Rare and Hungry: Feeding Ecology of the Golden Alpine Salamander, an Endangered Amphibian in the Alps
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Taxon
2.2. Study Area
2.3. Sampling Predators
2.4. Prey Availability (Potential Trophic Niche)
2.5. Data Analysis
2.5.1. Potential and Realized Trophic Niche
2.5.2. Trophic Strategy and Selectivity
2.5.3. Comparison with Other Studies
2.5.4. Inter-Individual Diet Variation
3. Results
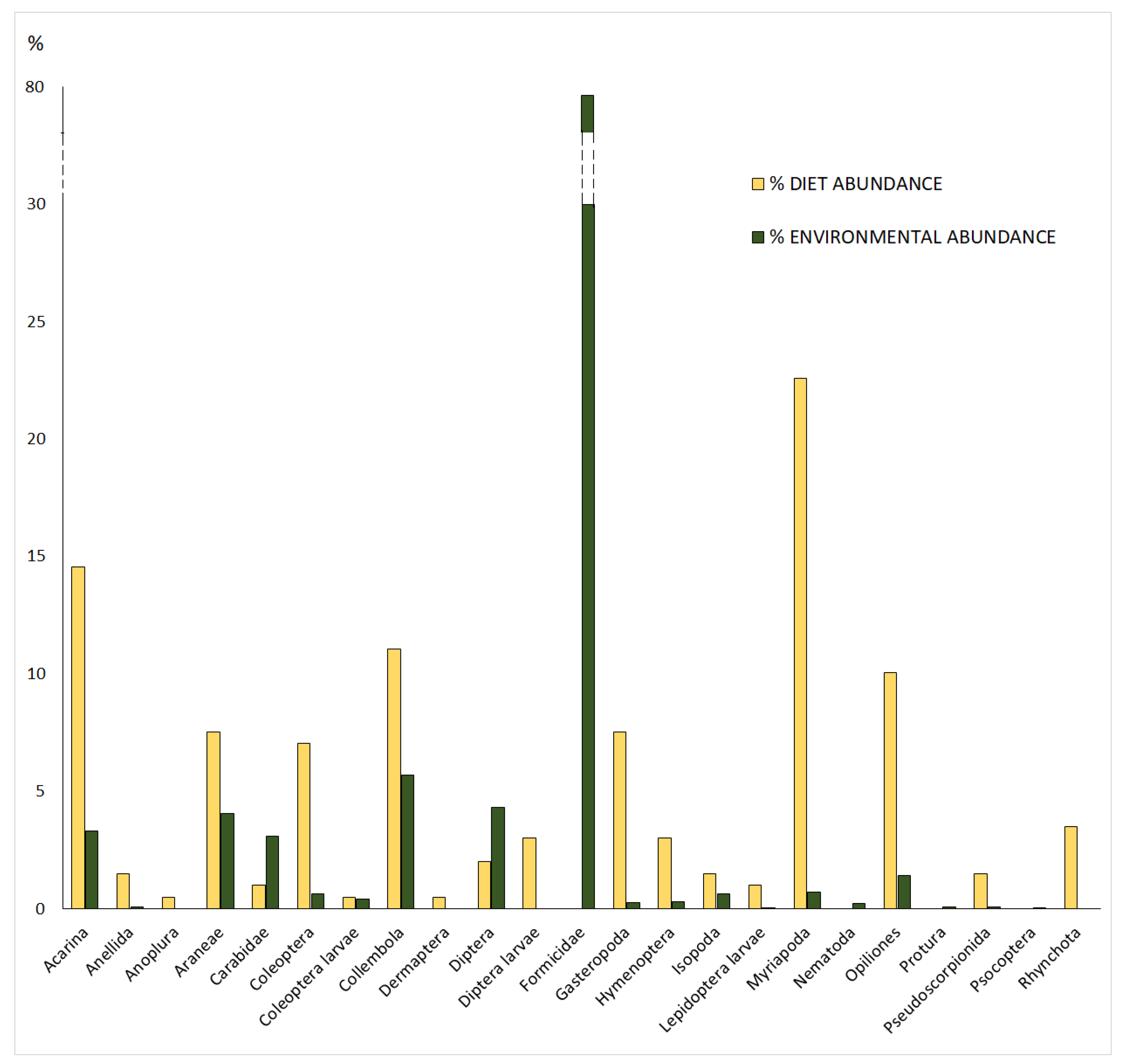
3.1. Potential and Realized Trophic Niche
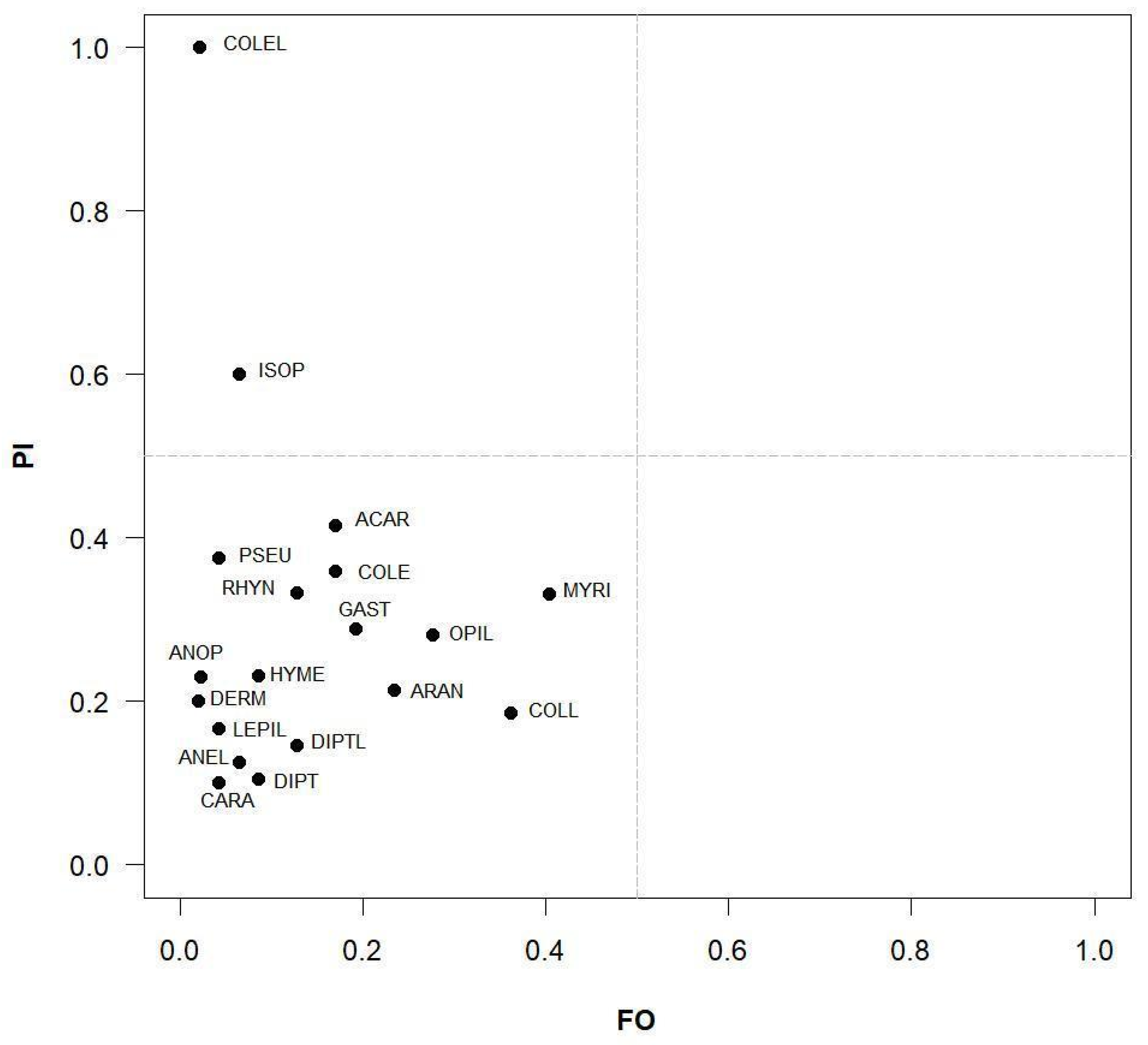
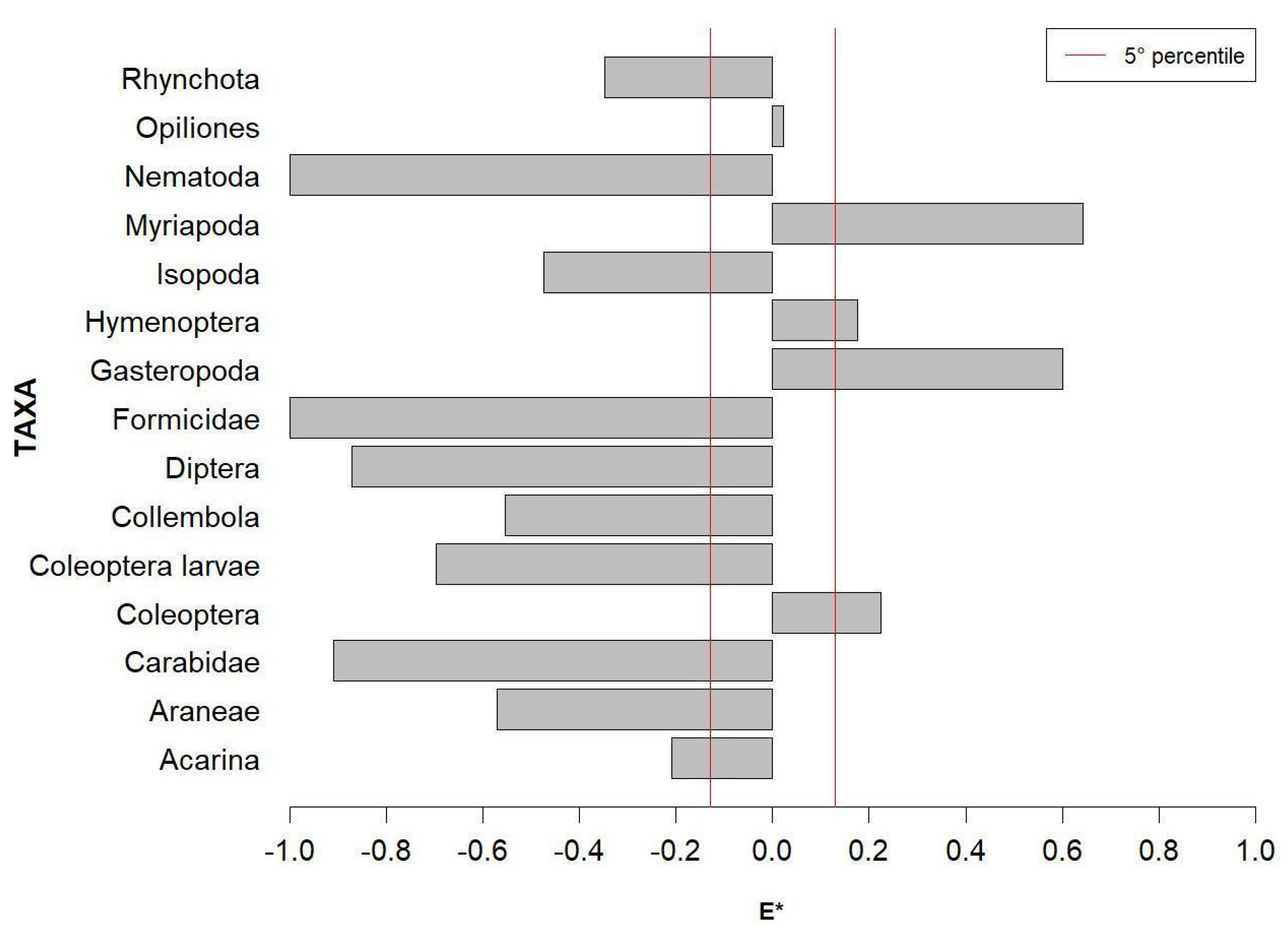
3.2. Trophic Selectivity
3.3. Comparison with Other Studies
3.4. Inter-Individual Diet Variation
4. Discussion
4.1. Prey Availability and Realized Trophic Niche
4.2. Trophic Selectivity
4.3. Inter-Individual Diet Variation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUCN Red List. 2023. Available online: https://www.iucnredlist.org/ (accessed on 5 March 2023).
