The Probiotic Phaeobacter inhibens Provokes Hypertrophic Growth via Activation of the IGF-1/Akt Pathway during the Process of Metamorphosis of Greater Amberjack (Seriola dumerili, Risso 1810)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Application of Biofilters with Phaeobacter inhibens to Greater Amberjack Larval Rearing Conditions
2.2. Rearing of Greater Amberjack Larvae
2.3. SDS/PAGE and Immunoblot Analysis
2.4. Determination of Intermediate Metabolism Enzyme Activities
2.5. Microbiological Analysis of Rearing Water
2.6. Statistical Analysis
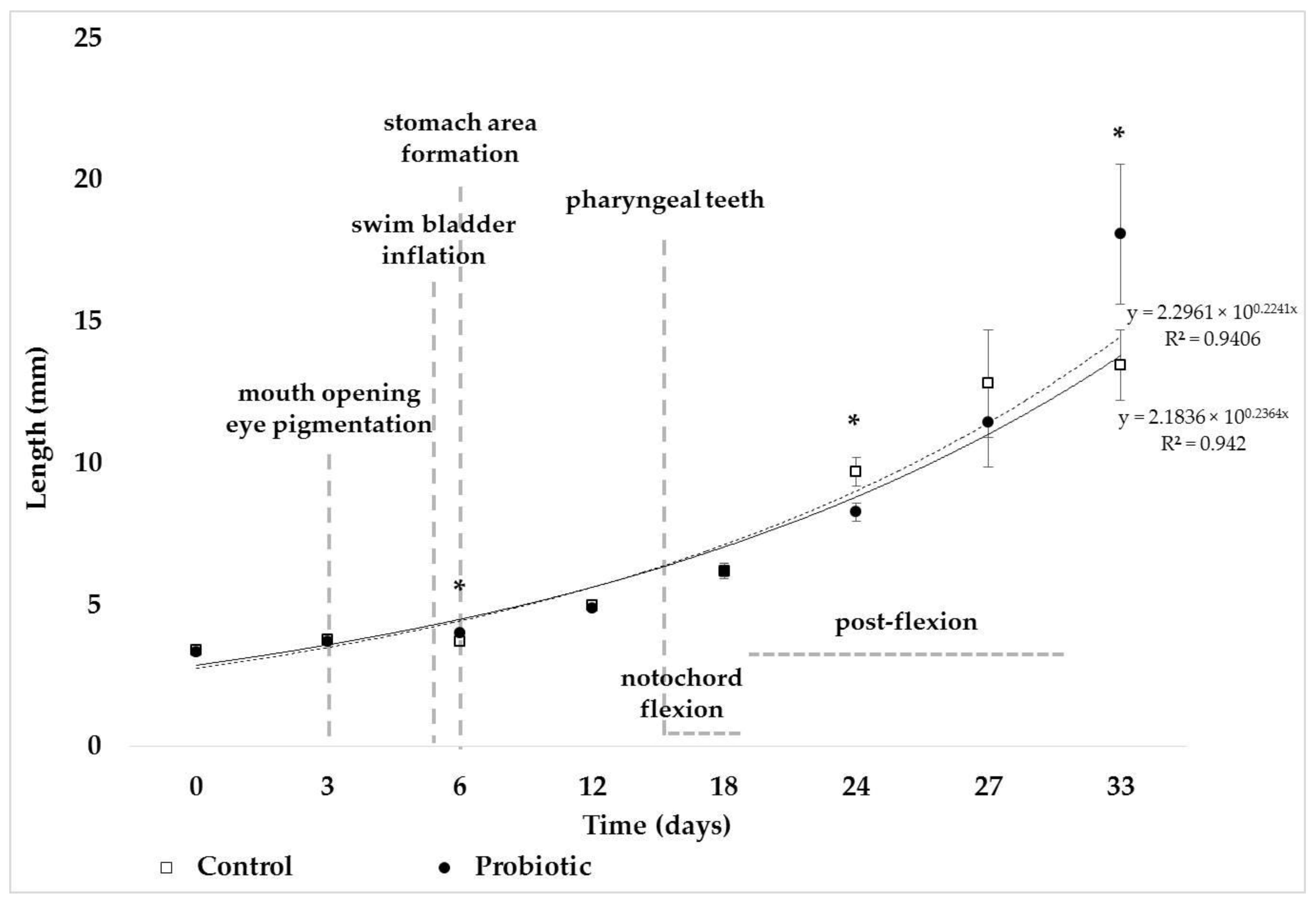
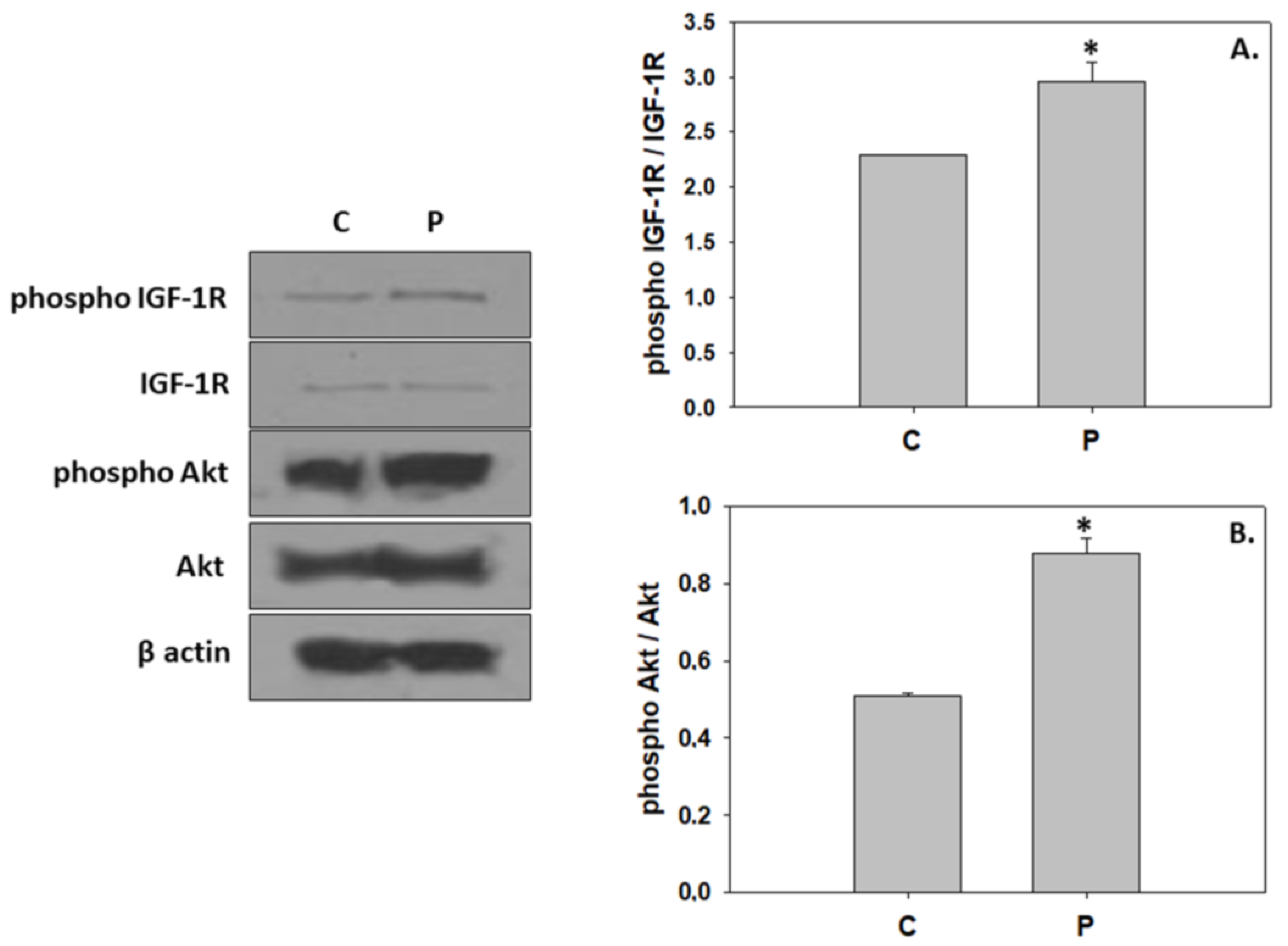
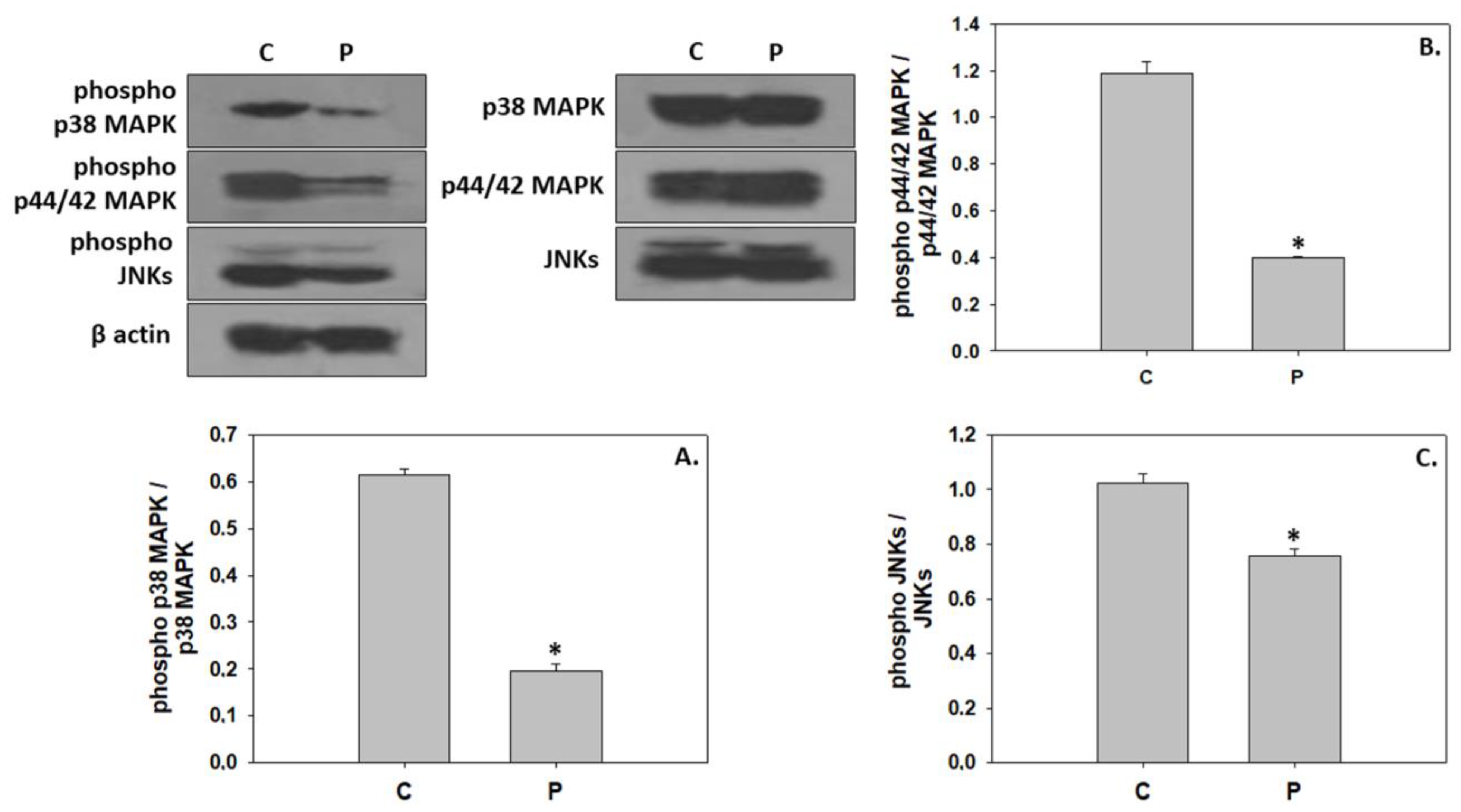
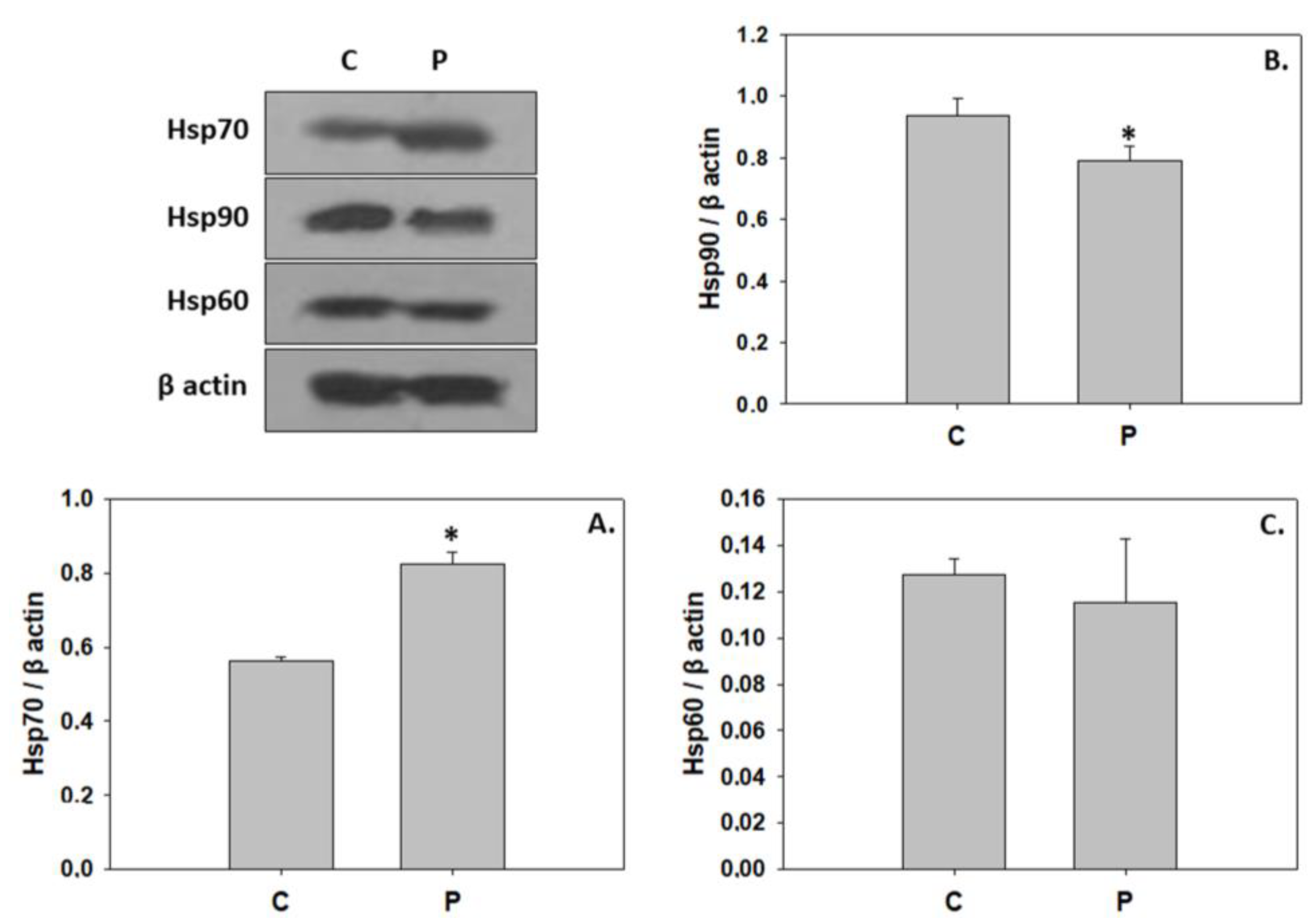
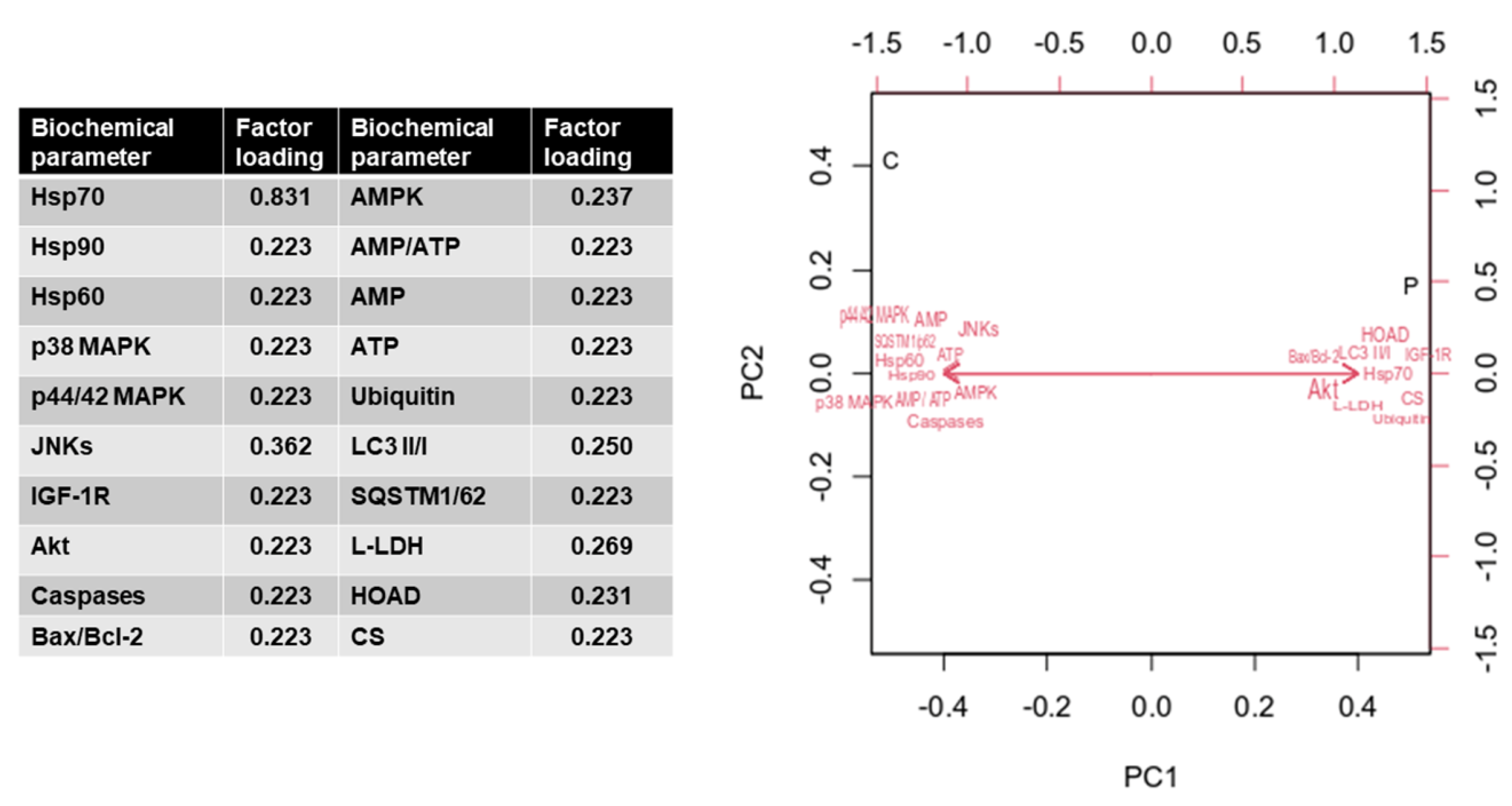
3. Results
4. Discussion
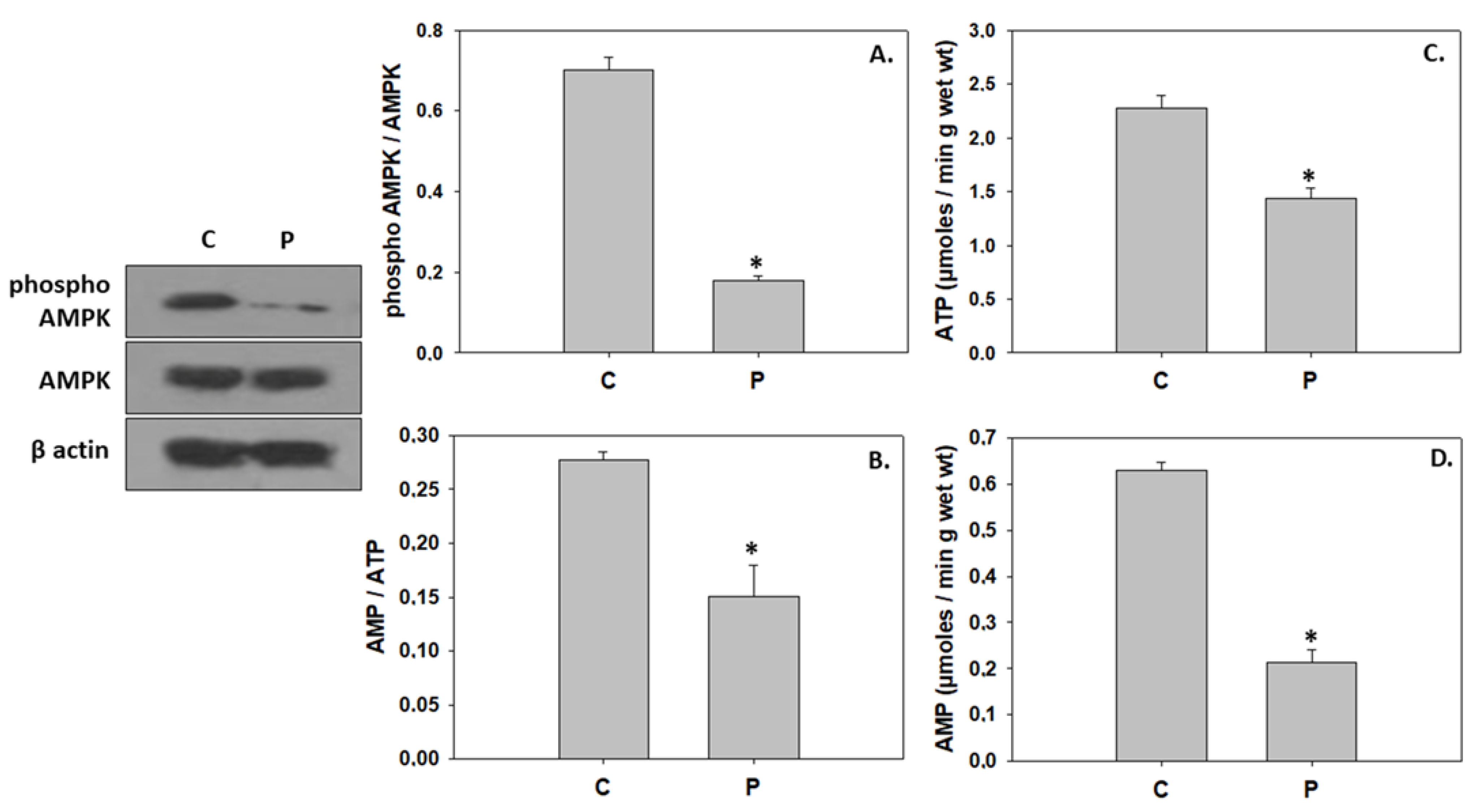
4.1. Induction of Hypertrophic Growth via Activation of IGF/Akt Pathway
4.2. Inhibition of Downstream Components in the IGF/MAPK Signaling
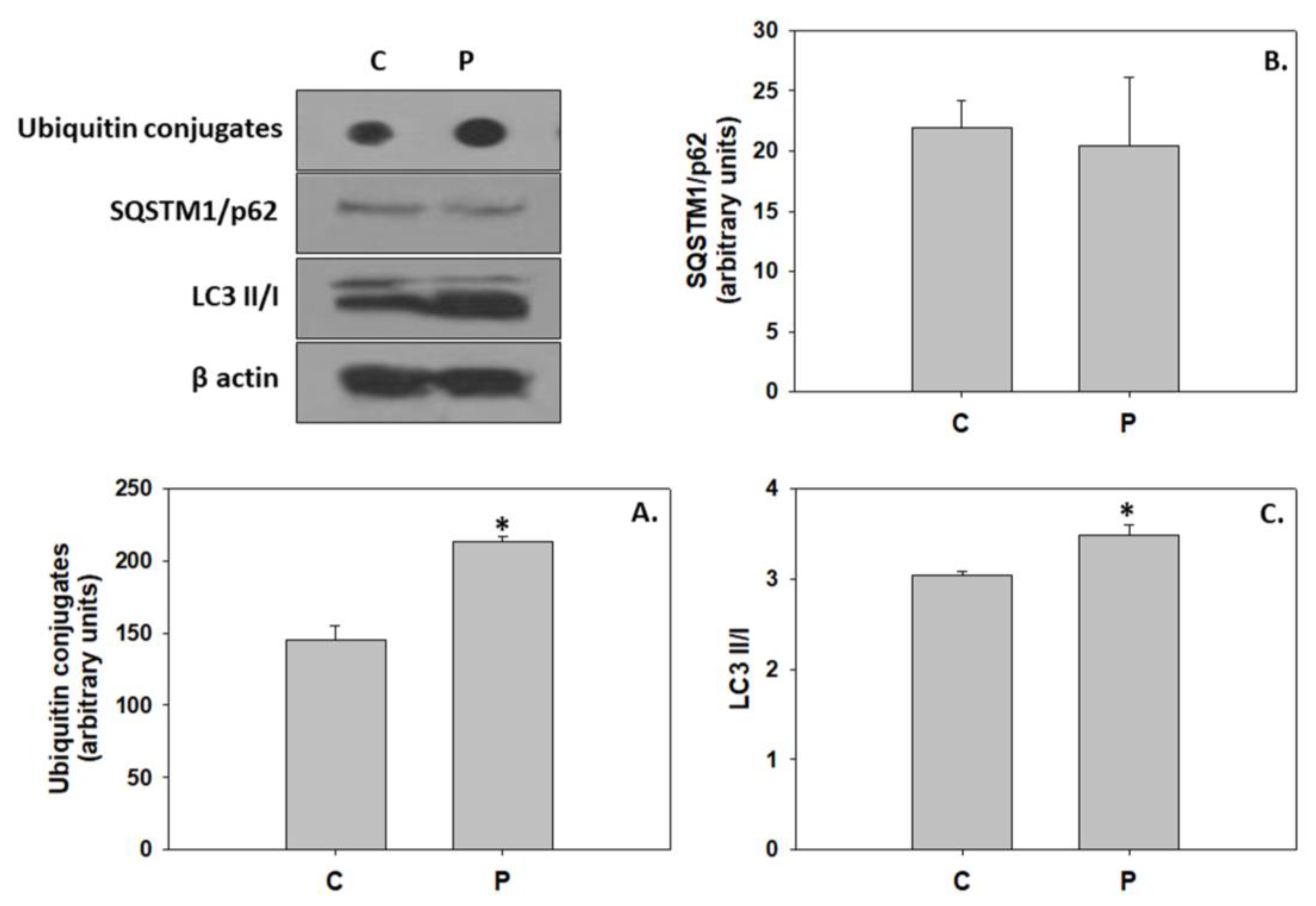
4.3. P. inhibens-Induced Developmental Cell Death
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMenamin, S.K.; Parichy, D.M. Chapter Five—Metamorphosis in Teleosts. In Current Topics in Developmental Biology; Shi, Y.-B., Ed.; Animal Metamorphosis; Academic Press: Cambridge, MA, USA, 2013; Volume 103, pp. 127–165. [Google Scholar]
