Sirard, M.-A.; Schellander, K.; Tesfaye, D. Large-scale transcriptional analysis of bovine embryo biopsies in relation to pregnancy success after transfer to recipients. Physiol. Genom. 2006, 28, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, N.; Wondim, D.S.; Gad, A.; Tesfaye, D.; Phatsara, C.; Tholen, E.; Looft, C.; Schellander, K.; Hoelker, M. Bovine blastocysts with developmental competence to term share similar expression of developmentally important genes although derived from different culture environments. Reproduction 2011, 142, 551–564. [Google Scholar] [CrossRef] [Green Version]
- Hoelker, M.; Rings, F.; Lund, Q.; Ghanem, N.; Phatsara, C.; Griese, J.; Schellander, K.; Tesfaye, D. Effect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitro. Reproduction 2009, 137, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Kepková, K.V.; Vodicka, P.; Toralova, T.; Lopatarova, M.; Cech, S.; Dolezel, R.; Havlicek, V.; Besenfelder, U.; Kuzmany, A.; Sirard, M.A.; et al. Transcriptomic analysis of in vivo and in vitro produced bovine embryos revealed a developmental change in cullin 1 expression during maternal-to-embryonic transition. Theriogenology 2011, 75, 1582–1595. [Google Scholar] [CrossRef]
- Labrecque, R.; Sirard, M.-A. Gene expression analysis of bovine blastocysts produced by parthenogenic activation or fertilisation. Reprod. Fertil. Dev. 2011, 23, 591–602. [Google Scholar] [CrossRef]
- Sudano, M.J.; Caixeta, E.S.; Paschoal, D.M.; Rascado, T.S.; Crocomo, L.F.; Maziero, R.R.; Magalhães, L.C.O.; Guastali, M.D.; Vergara, L.E.; Monteiro, B.A.; et al. Microarray Analysis of In Vitro-Produced Bovine Embryos with Phenazine Ethosulfate. Biol. Reprod. 2012, 87, 215. [Google Scholar] [CrossRef]
- Sudano, M.J.; Paschoal, D.M.; Caixeta, E.S.; Maziero, R.R.; Guastali, M.D.; Crocomo, L.F.; Rascado, T.S.; Magalhães, L.C.O.; Monteiro, B.A.; Martins, A.; et al. 82 Effect of High Fetal Calf Serum Concentration in the Gene Expression Pattern of In Vitro Produced Bovine Embryos. Reprod. Fertil. Dev. 2013, 26, 155. [Google Scholar] [CrossRef] [Green Version]
- Misirlioglu, M.; Page, G.P.; Sagirkaya, H.; Kaya, A.; Parrish, J.J.; First, N.L.; Memili, E. Dynamics of global transcriptome in bovine matured oocytes and preimplantation embryos. Proc. Natl. Acad. Sci. USA 2006, 103, 18905–18910. [Google Scholar] [CrossRef] [Green Version]
- Kues, W.A.; Sudheer, S.; Herrmann, D.; Carnwath, J.W.; Havlicek, V.; Besenfelder, U.; Lehrach, H.; Adjaye, J.; Niemann, H. Genome-wide expression profiling reveals distinct clusters of transcriptional regulation during bovine preimplantation development in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 19768–19773. [Google Scholar] [CrossRef] [Green Version]
- Carter, F.; Rings, F.; Mamo, S.; Hölker, M.; Kuzmany, A.; Besenfelder, U.; Havlicek, V.; Mehta, J.P.; Tesfaye, D.; Schellander, K.; et al. Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct. Biol. Reprod. 2010, 83, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yandell, B.S.; Khatib, H. Transcriptomic profiling of bovine IVF embryos revealed candidate genes and pathways involved in early embryonic development. BMC Genom. 2010, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Bermejo-Alvarez, P.; Rizos, D.; Rath, D.; Lonergan, P.; Gutierrez-Adan, A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc. Natl. Acad. Sci. USA 2010, 107, 3394–3399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salilew-Wondim, D.; Hölker, M.; Rings, F.; Ghanem, N.; Ulas-Cinar, M.; Peippo, J.; Tholen, E.; Looft, C.; Schellander, K.; Tesfaye, D. Bovine pretransfer endometrium and embryo transcriptome fingerprints as predictors of pregnancy success after embryo transfer. Physiol. Genom. 2010, 42, 201–218. [Google Scholar] [CrossRef] [Green Version]
- Gad, A.; Besenfelder, U.; Rings, F.; Ghanem, N.; Salilew-Wondim, D.; Hossain, M.; Tesfaye, D.; Lonergan, P.; Becker, A.; Cinar, U.; et al. Effect of reproductive tract environment following controlled ovarian hyperstimulation treatment on embryo development and global transcriptome profile of blastocysts: Implications for animal breeding and human assisted reproduction. Hum. Reprod. 2011, 26, 1693–1707. [Google Scholar] [CrossRef] [Green Version]
- Clemente, M.; Lopez-Vidriero, I.; O’Gaora, P.; Mehta, J.; Forde, N.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Transcriptome Changes at the Initiation of Elongation in the Bovine Conceptus. Biol. Reprod. 2011, 85, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagatomo, H.; Kagawa, S.; Kishi, Y.; Takuma, T.; Sada, A.; Yamanaka, K.-I.; Abe, Y.; Wada, Y.; Takahashi, M.; Kono, T.; et al. Transcriptional Wiring for Establishing Cell Lineage Specification at the Blastocyst Stage in Cattle. Biol. Reprod. 2013, 88, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gad, A.; Hoelker, M.; Besenfelder, U.; Havlicek, V.; Cinar, U.; Rings, F.; Held, E.; Dufort, I.; Sirard, M.A.; Schellander, K.; et al. Molecular Mechanisms and Pathways Involved in Bovine Embryonic Genome Activation and Their Regulation by Alternative In Vivo and In Vitro Culture Conditions. Biol. Reprod. 2012, 87, 100. [Google Scholar] [CrossRef] [PubMed]
- Cagnone, G.; Dufort, I.; Vigneault, C.; Sirard, M.-A. Differential Gene Expression Profile in Bovine Blastocysts Resulting from Hyperglycemia Exposure During Early Cleavage Stages. Biol. Reprod. 2012, 86, 50. [Google Scholar] [CrossRef] [PubMed]