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Cons. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; Chicago University Press: Chicago, IL, USA, 2010. [Google Scholar] [CrossRef]
- Davic, R.D.; Welsh, H.H., Jr. On the ecological roles of salamanders. Ann. Rev. Ecol. Evol. System. 2004, 35, 405–434. [Google Scholar] [CrossRef]
- Hairston, N.G. Community Ecology and Salamander Guilds; Cambridge University Press: Cambridge, UK, 1987; ISBN 0521325781 9780521325783. [Google Scholar]
- Sillero, N.; Campos, J.; Bonardi, A.; Corti, C.; Creemers, R.; Crochet, P.A.; Isailovic, J.C.; Denoël, M.; Ficetola, G.F.; Gonçalves, J.; et al. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphib. Reptil. 2014, 35, 1–31. [Google Scholar] [CrossRef]
- Bonato, L.; Steinfartz, S. Evolution of the melanistic colour in the Alpine salamander Salamandra atra as revealed by a new subspecies from the Venetian Prealps. Ital. J. Zool. 2005, 72, 253–260. [Google Scholar] [CrossRef]
- Romano, A.; Costa, A.; Salvidio, S.; Menegon, M.; Garollo, E.; Tabarelli de Fatis, K.; Miserocchi, D.; Matteucci, G.; Pedrini, P. Forest management and conservation of an elusive amphibian in the Alps: Habitat selection by the Golden Alpine Salamander reveals the importance of Fine Woody Debris. For. Ecol. Manag. 2018, 424, 338–344. [Google Scholar] [CrossRef]
- Fachbach, G.; Kolossau, I.; Ortner, A. Zur Ernährungsbiologie von Salamandra, S. salamandra und Salamandra atra. Salamandra 1975, 11, 136–144. [Google Scholar]
- Roner, L.; Costa, A.; Pedrini, P.; Matteucci, G.; Leonardi, S.; Romano, A. A Midsummer night’s diet: Snapshot on trophic strategy of the Alpine salamander, Salamandra atra. Diversity 2020, 12, 202. [Google Scholar] [CrossRef]
- Šunje, E.; Courant, J.; Vesnić, A.; Koren, T.; Bilela, L.L.; Van Damme, R. Patterns of variation in dietary composition among four populations of Alpine salamanders (Salamandra atra prenjensis). Amphib.-Reptil. 2022, 43, 1–15. [Google Scholar] [CrossRef]
- Roner, L.; Romano, A.; Trenti, M.; Pagani, A.; Melchiori, G.; Bombieri, G.; Tabarelli de Fatis, K.; Pedrini, P. The calm after the storm? The golden alpine salamander, Salamandra atra aurorae, and VAIA storm in Trentino: Past, present and future. In Proceeding of the XIV National Congress Societas Herpetologica Italica, Torino, Italy, 13–17 September 2022. [Google Scholar]
- Rondinini, C.; Battistoni, A.; Teofili, C. (Eds.) Lista Rossa IUCN dei Vertebrati Italiani 2022; Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica: Rome, Italy, 2022. [Google Scholar]
- Burgon, J.D.; Vences, M.; Steinfartz, S.; Bogaerts, S.; Bonato, L.; Donaire-Barroso, D.; Martínez-Solano, I.; Velo-Antón, G.; Vieites, D.R.; Mable, B.K.; et al. Phylogenomic inference of species and subspecies diversity in the Palearctic salamander genus Salamandra. Mol. Phylogenet. Evol. 2021, 157, 107063. [Google Scholar] [CrossRef]
- Romanazzi, E.; Bonato, L. Updating the range of the narrowly distributed endemites Salamandra atra aurorae and S. atra pasubiensis. Amphib.-Reptil. 2014, 35, 123–128. [Google Scholar] [CrossRef]
- Bonato, L.; Fracasso, G. Epigean habitat of a population of Salamandra atra aurorae: A preliminary analysis. In Proceedings of the X Congresso Nazionale della Societas Herpetologica Italica, Genova, Italy, 15–18 October 2014; Doria, G., Poggi, R., Salvidio, S., Tavano, M., Eds.; Ianieri Edition: Pescara, Italy, 2015; Volume 47–55. [Google Scholar]
- Bonato, L.; Fracasso, G. Salamandra alpina, Salamandra atra Laurenti, 1768; Salamandra di Aurora, Salamandra atra aurorae Trevisan, 1982. In Atlante degli Anfibi e dei Rettili della Provincia di Vicenza; Gilberto Padovan Editore: Vicenza, Italy, 2000; pp. 43–47. [Google Scholar]