- Schreiber, A.M.; Specker, J.L. Metamorphosis in the Summer Flounder, Paralichthys dentatus: Thyroidal Status Influences Gill Mitochondria-Rich Cells. Gen. Comp. Endocrinol. 2000, 117, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Vélez, E.J.; Lutfi, E.; Azizi, S.; Perelló, M.; Salmerón, C.; Riera-Codina, M.; Ibarz, A.; Fernández-Borràs, J.; Blasco, J.; Capilla, E.; et al. Understanding Fish Muscle Growth Regulation to Optimize Aquaculture Production. Aquaculture 2017, 467, 28–40. [Google Scholar] [CrossRef]
- Campinho, M.A. Teleost Metamorphosis: The Role of Thyroid Hormone. Front. Endocrinol. 2019, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, E.G.; Hirano, T.; Inui, Y. Flounder Metamorphosis: Its Regulation by Various Hormones. Fish Physiol. Biochem. 1993, 11, 323–328. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Valdés, J.A.; Molina, A.; Björnsson, B.T. Regulation of skeletal muscle growth in fish by the growth hormone–insulin-like growth factor system. Gen. Comp. Endocrinol. 2013, 192, 136–148. [Google Scholar] [CrossRef]
- Hildahl, J.; Power, D.M.; Björnsson, B.T.; Einarsdóttir, I.E. Involvement of Growth Hormone-Insulin-like Growth Factor I System in Cranial Remodeling during Halibut Metamorphosis as Indicated by Tissue- and Stage-Specific Receptor Gene Expression and the Presence of Growth Hormone Receptor Protein. Cell Tissue Res. 2008, 332, 211–225. [Google Scholar] [CrossRef]