- Cagnone, G.L.; Sirard, M.-A. Transcriptomic signature to oxidative stress exposure at the time of embryonic genome activation in bovine blastocysts. Mol. Reprod. Dev. 2013, 80, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Dufort, I.; Caballero, J.; Moulavi, F.; Ghanaei, H.R.; Sirard, M.A. Transcriptome profiling of bovine inner cell mass and trophectoderm derived from in vivo generated blastocysts. BMC Dev. Biol. 2015, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmet, K.L.J.; Van Hoeck, V.; Gagné, D.; Fournier, E.; Thakur, A.; O’doherty, A.M.; Walsh, C.P.; Sirard, M.A.; Bols, P.E.J.; Leroy, J.L.M.R. Exposure of bovine oocytes and embryos to elevated non-esterified fatty acid concentrations: Integration of epigenetic and transcriptomic signatures in resultant blastocysts. BMC Genom. 2016, 17, 1004. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Singh, J.; Dufort, I.; Robert, C.; Dias, F.; Anzar, M. Transcriptomic difference in bovine blastocysts following vitrification and slow freezing at morula stage. PLoS ONE 2017, 12, e0187268. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Blondin, P.; Vigneault, C.; Labrecque, R.; Sirard, M.-A. The age of the bull influences the transcriptome and epigenome of blastocysts produced by IVF. Theriogenology 2019, 144, 122–131. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Tesfaye, D.; Rings, F.; Held-Hoelker, E.; Miskel, D.; Sirard, M.-A.; Tholen, E.; Schellander, K.; Hoelker, M. The global gene expression outline of the bovine blastocyst: Reflector of environmental conditions and predictor of developmental capacity. BMC Genom. 2021, 22, 408. [Google Scholar] [CrossRef]
- Hallberg, I.; Persson, S.; Olovsson, M.; Sirard, M.-A.; Damdimopoulou, P.; Rüegg, J.; Sjunnesson, Y.C. Perfluorooctane sulfonate (PFOS) exposure of bovine oocytes affects early embryonic development at human-relevant levels in an in vitro model. Toxicology 2021, 464, 153028. [Google Scholar] [CrossRef]
- Rabaglino, M.B.; Salilew-Wondim, D.; Zolini, A.; Tesfaye, D.; Hoelker, M.; Lonergan, P.; Hansen, P.J. Machine-learning methods applied to integrated transcriptomic data from bovine blastocysts and elongating conceptuses to identify genes predictive of embryonic competence. FASEB J. 2023, 37, e22809. [Google Scholar] [CrossRef]
- Brinkhof, B.; Van Tol, H.T.A.; Koerkamp, M.J.A.G.; Wubbolts, R.W.; Haagsman, H.P.; Roelen, B.A.J. Characterization of bovine embryos cultured under conditions appropriate for sustaining human naïve pluripotency. PLoS ONE 2017, 12, e0172920. [Google Scholar] [CrossRef] [Green Version]
- Ushizawa, K.; Herath, C.B.; Kaneyama, K.; Shiojima, S.; Hirasawa, A.; Takahashi, T.; Imai, K.; Ochiai, K.; Tokunaga, T.; Tsunoda, Y.; et al. cDNA microarray analysis of bovine embryo gene expression profiles during the pre-implantation period. Reprod. Biol. Endocrinol. 2004, 2, 77. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.L.; Everts, R.E.; Tian, X.C.; Du, F.; Sung, L.-Y.; Rodriguez-Zas, S.L.; Jeong, B.-S.; Renard, J.-P.; Lewin, H.A.; Yang, X. Global gene expression profiles reveal significant nuclear reprogramming by the blastocyst stage after cloning. Proc. Natl. Acad. Sci. USA 2005, 102, 17582–17587. [Google Scholar] [CrossRef] [Green Version]
- Pfister-Genskow, M.; Myers, C.; Childs, L.A.; Lacson, J.C.; Patterson, T.; Betthauser, J.M.; Goueleke, P.J.; Koppang, R.W.; Lange, G.; Fisher, P.; et al. Identification of Differentially Expressed Genes in Individual Bovine Preimplantation Embryos Produced by Nuclear Transfer: Improper Reprogramming of Genes Required for Development. Biol. Reprod. 2005, 72, 546–555. [Google Scholar] [CrossRef]
- Somers, J.; Smith, C.; Donnison, M.; Wells, D.; Henderson, H.; McLeay, L.; Pfeffer, P. Gene expression profiling of individual bovine nuclear transfer blastocysts. Reproduction 2006, 131, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, B.; Bushel, P.R.; Thierry-Mieg, J.; Thierry-Mieg, D.; Xu, J.; Fang, H.; Hong, H.; Shen, J.; Su, Z.; et al. The concordance between RNA-seq and microarray data depends on chemical treatment and transcript abundance. Nat. Biotechnol. 2014, 32, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver from Short-Term Rat Toxicity Studies. Front. Genet. 2019, 9, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Khatib, H. Comparison of transcriptomic landscapes of bovine embryos using RNA-Seq. BMC Genom. 2010, 11, 711. [Google Scholar] [CrossRef] [Green Version]
- Mamo, S.; Mehta, J.P.; McGettigan, P.; Fair, T.; Spencer, T.E.; Bazer, F.W.; Lonergan, P. RNA Sequencing Reveals Novel Gene Clusters in Bovine Conceptuses Associated with Maternal Recognition of Pregnancy and Implantation. Biol. Reprod. 2011, 85, 1143–1151. [Google Scholar] [CrossRef]
- Graf, A.; Krebs, S.; Zakhartchenko, V.; Schwalb, B.; Blum, H.; Wolf, E. Fine mapping of genome activation in bovine embryos by RNA sequencing. Proc. Natl. Acad. Sci. USA 2014, 111, 4139–4144. [Google Scholar] [CrossRef] [Green Version]
- Chitwood, J.L.; Rincon, G.; Kaiser, G.G.; Medrano, J.F.; Ross, P.J. RNA-seq analysis of single bovine blastocysts. BMC Genom. 2013, 14, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Shi, Y.; Wang, H.; Wang, Z.; Dang, Y.; Li, S.; Wang, S.; Zhang, K. Base editing in bovine embryos reveals a species-specific role of SOX2 in regulation of pluripotency. PLoS Genet. 2022, 18, e1010307. [Google Scholar] [CrossRef] [PubMed]
- Zolini, A.M.; Block, J.; Rabaglino, M.B.; Rincon, G.; Hoelker, M.; Bromfield, J.J.; Salilew-Wondim, D.; Hansen, P.J. Genes associated with survival of female bovine blastocysts produced in vivo. Cell Tissue Res. 2020, 382, 665–678. [Google Scholar] [CrossRef]