- Bonato, L.; Fracasso, G. Aspetti morfologici ed ecologici di una popolazione di Salamandra atra aurorae: Risultati preliminari. In Proceedings of the 2th Convegno dei Faunisti Veneti, Padua, Italy, 25–26 October 1997; Bon, M., Mezzavilla, F., Eds.; Museo Civico di Storia Naturale di Venezia: Venezia, Italy, 1999; Volume 48, pp. 31–35. [Google Scholar]
- Roner, L.; Trenti, M.; Salvidio, S.; Costa, C.; Pedrini, P.; Romano, A. Il monitoraggio della salamandra alpina Salamandra atra in Trentino: Applicazione e validità del metodo del doppio osservatore in diverse condizioni meteorologiche. In Proceedings of the XIII Congresso Nazionale Societas Herpetologica Italica, Lipari, Italy, 22–26 September 2021; Biaggini, M., Corti, C., Giacobbe, D., Lo Cascio, P., Restivo, S., Eds.; Naturalista siciliano: Palermo, Italy, 2022; Volume 46, pp. 361–368. [Google Scholar]
- Eccel, E.; Saibanti, S. Inquadramento climatico dell’Altopiano di Lavarone-Vezzena nel contesto generale Trentino. Studi Trent. Sci. Nat. Acta Geol. 2005, 82, 111–121. [Google Scholar]
- Bonato, L.; Fracasso, G. Movements, distribution pattern and density in a population of Salamandra atra aurorae (Caudata: Salamandridae). Amphib.-Reptil. 2003, 24, 251–260. [Google Scholar] [CrossRef]
- Schabetsberger, R. Gastric evacuation rates of adult and larval Alpine newts (Triturus alpestris) under laboratory and field conditions. Freshw. Biol. 1994, 31, 143–151. [Google Scholar] [CrossRef]
- Solé, M.; Beckmann, O.; Pelz, B.; Kwet, A.; Engels, W. Stomach–flushing for diet analysis in anurans: An improved protocol evaluated in a case study in Araucaria forests, southern Brazil. Stud. Neotrop. Fauna Environ. 2005, 40, 23–28. [Google Scholar] [CrossRef]
- Costa, A.; Salvidio, S.; Posillico, M.; Altea, T.; Matteucci, G.; Romano, A. What goes in does not come out: Different nonlethal dietary methods give contradictory interpretations of prey selectivity in amphibians. Amphib.-Reptil. 2014, 35, 255–262. [Google Scholar] [CrossRef]
- Klewen, R.F. Die Landsalamander Europas 1: Die Gattungen Salamandra und Mertensiella; A. Ziemsen Verlag: Wittenberg Lutherstadt, Germany, 1988. [Google Scholar]
- Klewen, R.F. Untersuchungen zur Verbreitung, Öko-Ethologie und Innerartlichen Gliederung von Salamandra atra (Laurenti 1768). Ph.D. Thesis, Universität Köln, Köln, Germany, 1986. [Google Scholar]
- Fraser, D.F. Coexistence of salamanders in the genus Plethodon: A variation of the Santa Rosalia theme. Ecology 1976, 55, 238–251. [Google Scholar] [CrossRef]
- Salvidio, S. Diet and food utilization in a rockface population of Speleomantes ambrosii (Amphibia, Caudata, Plethodontidae). Vie Milieu 1992, 42, 35–39. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006; ISBN 1139458019 9781139458016. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Wiley-Blackwell: Oxford, UK, 2000. [Google Scholar]
- Ausden, M. Invertebrates. In Ecological Census Techniques: A Handbook; Sutherland, W.J., Ed.; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; Mc Graw-Hill Book Company: New York, NY, USA, 1986. [Google Scholar]
- Solé, M.; Rödder, D. Dietary assessments of adult amphibians. In Amphibian Ecology and Conservation: A Handbook of Techniques; Dodd, J., Ed.; Oxford University Press: Oxford, UK, 2010; pp. 167–184. ISBN 0199541191 9780199541195. [Google Scholar]
- Scheller, H.V. Pitfall trapping as the basis for studying ground beetle predation in spring barley. Tidsskr. Planteavl. 1984, 88, 317–324. [Google Scholar]
- Woodcock, B.A. Pitfall trapping in ecological studies. In Insect Sampling in Forest Ecosystems; Leather, S.R., Ed.; Blackwell Publishing Co.: Hoboken, NJ, USA, 2005; pp. 37–56. ISBN 1405140291 9781405140294. [Google Scholar]