- Jia, Y. Roles of Insulin-like Growth Factors in Metamorphic Development of Turbot (Scophthalmus maximus). Gen. Comp. Endocrinol. 2018, 265, 61–63. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Cheng, Q.; Chen, X. Expression of Insulin-like Growth Factor I Receptors at MRNA and Protein Levels during Metamorphosis of Japanese Flounder (Paralichthys olivaceus). Gen. Comp. Endocrinol. 2011, 173, 78–85. [Google Scholar] [CrossRef]
- Moriyama, S.; Ayson, F.G.; Kawauchi, H. Growth Regulation by Insulin-like Growth Factor-I in Fish. Biosci. Biotech. Biochem. 2000, 64, 1553–1562. [Google Scholar] [CrossRef]
- Reinecke, M.; Björnsson, B.T.; Dickhoff, W.W.; McCormick, S.D.; Navarro, I.; Power, D.M.; Gutiérrez, J. Growth Hormone and Insulin-like Growth Factors in Fish: Where We Are and Where to Go. Gen. Comp. Endocrinol. 2005, 142, 20–24. [Google Scholar] [CrossRef]
- Duan, C. Nutritional and Developmental Regulation of Insulin-like Growth Factors in Fish. J. Nutr. 1998, 128, 306S–314S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, J.; LeRoith, D. Insulin and Insulin-Like Growth Factor I Receptors: Similarities and Differences in Signal Transduction. Horm. Res. Paediatr. 2001, 55, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Favelyukis, S.; Till, J.H.; Hubbard, S.R.; Miller, W.T. Structure and Autoregulation of the Insulin-like Growth Factor 1 Receptor Kinase. Nat. Struct. Mol. Biol. 2001, 8, 1058–1063. [Google Scholar] [CrossRef]