- Zolini, A.M.; Block, J.; Rabaglino, M.B.; Tríbulo, P.; Hoelker, M.; Rincon, G.; Bromfield, J.J.; Hansen, P.J. Molecular fingerprint of female bovine embryos produced in vitro with high competence to establish and maintain pregnancy†. Biol. Reprod. 2019, 102, 292–305. [Google Scholar] [CrossRef]
- Nõmm, M.; Ivask, M.; Pärn, P.; Reimann, E.; Kõks, S.; Jaakma, Ü. Detecting Embryo Developmental Potential by Single Blastomere RNA-Seq. Genes 2023, 14, 569. [Google Scholar] [CrossRef] [PubMed]
- Lavagi, I.; Krebs, S.; Simmet, K.; Beck, A.; Zakhartchenko, V.; Wolf, E.; Blum, H. Single-cell RNA sequencing reveals developmental heterogeneity of blastomeres during major genome activation in bovine embryos. Sci. Rep. 2018, 8, 4071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kropp, J.; Khatib, H. Characterization of microRNA in bovine in vitro culture media associated with embryo quality and development. J. Dairy Sci. 2015, 98, 6552–6563. [Google Scholar] [CrossRef] [Green Version]
- Simmet, K.; Kurome, M.; Zakhartchenko, V.; Reichenbach, H.-D.; Springer, C.; Bähr, A.; Blum, H.; Philippou-Massier, J.; Wolf, E. The second lineage differentiation of bovine embryos fails in the absence of OCT4/POU5F. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Cui, L.-S.; Hao, H.-S.; Wang, H.-Y.; Zhao, S.-J.; Du, W.-H.; Wang, D.; Liu, Y.; Zhu, H.-B. Transcriptome analyses of inner cell mass and trophectoderm cells isolated by magnetic-activated cell sorting from bovine blastocysts using single cell RNA-seq. Reprod. Domest. Anim. 2016, 51, 726–735. [Google Scholar] [CrossRef]
- Kropp, J.; Carrillo, J.A.; Namous, H.; Daniels, A.; Salih, S.M.; Song, J.; Khatib, H. Male fertility status is associated with DNA methylation signatures in sperm and transcriptomic profiles of bovine preimplantation embryos. BMC Genom. 2017, 18, 280. [Google Scholar] [CrossRef] [Green Version]
- Gilchrist, G.C.; Tscherner, A.; Nalpathamkalam, T.; Merico, D.; LaMarre, J. MicroRNA Expression during Bovine Oocyte Maturation and Fertilization. Int. J. Mol. Sci. 2016, 17, 396. [Google Scholar] [CrossRef] [Green Version]
- Tríbulo, P.; Rabaglino, M.B.; Bo, M.B.; Carvalheira, L.D.R.; Bishop, J.V.; Hansen, T.R.; Hansen, P.J. Dickkopf-related protein 1 is a progestomedin acting on the bovine embryo during the morula-to-blastocyst transition to program trophoblast elongation. Sci. Rep. 2019, 9, 11816. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Lundahl, S.; Sundaram, A.Y.; Gillund, P.; Gilfillan, G.D.; Olsaker, I.; Krogenæs, A. Gene Expression in Embryos From Norwegian Red Bulls With High or Low Non Return Rate: An RNA-Seq Study of in vivo-Produced Single Embryos. Front. Genet. 2022, 12, 780113. [Google Scholar] [CrossRef] [PubMed]
- Halstead, M.M.; Ma, X.; Zhou, C.; Schultz, R.M.; Ross, P.J. Chromatin remodeling in bovine embryos indicates species-specific regulation of genome activation. Nat. Commun. 2020, 11, 4654. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.; Strillacci, M.G.; Peñagaricano, F.; Khatib, H. Characterization and functional roles of paternal RNAs in 2–4 cell bovine embryos. Sci. Rep. 2019, 9, 20347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zhao, P.; Dang, Y.; Li, S.; Luo, L.; Hu, B.; Wang, S.; Wang, H.; Zhang, K. Functional roles of the chromatin remodeler SMARCA5 in mouse and bovine preimplantation embryos†. Biol. Reprod. 2021, 105, 359–370. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Dang, Y.; Luo, L.; Hu, B.; Wang, S.; Wang, H.; Zhang, K. NOTCH signaling pathway is required for bovine early embryonic development. Biol. Reprod. 2021, 105, 332–344. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, T.; Iyyappan, R.; Ming, H.; Dvoran, M.; Wang, Y.; Chen, Q.; Roberts, R.M.; Susor, A.; Jiang, Z. High-resolution ribosome profiling reveals translational selectivity for transcripts in bovine preimplantation embryo development. Development 2022, 149, dev.200819. [Google Scholar] [CrossRef]
- Kajdasz, A.; Warzych, E.; Derebecka, N.; Madeja, Z.E.; Lechniak, D.; Wesoly, J.; Pawlak, P. Lipid Stores and Lipid Metabolism Associated Gene Expression in Porcine and Bovine Parthenogenetic Embryos Revealed by Fluorescent Staining and RNA-seq. Int. J. Mol. Sci. 2020, 21, 6488. [Google Scholar] [CrossRef]
- Nix, J.L.; Schettini, G.P.; Biase, F.H. Sexing of cattle embryos using RNA-sequencing data or polymerase chain reaction based on a complete sequence of cattle chromosome Y. Front. Genet. 2023, 14, 569. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, J.; Dong, H.; Luo, O.; Zheng, X.; Obergfell, C.; Tang, Y.; Bi, J.; O’neill, R.; Ruan, Y.; et al. Transcriptional profiles of bovine in vivo pre-implantation development. BMC Genom. 2014, 15, 756. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Dong, H.; Zheng, X.; Marjani, S.L.; Donovan, D.M.; Chen, J.; Tian, X. mRNA Levels of Imprinted Genes in Bovine In Vivo Oocytes, Embryos and Cross Species Comparisons with Humans, Mice and Pigs. Sci. Rep. 2015, 5, 17898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banliat, C.; Mahé, C.; Lavigne, R.; Com, E.; Pineau, C.; Labas, V.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. Dynamic changes in the proteome of early bovine embryos developed in vivo. Front. Cell Dev. Biol. 2022, 10, 863700. [Google Scholar] [CrossRef]