- Salvidio, S.; Pasmans, F.; Bogaerts, S.; Martel, A.; van de Loo, M.; Romano, A. Consistency in trophic strategies between populations of the Sardinian endemic salamander Speleomantes imperialis. Anim. Biol. 2017, 67, 1–16. [Google Scholar] [CrossRef]
- Costa, A.; Salvidio, S.; Posillico, M.; Matteucci, G.; De Cinti, B.; Romano, A. Generalisation within specialization: Inter–individual diet variation in the only specialized salamander in the world. Sci. Rep. 2015, 5, 13260. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Clarke, K.R. Non–parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Costello, M.J. Predator feeding strategy and prey importance: A new graphical analysis. J. Fish Biol. 1990, 36, 261–263. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Gabler, H.M.; Staldvik, F.J. A new approach to graphical analysis of feeding strategy from stomach contents data—Modification of the Costello (1990) method. J. Fish Biol. 1996, 48, 607–614. [Google Scholar] [CrossRef]
- Vignoli, L.; Luiselli, L.; Bologna, M.A. Dietary patterns and overlap in an amphibian assemblage at a pond in Mediterranean central Italy. Vie Milieu 2009, 59, 47–57. [Google Scholar]
- Romano, A.; Salvidio, S.; Palozzi, R.; Sbordoni, V. Diet of the newt, Triturus carnifex (Laurenti, 1768), in a flooded karstic sinkhole (“Pozzo del Merro”, Central Italy). J. Cave Karst Stud. 2012, 74, 271–277. [Google Scholar] [CrossRef]
- Salvidio, S.; Romano, A.; Oneto, F.; Ottonello, D.; Michelon, R. Different season, different strategies: Feeding ecology of two syntopic forest dwelling salamanders. Acta Oecol. 2012, 43, 42–50. [Google Scholar] [CrossRef]
- Bounaceur, F.; Benamor, N.; Bissaad, F.Z.; Lasgaa, F.; Baghadid, S.; Rezigua, F.; Aulagnier, S. Feeding ecology of the vulnerable aoudad (Ammotragus lervia) in north-western Sahara. Afr. J. Ecol. 2023, 61, 28–36. [Google Scholar] [CrossRef]
- Castelló y Tickell, S.; Low, N.H.N.; Lamb, R.W.; Brandt, M.; Witman, J.D. Distribution and feeding ecology of sea stars in the Galàpagos rocky subtidal zone. J. Ex. Mar. Biol. Ecol. 2022, 553, 151754. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Scavia, D. Calculation and use of selectivity coefficients of feeding: Zooplankton grazing. Ecol. Mod. 1979, 7, 135–149. [Google Scholar] [CrossRef]
- Lechowicz, M.J. The sampling characteristics of electivity indices. Oecologia 1982, 52, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ramos–Jiliberto, R.; Valdovinos, F.S.; Arias, J.; Alcaraz, C.; García–Berthou, E. A network–based approach to the analysis of ontogenetic diet shifts: An example with an endangered, small–sized fish. Ecol. Compl. 2011, 8, 123–129. [Google Scholar] [CrossRef]
- Davidson, R.; Harel, D. Drawing graphs nicely using simulated annealing. Acm. T. Graphic 1996, 15, 301–331. [Google Scholar] [CrossRef]
- Araújo, M.S.; Guimarães, P.R., Jr.; Svanbäck, R.; Pinheiro, A.; Guimarães, P.; Reis, S.F.D.; Bolnick, D.I. Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology 2008, 89, 1981–1993. [Google Scholar] [CrossRef]
- Almeida–Neto, M.; Guimaraes, P.; Guimaraes, P.R., Jr.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and quantification. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Beckett, S.J. Improved community detection in weighted bipartite networks. R. Soc. Open Sci. 2016, 3, 140536. [Google Scholar] [CrossRef]
- Bonato, L.; Fracasso, G.; Luiselli, L. Salamandra atra Laurenti, 1768. In Fauna d’Italia; Lanza, B., Andreone, F., Bologna, M.A., Corti, C., Razzetti, E., Eds.; Calderini Edition: Bologna, Italy, 2007; Volume 42, pp. 97–211. ISBN 9788850652563. [Google Scholar]