- Coolican, S.A.; Samuel, D.S.; Ewton, D.Z.; McWade, F.J.; Florini, J.R. The Mitogenic and Myogenic Actions of Insulin-like Growth Factors Utilize Distinct Signaling Pathways. J. Biol. Chem. 1997, 272, 6653–6662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/MTOR Pathway Is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy in Vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-Induced Skeletal Myotube Hypertrophy by PI(3)K/Akt/MTOR and PI(3)K/Akt/GSK3 Pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef]
- Codina, M.; García de la Serrana, D.; Sánchez-Gurmaches, J.; Montserrat, N.; Chistyakova, O.; Navarro, I.; Gutiérrez, J. Metabolic and Mitogenic Effects of IGF-II in Rainbow Trout (Oncorhynchus mykiss) Myocytes in Culture and the Role of IGF-II in the PI3K/Akt and MAPK Signalling Pathways. Gen. Comp. Endocrinol. 2008, 157, 116–124. [Google Scholar] [CrossRef]
- Li, J.; Johnson, S.E. ERK2 Is Required for Efficient Terminal Differentiation of Skeletal Myoblasts. Biochem. Biophys. Res. Commun. 2006, 345, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Feidantsis, K.; Pörtner, H.O.; Markou, T.; Lazou, A.; Michaelidis, B. Involvement of P38 MAPK in the Induction of Hsp70 during Acute Thermal Stress in Red Blood Cells of the Gilthead Sea Bream, Sparus aurata. J. Exp. Zool. A Ecol. Genet. Physiol. 2012, 317, 303–310. [Google Scholar] [CrossRef]
- Sun, X.-C.; Xian, X.-H.; Li, W.-B.; Li, L.; Yan, C.-Z.; Li, Q.-J.; Zhang, M. Activation of P38 MAPK Participates in Brain Ischemic Tolerance Induced by Limb Ischemic Preconditioning by Up-Regulating HSP 70. Exp. Neurol. 2010, 224, 347–355. [Google Scholar] [CrossRef]
- Krone, P.H.; Sass, J.B. Hsp 90α and Hsp 90β Genes Are Present in the Zebrafish and Are Differentially Regulated in Developing Embryos. Biochem. Biophys. Res. Commun. 1994, 204, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Manchado, M.; Salas-Leiton, E.; Infante, C.; Ponce, M.; Asensio, E.; Crespo, A.; Zuasti, E.; Cañavate, J.P. Molecular Characterization, Gene Expression and Transcriptional Regulation of Cytosolic HSP90 Genes in the Flatfish Senegalese Sole (Solea senegalensis Kaup). Gene 2008, 416, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kroiher, M.; Walther, M.; Berking, S. Heat Shock as Inducer of Metamorphosis in Marine Invertebrates. Roux’s Arch. Dev. Biol. 1992, 201, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Degnan, S.M. Nitric Oxide Acts as a Positive Regulator to Induce Metamorphosis of the Ascidian Herdmania momus. PLoS ONE 2013, 8, e72797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiess, W.; Gallaher, B. Hormonal Control of Programmed Cell Death/Apoptosis. Eur. J. Endocrinol. 