- Demant, M.; Deutsch, D.R.; Fröhlich, T.; Wolf, E.; Arnold, G.J. Proteome analysis of early lineage specification in bovine embryos. Proteomics 2014, 15, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.R.; Fröhlich, T.; Otte, K.A.; Beck, A.; Habermann, F.A.; Wolf, E.; Arnold, G.J. Stage-Specific Proteome Signatures in Early Bovine Embryo Development. J. Proteome Res. 2014, 13, 4363–4376. [Google Scholar] [CrossRef]
- Banliat, C.; Mahé, C.; Lavigne, R.; Com, E.; Pineau, C.; Labas, V.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. The proteomic analysis of bovine embryos developed in vivo or in vitro reveals the contribution of the maternal environment to early embryo. BMC Genom. 2022, 23, 839. [Google Scholar] [CrossRef]
- Jensen, P.L.; Grøndahl, M.L.; Beck, H.C.; Petersen, J.; Stroebech, L.; Christensen, S.T.; Andersen, C.Y. Proteomic analysis of bovine blastocoel fluid and blastocyst cells. Syst. Biol. Reprod. Med. 2014, 60, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Jensen, P.L.; Beck, H.C.; Petersen, T.S.; Stroebech, L.; Schmidt, M.; Rasmussen, L.M.; Hyttel, P. Proteomic analysis of the early bovine yolk sac fluid and cells from the day 13 ovoid and elongated preimplantation embryos. Theriogenology 2014, 82, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.C.; Powell, A.M.; Caperna, T.J.; Garrett, W.M. Proteomic analysis of the major cellular proteins of bovine trophectoderm cell lines derived from IVP, parthenogenetic and nuclear transfer embryos: Reduced expression of annexins I and II in nuclear transfer-derived cell lines. Anim. Reprod. Sci. 2010, 120, 187–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raes, A.; Wydooghe, E.; Pavani, K.C.; Pascottini, O.B.; Van Steendam, K.; Dhaenens, M.; Boel, A.; Heras, S.; Heindryckx, B.; Peelman, L.; et al. Cathepsin-L Secreted by High-Quality Bovine Embryos Exerts an Embryotrophic Effect In Vitro. Int. J. Mol. Sci. 2023, 24, 6563. [Google Scholar] [CrossRef]
- Nel-Themaat, L.; Nagy, Z. A review of the promises and pitfalls of oocyte and embryo metabolomics. Placenta 2011, 32, S257–S263. [Google Scholar] [CrossRef] [PubMed]
- Rieger, D.; Guay, P. Measurement of the metabolism of energy substrates in individual bovine blastocysts. Reproduction 1988, 83, 585–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.G.; McNaughton, C.; Gasparrini, B.; McGowan, L.T.; Tervit, H.R. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J. Reprod. Fertil. 2000, 118, 47–56. [Google Scholar] [CrossRef]
- Leese, H.J. What does an embryo need? Hum. Fertil. 2003, 6, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.; Pawelczynski, M.; Trounson, A. Nutrient uptake and utilization can be used to select viable day 7 bovine blastocysts after cryopreservation. Mol. Reprod. Dev. 1996, 44, 472–475. [Google Scholar] [CrossRef]
- Partridge, R.; Leese, H. Consumption of amino acids by bovine preimplantation embryos. Reprod. Fertil. Dev. 1996, 8, 945–950. [Google Scholar] [CrossRef]
- Lopes, A.; Madsen, S.; Ramsing, N.; Løvendahl, P.; Greve, T.; Callesen, H. Investigation of respiration of individual bovine embryos produced in vivo and in vitro and correlation with viability following transfer. Hum. Reprod. 2006, 22, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Lopes, A.S.; Lane, M.; Thompson, J.G. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum. Reprod. 2010, 25, 2762–2773. [Google Scholar] [CrossRef] [Green Version]
- Obeidat, Y.; Catandi, G.; Carnevale, E.; Chicco, A.J.; DeMann, A.; Field, S.; Chen, T. A multi-sensor system for measuring bovine embryo metabolism. Biosens. Bioelectron. 2018, 126, 615–623. [Google Scholar] [CrossRef]
- Gómez, E.; Muñoz, M.; Simó, C.; Ibáñez, C.; Carrocera, S.; Martín-González, D.; Cifuentes, A. Non-invasive metabolomics for improved determination of embryonic sex markers in chemically defined culture medium. J. Chromatogr. A 2016, 1474, 138–144. [Google Scholar] [CrossRef]
- Gómez, E.; Carrocera, S.; Martin, D.; Herrero, P.; Canela, N.; Muñoz, M. Differential release of cell-signaling metabolites by male and female bovine embryos cultured in vitro. Theriogenology 2018, 114, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Uyar, A.; Correia, E.; Díez, C.; Fernandez-Gonzalez, A.; Caamaño, J.N.; Trigal, B.; Carrocera, S.; Seli, E.; Gomez, E. Non-invasive assessment of embryonic sex in cattle by metabolic fingerprinting of in vitro culture medium. Metabolomics 2014, 10, 443–451. [Google Scholar] [CrossRef]
- dos Santos, É.; da Silva Martinho, H.; Annes, K.; da Silva, T.; Soares, C.; Leite, R.F.; Milazzotto, M. Raman-based noninvasive metabolic profile evaluation of in vitro bovine embryos. J. Biomed. Opt. 2016, 21, 075002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubessa, M.; Ambrosi, A.; Gonzalez-Pena, D.; Polkoff, K.M.; Wheeler, M.B. Non-invasive nuclear magnetic resonance analysis of male and female embryo metabolites during in vitro embryo culture. Metabolomics 2018, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Perkel, K.J.; Madan, P. Spent culture medium analysis from individually cultured bovine embryos demonstrates metabolomic differences. Zygote 2017, 25, 662–674. [Google Scholar] [CrossRef]
- Sturmey, R.; Bermejo-Alvarez, P.; Gutierrez-Adan, A.; Rizos, D.; Leese, H.; Lonergan, P. Amino acid metabolism of bovine blastocysts: A biomarker of sex and viability. Mol. Reprod. Dev. 2010, 77, 285–296. [Google Scholar] [CrossRef]
- Tiffin, G.J.; Rieger, D.; Betteridge, K.J.; Yadav, B.R.; King, W.A. Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. Reproduction 1991, 93, 125–132. [Google Scholar] [CrossRef]