- Sparreboom, M. Salamanders of the Old World: The Salamanders of Europe, Asia and Northern Africa; BRILL: Zeist, The Netherlands, 2014; ISBN 9004285628 9789004285620. [Google Scholar]
- Pereira, L.B.; Botta, S.; Teixeira, C.R.; Fruet, P.; Simões-Lopes, P.C.; Daura-Jorge, F.G. Feeding ecology of two subspecies of bottlenose dolphin: A tooth tale. Aquat. Ecol. 2020, 54, 941–955. [Google Scholar] [CrossRef]
- Corrêa, D.N.; Quintela, F.M.; Loebmann, D. Feeding ecology of Erythrolamprus jaegeri jaegeri (Günter, 1858) and Erythrolamprus poecilogyrus sublineatus (Cope, 1860) in the coastal zone of Subtropical Brazil (Serpentes, Dipsadidae). An. Ac. Bras. Ciênc. 2016, 88, 293–308. [Google Scholar] [CrossRef]
- Perera, A.; Pérez-Mellado, V.; Carretero, M.A.; Harris, D.J. Variation between populations in the diet of the Mediterranean lizard Lacerta perspicillata. Herpetol. J. 2006, 16, 107–113. [Google Scholar]
- Andreone, F.; De Michelis, S.; Clima, V. A montane amphibian and its feeding habits: Salamandra lanzai (Caudata, Salamandridae) in the Alps of northwestern Italy. Ital. J. Zool. 1999, 66, 45–49. [Google Scholar] [CrossRef]
- Krebs, J.R. Optimal foraging: Decision rules for predators. In Behavioural Ecology: An Evolutionary Approach; Krebs, J.R., Davies, N.B., Eds.; Backwell Scientific Publications: Oxford, UK, 1978; pp. 23–63. [Google Scholar]
- Stephens, D.W. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1986; ISBN 9780691084428. [Google Scholar]
- Maiorana, V.C. Behavior of an Unobservable Species: Diet Selection by a Salamander. Copeia 1978, 4, 664–672. [Google Scholar] [CrossRef]
- Sites, J.W. The foraging strategy of the dusky salamander, Desmognathus fuscus (Amphibia, Urodela, Plethodontidae): An empirical approach to predation theory. J. Herpetol. 1978, 12, 373–383. [Google Scholar] [CrossRef]
- Jaeger, R.G. Fluctuations in prey availability and food limitation for a terrestrial salamander. Oecologia 1980, 44, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Salvidio, S.; Oneto, F.; Ottonello, D.; Costa, A.; Romano, A. Trophic specialization at the individual level in a terrestrial generalist salamander. Can. J. Zool. 2015, 93, 79–83. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990; ISBN 0674040759 9780674040755. [Google Scholar]
- Hopkin, S.P. Collembola. In Encyclopaedia of Soil Science; Dekker, M., Ed.; R. LAL: New York, NY, USA, 2002; pp. 207–210. [Google Scholar]
- Crovetto, F.; Romano, A.; Salvidio, S. Comparison of two non-lethal methods for dietary studies in terrestrial salamanders. Wildl. Res. 2012, 39, 266–270. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997; ISBN 019158925X 9780191589256. [Google Scholar]
- Whitaker, J.O., Jr.; Rubin, D.C. Food habits of Plethodon jordani metcalfi and Plethodon jordani shermani from North Carolina. Herpetologica 1971, 27, 81–86. [Google Scholar]
- Parisi, F.; Pioli, S.; Lombardi, F.; Fravolini, G.; Marchetti, M.; Tognetti, R. Linking deadwood traits with saproxylic invertebrates and fungi in European forests—A review. iForest 2018, 11, 423–436. [Google Scholar] [CrossRef]
- Jabin, M.; Mohr, D.; Kappes, H.; Topp, W. Influence of deadwood on density of soil macro-arthropods in a managed oakbeechforest. For. Ecol. Manag. 2004, 194, 61–69. [Google Scholar] [CrossRef]
- Dajoz, R. Insects and Forests: The Role and Diversity of Insects in the Forest Environment; Intercept Limited: Paris, France, 2000; p. 620. [Google Scholar]