1998, 138, 482–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizuya-Oka, A.; Hasebe, T.; Shi, Y.-B. Apoptosis in Amphibian Organs during Metamorphosis. Apoptosis 2010, 15, 350–364. [Google Scholar] [CrossRef] [Green Version]
- Meier, P.; Finch, A.; Evan, G. Apoptosis in Development. Nature 2000, 407, 796–801. [Google Scholar] [CrossRef]
- Fuchs, Y.; Steller, H. Programmed Cell Death in Animal Development and Disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [Green Version]
- Takle, H.; Andersen, Ø. Caspases and Apoptosis in Fish. J. Fish Biol. 2007, 71, 326–349. [Google Scholar] [CrossRef]
- Lee, E.; Koo, Y.; Ng, A.; Wei, Y.; Luby-Phelps, K.; Juraszek, A.; Xavier, R.J.; Cleaver, O.; Levine, B.; Amatruda, J.F. Autophagy Is Essential for Cardiac Morphogenesis during Vertebrate Development. Autophagy 2014, 10, 572–587. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Levine, B. Autophagy in Mammalian Development and Differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef]
- Fernández-Díaz, C.; Yýfera, M.; Cañavate, J.P.; Moyano, F.J.; Alarcón, F.J.; Díaz, M. Growth and Physiological Changes during Metamorphosis of Senegal Sole Reared in the Laboratory. J. Fish Biol. 2001, 58, 1086–1097. [Google Scholar] [CrossRef]