- Rieger, D.; Loskutoff, N.; Betteridge, K. Developmentally related changes in the uptake and metabolism of glucose, glutamine and pyruvate by cattle embryos produced in vitro. Reprod. Fertil. Dev. 1992, 4, 547–557. [Google Scholar] [CrossRef]
- Donnay, I.; Leese, H. Embryo metabolism during the expansion of the bovine blastocyst. Mol. Reprod. Dev. 1999, 53, 171–178. [Google Scholar] [CrossRef]
- Khurana, N.K.; Niemann, H. Energy Metabolism in Preimplantation Bovine Embryos Derived In Vitro or In Vivo. Biol. Reprod. 2000, 62, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Rubessa, M.; Ambrosi, A.; Gonzalez-Pena, D.; Polkoff, K.M.; Denmark, S.E.; Wheeler, M.B. Non-invasive analysis of bovine embryo metabolites during in vitro embryo culture using nuclear magnetic resonance. AIMS Bioeng. 2016, 3, 538–551. [Google Scholar] [CrossRef]
- Steeves, T.; Gardner, D. Temporal and Differential Effects of Amino Acids on Bovine Embryo Development in Culture. Biol. Reprod. 1999, 61, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Nõmm, M.; Porosk, R.; Pärn, P.; Kilk, K.; Soomets, U.; Kõks, S.; Jaakma, Ü. In vitro culture and non-invasive metabolic profiling of single bovine embryos. Reprod. Fertil. Dev. 2019, 31, 306. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.M.; Leese, H.J. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2006, 73, 1195–1201. [Google Scholar] [CrossRef]
- Sudano, M.J.; Rascado, T.D.; Tata, A.; Belaz, K.R.; Santos, V.G.; Valente, R.S.; Mesquita, F.S.; Ferreira, C.R.; Araújo, J.P.; Eberlin, M.N.; et al. Lipidome signatures in early bovine embryo development. Theriogenology 2016, 86, 472–484.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurosawa, H.; Utsunomiya, H.; Shiga, N.; Takahashi, A.; Ihara, M.; Ishibashi, M.; Nishimoto, M.; Watanabe, Z.; Abe, H.; Kumagai, J.; et al. Development of a new clinically applicable device for embryo evaluation which measures embryo oxygen consumption. Hum. Reprod. 2016, 31, 2321–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asampille, G.; Cheredath, A.; Joseph, D.; Adiga, S.K.; Atreya, H.S. The utility of nuclear magnetic resonance spectroscopy in assisted reproduction. Open Biol. 2020, 10, 200092. [Google Scholar] [CrossRef]
- Sivelli, G.; Conley, G.M.; Herrera, C.; Marable, K.; Rodriguez, K.J.; Bollwein, H.; Sudano, M.J.; Brugger, J.; Simpson, A.J.; Boero, G.; et al. NMR spectroscopy of a single mammalian early stage embryo. J. Magn. Reson. 2022, 335, 107142. [Google Scholar] [CrossRef]
- Pomar, F.R.; Teerds, K.; Kidson, A.; Colenbrander, B.; Tharasanit, T.; Aguilar, B.; Roelen, B. Differences in the incidence of apoptosis between in vivo and in vitro produced blastocysts of farm animal species: A comparative study. Theriogenology 2005, 63, 2254–2268. [Google Scholar] [CrossRef]
- Sagirkaya, H.; Misirlioglu, M.; Kaya, A.; First, N.L.; Parrish, J.; Memili, E. Developmental and molecular correlates of bovine preimplantation embryos. Reproduction 2006, 131, 895–904. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Gimeno, I.; Cañón-Beltrán, K.; Gutiérrez-Adán, A.; Rizos, D.; Gómez, E. Senescence and Apoptosis during in vitro Embryo Development in a Bovine Model. Front. Cell Dev. Biol. 2020, 8, 619902. [Google Scholar] [CrossRef] [PubMed]
- Wondim, D.S.; Fournier, E.; Hoelker, M.; Saeed-Zidane, M.; Tholen, E.; Looft, C.; Neuhoff, C.; Besenfelder, U.; Havlicek, V.; Rings, F.; et al. Genome-Wide DNA Methylation Patterns of Bovine Blastocysts Developed In Vivo from Embryos Completed Different Stages of Development In Vitro. PLoS ONE 2015, 10, e0140467. [Google Scholar] [CrossRef]
- Dobbs, K.B.; Rodriguez, M.; Sudano, M.J.; Ortega, M.S.; Hansen, P.J. Dynamics of DNA methylation during early development of the preimplantation bovine embryo. PLoS ONE 2013, 8, e66230. [Google Scholar] [CrossRef] [PubMed]
- Morin-Doré, L.; Blondin, P.; Vigneault, C.; Grand, F.; Labrecque, R.; Sirard, M. DNA methylation status of bovine blastocysts obtained from peripubertal oocyte donors. Mol. Reprod. Dev. 2020, 87, 910–924. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, D.; Humblot, P.; Sirard, M.-A.; Sjunnesson, Y.; Jhamat, N.; Båge, R.; Andersson, G. DNA methylation pattern of bovine blastocysts associated with hyperinsulinemia in vitro. Mol. Reprod. Dev. 2018, 85, 599–611. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Saeed-Zidane, M.; Hoelker, M.; Gebremedhn, S.; Poirier, M.; Pandey, H.O.; Tholen, E.; Neuhoff, C.; Held, E.; Besenfelder, U.; et al. Genome-wide DNA methylation patterns of bovine blastocysts derived from in vivo embryos subjected to in vitro culture before, during or after embryonic genome activation. BMC Genom. 2018, 19, 424. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.E.; Jiang, Z.C.; Alqahtani, F.; Mandoiu, I.; Dong, H.; Zheng, X.; Marjani, S.L.; Chen, J.; Tian, X.C. Methylome Dynamics of Bovine Gametes and in vivo Early Embryos. Front. Genet. 2019, 10, 512. [Google Scholar] [CrossRef] [Green Version]
- Ming, H.; Sun, J.; Pasquariello, R.; Gatenby, L.; Herrick, J.R.; Yuan, Y.; Pinto, C.R.; Bondioli, K.R.; Krisher, R.L.; Jiang, Z. The landscape of accessible chromatin in bovine oocytes and early embryos. Epigenetics 2020, 16, 300–312. [Google Scholar] [CrossRef]
- Kurosaka, S.; Eckardt, S.; McLaughlin, K.J. Pluripotent Lineage Definition in Bovine Embryos by Oct4 Transcript Localization. Biol. Reprod. 2004, 71, 1578–1582. [Google Scholar] [CrossRef] [Green Version]