- Jonsell, M.; Vårdal, H.; Forshage, M.; Stigenberg, J. Saproxylic Hymenoptera in dead wood retained on clear cuts, relation to wood parameters and their degree of specialisation. J. Insect Conserv. 2023, 27, 347–359. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Yang, L.H.; Fordyce, J.A.; Davis, J.M.; Svanback, R. Measuring individual–level resource specialization. Ecology 2002, 83, 2936–2941. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanback, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; Corti, C.; Biaggini, M.; Zhao, Y.; Cianferoni, F. The Trophic Niche of Two Sympatric Species of Salamanders (Plethodontidae and Salamandridae) from Italy. Animals 2022, 12, 2221. [Google Scholar] [CrossRef] [PubMed]
- Tinker, M.; Guimarães, P.R.; Novak, M.; Marquitti, F.M.D.; Bodkin, M.S.; Bentall, G.; Estes, J.A. Structure and mechanism of diet specialisation: Testing models of individual variation in resource use with sea otters. Ecol. Lett. 2012, 15, 475–483. [Google Scholar] [CrossRef]
- Pires, L.P.; De Melo, C. Individual–resource networks reveal distinct fruit preferences of selective individuals from a generalist population of the Helmeted Manakin. Ibis 2020, 162, 713–722. [Google Scholar] [CrossRef]
- Pires, M.M.; Guimarães, P.R.; Araújo, M.S.; Giaretta, A.A.; Costa, J.C.L.; Dos Reis, S.F. The nested assembly of individual-resource networks. J. Anim. Ecol. 2011, 80, 896–903. [Google Scholar] [CrossRef]
- Lemos-Costa, P.; Pires, M.M.; Araújo, M.S.; De Aguiar, M.A.M.; Guimarães, P.R. Network analyses support the role of prey preferences in shaping resource use patterns within five animal populations. Oikos 2016, 125, 492–501. [Google Scholar] [CrossRef]
- Duelli, P.; Obrist, M.K.; Schmatz, D.R. Biodiversity evaluation in agricultural landscapes: Above-ground insects. Agric. Ecosyst. Environ. 1999, 74, 33–64. [Google Scholar] [CrossRef]
- Duelli, P.; Obrist, M.K.; Wermelinger, B. Wind-throw induces changes of faunistic biodiversity in alpine spruce forests. For. Snow Landsc. Res. 2002, 77, 117–131. [Google Scholar]
- Rota, N.; Canedoli, C.; Ferrè, C.; Ficetola, G.F.; Guerrieri, A.; Padoa-Schioppa, E. Evaluation of Soil Biodiversity in Alpine Habitats through eDNA Metabarcoding and Relationships with Environmental Features. Forests 2020, 11, 738. [Google Scholar] [CrossRef]
- Nitzu, E.; Nae, A.; Băncilă, R.; Popa, I.; Giurginca, A.; Plăiaşu, R. Scree habitats: Ecological function, species conservation and spatial-temporal variation in the arthropod community. Syst. Biodivers. 2014, 12, 65–75. [Google Scholar] [CrossRef]
- Costa, A.; Romano, A.; Rosa, G.; Salvidio, S. Weighted individual-resource networks in prey–predator systems: The role of prey availability on the emergence of modular structures. Integr. Zool. 2021, 17, 115–127. [Google Scholar] [CrossRef] [PubMed]


| Simpson Diversity Index | 1-D (95% C.I.) | Taxa |
|---|---|---|
| Environmental availability | 0.44 (0.42–0.46) | 17 |
| Stomach content | 0.88 (0.86–0.90) | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centomo, E.; Roner, L.; Salvatori, M.; Pedrini, P.; Romano, A. Rare and Hungry: Feeding Ecology of the Golden Alpine Salamander, an Endangered Amphibian in the Alps. Animals 2023, 13, 2135. https://doi.org/10.3390/ani13132135
Centomo E, Roner L, Salvatori M, Pedrini P, Romano A. Rare and Hungry: Feeding Ecology of the Golden Alpine Salamander, an Endangered Amphibian in the Alps. Animals. 2023; 13(13):2135. https://doi.org/10.3390/ani13132135
Chicago/Turabian StyleCentomo, Emma, Luca Roner, Marco Salvatori, Paolo Pedrini, and Antonio Romano. 2023. "Rare and Hungry: Feeding Ecology of the Golden Alpine Salamander, an Endangered Amphibian in the Alps" Animals 13, no. 13: 2135. https://doi.org/10.3390/ani13132135
APA StyleCentomo, E., Roner, L., Salvatori, M., Pedrini, P., & Romano, A. (2023). Rare and Hungry: Feeding Ecology of the Golden Alpine Salamander, an Endangered Amphibian in the Alps. Animals, 13(13), 2135. https://doi.org/10.3390/ani13132135