- Hyttel, P.; Viuff, D.; Laurincik, J.; Schmidt, M.; Thomsen, P.; Avery, B.; Callesen, H.; Rath, D.; Niemann, H.; Rosenkranz, H.; et al. Risks of in-vitro production of cattle and swine embryos: Aberrations in chromosome numbers, ribosomal RNA gene activation and perinatal physiology. Hum. Reprod. 2000, 15, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Demyda-Peyrás, S.; Dorado, J.; Hidalgo, M.; Anter, J.; De Luca, L.; Genero, E.R.; Moreno-Millán, M. Effects of oocyte quality, incubation time and maturation environment on the number of chromosomal abnormalities in IVF-derived early bovine embryos. Reprod. Fertil. Dev. 2013, 25, 1077–1084. [Google Scholar] [CrossRef]
- Guilherme, V.B.; Pronunciate, M.; dos Santos, P.H.; Ciniciato, D.D.S.; Takahashi, M.B.; Rocha, J.C.; Nogueira, M.F.G. Distinct Sources of a Bovine Blastocyst Digital Image Do not Produce the Same Classification by a Previously Trained Software Using Artificial Neural Network. bioRxiv 2018, 424028. [Google Scholar] [CrossRef]
- Rocha, J.C.; Passalia, F.J.; Matos, F.D.; Takahashi, M.B.; Ciniciato, D.D.S.; Maserati, M.P.; Alves, M.F.; de Almeida, T.G.; Cardoso, B.L.; Basso, A.C.; et al. A method based on artificial intelligence to fully automatize the evaluation of bovine blastocyst images. Sci. Rep. 2017, 7, 7659. [Google Scholar] [CrossRef] [Green Version]
- Melo, D.H.; Nascimento, M.Z.; Oliveira, D.L.; Neves, L.A.; Annes, K. Algorithms for automatic segmentation of bovine embryos produced in vitro. J. Phys. Conf. Ser. 2014, 490, 012125. [Google Scholar] [CrossRef]
- Ciniciato, D.D.S.; Takahashi, M.B.; Nogueira, M.F.G.; Rocha, J.C. Potential Use of Smartphone as a Tool to Capture Embryo Digital Images from Stereomicroscope and to Evaluate Them by an Artificial Neural Network. In Proceedings of the International Conference on Computer-Human Interaction Research and Applications, Funchal, Portugal, 31 October–2 November 2017; pp. 185–189. [Google Scholar] [CrossRef]
- Nogueira, M.F.G.; Guilherme, V.B.; Pronunciate, M.; Dos Santos, P.H.; da Silva, D.L.B.; Rocha, J.C. Artificial Intelligence-Based Grading Quality of Bovine Blastocyst Digital Images: Direct Capture with Juxtaposed Lenses of Smartphone Camera and Stereomicroscope Ocular Lens. Sensors 2018, 18, 4440. [Google Scholar] [CrossRef] [Green Version]
- Workman, A.M.; Heaton, M.P.; Ley, B.L.V.; Webster, D.A.; Sherry, L.; Larson, S.; Kalbfleisch, T.S.; Harhay, G.P.; Jobman, E.E.; Carlson, D.F.; et al. First gene-edited calf with reduced susceptibility to a major viral pathogen. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Mellisho, E.; Briones, M.A.; Velasquez, A.E.; Cabezas, J.; Castro, F.O.; Rodriguez-Alvarez, L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction 2019, 158, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Mellisho, E.A.; Velásquez, A.E.; Nuñez, M.J.; Cabezas, J.G.; Cueto, J.A.; Fader, C.; Castro, F.O.; Rodríguez-Álvarez, L. Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro. PLoS ONE 2017, 12, e0178306. [Google Scholar] [CrossRef] [Green Version]
- Saadeldin, I.M.; Kim, S.J.; Bin Choi, Y.; Lee, B.C. Improvement of cloned embryos development by co-culturing with parthenotes: A possible role of exosomes/microvesicles for embryos paracrine communication. Cell. Reprogram. 2014, 16, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabaglino, M.B.; O’doherty, A.; Secher, J.B.-M.; Lonergan, P.; Hyttel, P.; Fair, T.; Kadarmideen, H.N. Application of multi-omics data integration and machine learning approaches to identify epigenetic and transcriptomic differences between in vitro and in vivo produced bovine embryos. PLoS ONE 2021, 16, e0252096. [Google Scholar] [CrossRef]
- Milazzotto, M.P.; Noonan, M.J.; Ferraz, M.D.A.M.M. Mining RNAseq data reveals dynamic metaboloepigenetic profiles in human, mouse and bovine pre-implantation embryos. iScience 2022, 25, 103904. [Google Scholar] [CrossRef] [PubMed]
- Dochi, O. Direct transfer of frozen-thawed bovine embryos and its application in cattle reproduction management. J. Reprod. Dev. 2019, 65, 389–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banik, S.; Melanthota, S.K.; Arbaaz; Vaz, J.M.; Kadambalithaya, V.M.; Hussain, I.; Dutta, S.; Mazumder, N. Recent trends in smartphone-based detection for biomedical applications: A review. Anal. Bioanal. Chem. 2021, 413, 2389–2406. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Dinnes, J.; Chuchu, N.; Takwoingi, Y.; Bayliss, S.E.; Matin, R.N.; Jain, A.; Walter, F.M.; Williams, H.C.; Deeks, J.J. Algorithm based smartphone apps to assess risk of skin cancer in adults: Systematic review of diagnostic accuracy studies. BMJ 2020, 368, m127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.K.; Nair, M.R.; Manjunath, A.; Mujib, B.R.A. Evaluation and comparison between smartphone and photomicrography based whole slide imaging. J. Fam. Med. Prim. Care 2020, 9, 2319–2323. [Google Scholar] [CrossRef]
- Russ, J.C.; Matey, J.R.; Mallinckrodt, A.J.; McKay, S. The Image Processing Handbook. Comput. Phys. 1994, 8, 177–178. [Google Scholar] [CrossRef] [Green Version]
| Source | Code | Criterion/Definition |
|---|---|---|
| [21] | 1 | 2-cell embryo |
| 2 | 4-cell embryo | |
| 3 | 8-cell embryo | |
| 4 | Morula (16–32 cells) | |
| 5 | Morula (32–64 cells) | |
| 6 | Blastocyst | |
| 7 | Hatched blastocyst | |
| [20] | 1 | Unfertilized |
| 2 | 2- to 16-cell embryo | |
| 3 | Early morula | |
| 4 | Morula | |
| 5 | Early blastocyst | |
| 6 | Blastocyst | |
| 7 | Expanded blastocyst | |
| 8 | Hatching blastocyst | |
| 9 | Hatched blastocyst |
| Applications of DIC in Bovine Embryo Analysis | Source |
|---|---|
| Visualization of pronuclei | [30] |
| Evaluation of embryos between days 4 and 8 of culture | [31] |
| Evaluation of cleavage | [32] |
| Visualization of lipid droplets | [33] |
| Comparison of 8-cell-stage embryos in different culture media and visualization of cytoplasmic lipid droplets | [34] |
| Visualization of DNA microinjection into the male pronucleus | [35,36] |
| Observation of the development of individual embryos | [37] |
| Observation of cytoplasmic lipid droplets | [38,39] |
| Applications of Confocal Microscopy in Bovine Embryo Analysis | Source |
|---|---|
| Using targeted fluorescent labeling | |
| TUNEL labeling of apoptotic nuclei | [37,71,72] |
| Intracellular localization of nucleolar proteins | [44] |
| Characterization of the zona pellucida | [73] |
| Intracellular localization of Nalp5/Mater | [74] |
| Study of DNA methylation patterns | [75] |
| Intracellular localization of IGF1R, IGF2, and IGF2R | [76] |
| Intracellular localization of pluripotency and lineage-specific markers NANOG, OCT4, CDX2, and GATA6 | [77,78,79,80] |
| Non-invasive long-term live-cell imaging of IVP embryos | [81] |
| Characterization of mitochondrial activity and distribution of lipid droplets | [82] |
| Using label-free imaging | |
| Detection of autofluorescence of FAD and NAD(P)H (to assess metabolic status) | [83] |
| Source | Type of Embryo | Number of Embryos | Pregnancy Rate (PR) | Observations |
|---|---|---|---|---|
| Mullaart and Wells, 2018 [125] | Biopsied (IVD) | 1190 | 46% | PR different at 5 months (p < 0.05) |
| Intact (IVD) | 13,067 | 54% | ||
| de Sousa, da Silva Cardoso, 2017 [126] | Biopsied (IVD) | 380 | 54% | PR not different at 60 days (p > 0.05) |
| Intact (IVD) | 229 | 56% | ||
| Biopsied (IVP) | 91 | 26% | PR not different at 60 days (p > 0.05) | |
| Intact (IVP) | 227 | 20% | ||
| Fisher, Hyndman, 2012 [127] | Biopsied (IVP) | 42 | 43% | PR not different at 65 days (p > 0.05) |
| Intact (IVP) | 42 | 38% |
| Microarray Platform Used | Objective/s of the Study | Source |
|---|---|---|
| Bovine cDNA microarray developed in-house | To compare IVD and IVP blastocysts | [142,143] |
| BlueChip cDNA microarray, Universite Laval, Quebec, Canada | To compare in vivo derived 2-cell embryos, 8-cell embryos, blastocysts, and GV oocytes | [144] |
| To discover genes that will predict the post-transfer fate of embryos | [145,146] | |
| To study the effects of embryo microenvironment using a well-of-the-well (WOW) system | [147] | |
| To compare IVD and IVP pre-implantation stages (2-, 4-, and 8-cell stages, morulae, and blastocysts) | [148] | |
| To compare blastocysts derived from IVP and parthenogenesis | [149] | |
| Affymetrix GeneChip® Bovine Genome Array, CA, USA | To study the effects of metabolic regulators on blastocysts | [150] |
| To test the effects of different culture media on IVP embryos | [151] | |
| To study transcriptomic dynamics at EGA (8-cell stage) | [152] | |
| To identify stage-specific expression patterns of in vivo developing 2-cell, 4-cell, and 8-cell stages and of morulae and blastocysts | [153] | |
| To study effects of elevated progesterone on blastocyst development | [154] | |
| To compare degenerated and healthy-looking IVP blastocysts | [155] | |
| To study sex-specific gene expression patterns of blastocysts | [156] | |
| To identify transcriptome fingerprints as predictors of pregnancy success after ET | [157] | |
| To study the effects of superovulation on embryo development | [158] | |
| To study the transition of a spherical blastocyst to an ovoid conceptus | [159] | |
| To study the effects of metabolic regulators on blastocysts | [150] | |
| To study cell lineage specification of blastocysts | [160] | |
| EmbryoGENE Bovine Microarray, Agilent, CA, USA | To study EGA and effects of in vivo and in vitro culture conditions on early embryo development | [161] |
| To study the effects of hyperglycemic stress on IVP blastocysts | [162] | |
| To study the effects of oxidative stress on IVP blastocysts | [163] | |
| To compare the ICM and TE between IVP and IVD embryos | [164] | |
| To study the effects of non-esterified fatty acid (NEFA) concentrations on blastocysts | [165] | |
| To compare effects of vitrification and slow freezing on morulae and blastocysts | [166] | |
| To study effects of bull age on blastocysts | [167] | |
| To identify transcriptomic predictors of pregnancy outcomes | [168] | |
| To study effects of perfluorooctane sulfonate (PFOS) exposure of bovine oocytes on early embryonic development | [169] | |
| To model a gene signature predictive of embryonic survival | [170] | |
| Bovine gene expression microarray V2, Agilent, CA, USA | To study the transcriptomic profile related to bovine pluripotency | [171] |
| 3995 bovine cDNA microarray developed in-house | To study temporal changes during peri-implantation developmental stages (7–28-day-old embryos) | [172] |
| 7872 bovine cDNA microarray developed by University of Illinois, Urbana-Champaign | To compare IVD and somatic cell nuclear transfer (SCNT)-derived blastocysts | [173] |
| 2640 bovine cDNA microarray developed in-house | To compare IVP- and SCNT-derived blastocysts | [174] |
| 5000 bovine cDNA microarray developed in-house | To compare IVP- and SCNT-derived blastocysts | [175] |
| RNA-Seq Platform Used | Objective/s of the Study | Source |
|---|---|---|
| Genome Analyzer, Illumina, CA, USA | To compare healthy-looking and degenerated IVP blastocysts | [178] |
| To study maternal recognition and implantation using 7-, 10-, 13-, 16-, and 19-day-old bovine embryos | [179] | |
| To study EGA using 4-cell, 8-cell, 16-cell, and blastocyst stages | [180] | |
| To demonstrate that RNA-seq can be carried out using RNA extracted from individual blastocysts | [181] | |
| To identify microRNA (miRNA) in culture media of embryos of differing developmental competence | [187] | |
| HiSeq 1500TM system, Illumina, CA, USA | To study the function of the lineage-specific gene OCT4 (by knocking it out using CRISPR/Cas-9 system) | [79] |
| To study EGA using single-blastomere RNA-seq | [186] | |
| To study the role of OCT4 in the second lineage differentiation | [188] | |
| HiSeq 2000TM system, Illumina, CA, USA | To compare morphologically similar IVD and IVP blastocysts | [25] |
| To demonstrate that RNA-seq can be carried out using RNA extracted from single ICM and TE cell “biopsies” | [189] | |
| To study the paternal genetic contribution on pre-implantation IVP blastocysts | [190] | |
| HiSeq 2500TM system, Illumina, CA, USA | To study miRNA expression in zygotes | [191] |
| HiSeq 3000TM system, Illumina, CA, USA | To test the effects of DKK1 (WNT antagonist) during the morula and blastocyst stages | [192] |
| HiSeq 4000TM system, Illumina, CA, USA | To study the paternal genetic contribution in pre-implantation IVD blastocysts | [193] |
| NextSeqTM system, Illumina, CA, USA | To identify transcriptomic signatures predictive of establishing and maintaining gestation | [183,184] |
| To study chromatin remodeling events during EGA | [194] | |
| HiSeq X TenTM system, Illumina, CA, USA | To study paternally contributed RNAs (sperm-derived RNAs) in pre-EGA (2-cell and 4-cell) embryos | [195] |
| To determine the role of the chromatin remodeler SMARCA5 in blastocysts | [196] | |
| To determine the role of the NOTCH signaling pathway in early embryonic development | [197] | |
| To study the spatiotemporal translational regulation during pre-implantation development | [198] | |
| NovaSeq6000TM system, Illumina, CA, USA | To study lipid-metabolism-associated gene expression in parthenogenetic embryos | [199] |
| To study the functional consequences of three critical lineage-specific genes (SOX2, OCT4, and CDX2) | [182] | |
| sexing of embryos | [200] | |
| 5500xl Genetic Analyzer, Applied Biosystems, CA, USA | To compare 2-cell to 16-cell embryos and early morulae and blastocysts to human and mice embryos | [201] |
| To quantify transcript abundance of imprinted genes | [202] | |
| To detect embryo developmental potential by single-blastomere RNA-seq | [185] |
| Marker Measured/Methodology | Equipment/Methodology Used | Source/s |
|---|---|---|
| Apoptosis | TUNEL assay | [71,239,240], reviewed by [241] |
| Ribosome profile | High-resolution ribosome fractionation, polysome profiling, RNA-seq | [198] |
| DNA methylation landscape | Anti-5-methylcytosine (5-MeC) antibody, methylation-sensitive high-resolution melting analysis (MS-HRM), bisulfite sequencing | [75,242,243,244,245,246,247,248] |
| mRNA localization | In situ hybridization | [63,80,249,250] |
| Chromosomal abnormalities | 5% Giemsa staining (1250× magnification) | [251] |
| Automation/artificial intelligence (AI)-based microscopy | Use of genetic algorithms, artificial neural networks, automatic feature extraction from images and supervised learning to formulate AI-based computer-assisted scoring systems (CASS)/predictive models | [1,252,253,254,255,256] |
| Gene knockdown | Cytosine base editing, CRISPR/Cas-9 gene editing, siRNA-mediated gene knockdown | [79,182,196,197,257] |
| Characterization of extracellular vesicles | Nanoparticle tracking analysis, electron microscopy, small RNA sequencing | [258,259,260] |
| Multi-omics approaches | Integrating transcriptomic and epigenetic data and integrating metabolomics and epigenetic data | [261,262] |
| Accuracy | Invasive Nature | Subjectivity | Initial Cost and/or Cost of Operation | Technical Complexity | Evaluation Complete by Blastulation | Need Extra Freezing Step | |
|---|---|---|---|---|---|---|---|
| Stereoscopy | Low | Low | High | Low | Low | No | No |
| Fluorescence microscopy | High | High | Low | High | High | N/A ** | N/A ** |
| Electron microscopy | High | High | Low | High | High | N/A ** | N/A ** |
| GLIM | High | Low | Low | High | High | No | No |
| TLM | High | Low | Low | High | Low | Yes | No |
| AI-based microscopy | High | Low | Low | Low | High | No | No |
| Genomics | High | High * | Low | High | High | No | Yes |
| Transcriptomics | High | High * | Low | High | High | No | Yes |
| Proteomics | High | Low | Low | High | High | No | Yes |
| Metabolomics | High | Low | Low | High | High | No | Yes |
| NMR | High | Low | Low | High | High | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabel, R.A.C.; Marchioretto, P.V.; Bangert, E.A.; Wilson, K.; Milner, D.J.; Wheeler, M.B. Pre-Implantation Bovine Embryo Evaluation—From Optics to Omics and Beyond. Animals 2023, 13, 2102. https://doi.org/10.3390/ani13132102
Rabel RAC, Marchioretto PV, Bangert EA, Wilson K, Milner DJ, Wheeler MB. Pre-Implantation Bovine Embryo Evaluation—From Optics to Omics and Beyond. Animals. 2023; 13(13):2102. https://doi.org/10.3390/ani13132102
Chicago/Turabian StyleRabel, R. A. Chanaka, Paula V. Marchioretto, Elizabeth A. Bangert, Kenneth Wilson, Derek J. Milner, and Matthew B. Wheeler. 2023. "Pre-Implantation Bovine Embryo Evaluation—From Optics to Omics and Beyond" Animals 13, no. 13: 2102. https://doi.org/10.3390/ani13132102