- Keefe, M.; Able, K.W. Patterns of Metamorphosis in Summer Flounder, Paralichthys dentatus. J. Fish Biol. 1993, 42, 713–728. [Google Scholar] [CrossRef]
- Hamre, K.; Moren, M.; Solbakken, J.; Opstad, I.; Pittman, K. The Impact of Nutrition on Metamorphosis in Atlantic Halibut (Hippoglossus hippoglossus L.). Aquaculture 2005, 250, 555–565. [Google Scholar] [CrossRef]
- Pinto, W.; Figueira, L.; Dinis, M.T.; Aragão, C. How Does Fish Metamorphosis Affect Aromatic Amino Acid Metabolism? Amino Acids 2009, 36, 177–183. [Google Scholar] [CrossRef]
- Gram, L.; Ringø, E. Chapter 17 Prospects of Fish Probiotics. In Biology of Growing Animals; Holzapfel, W.H., Naughton, P.J., Pierzynowski, S.G., Zabielski, R., Salek, E., Eds.; Microbial Ecology in Growing Animals; Elsevier: Amsterdam, The Netherlands, 2005; Volume 2, pp. 379–417. [Google Scholar]
- Merrifield, D.L.; Dimitroglou, A.; Foey, A.; Davies, S.J.; Baker, R.T.M.; Bøgwald, J.; Castex, M.; Ringø, E. The Current Status and Future Focus of Probiotic and Prebiotic Applications for Salmonids. Aquaculture 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Avella, M.A.; Olivotto, I.; Silvi, S.; Place, A.R.; Carnevali, O. Effect of Dietary Probiotics on Clownfish: A Molecular Approach to Define How Lactic Acid Bacteria Modulate Development in a Marine Fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R359–R371. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Dossou, S.; Moss, A.S. Effects of Dietary Supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the Growth, Gut Microbiota and Immune Responses of Red Sea Bream, Pagrus major. Fish Shellfish Immunol. 2016, 49, 275–285. [Google Scholar] [CrossRef]
- Gatesoupe, F.-J. Lactic Acid Bacteria Increase the Resistance of Turbot Larvae, Scophthalmus maximus, against Pathogenic Vibrio. Aquat. Living Resour. 1994, 7, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.X.; Feng, X.; Xie, L.L.; Peng, X.Y.; Yuan, J.; Chen, X.X. Effect of Probiotic Bacillus subtilis Ch9 for Grass Carp, Ctenopharyngodon idella (Valenciennes, 1844), on Growth Performance, Digestive Enzyme Activities and Intestinal Microflora. J. Appl. Ichthyol. 2012, 28, 721–727. [Google Scholar] [CrossRef]
- Makridis, P.; Kokou, F.; Bournakas, C.; Papandroulakis, N.; Sarropoulou, E. Isolation of Phaeobacter sp. from Larvae of Atlantic Bonito (Sarda sarda) in a Mesocosmos Unit, and Its Use for the Rearing of European Seabass Larvae (Dicentrarchus labrax L.). Microorganisms 2021, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, E.C.; Jimenez, G.; Castex, M.; Gram, L. The Roseobacter-group Bacterium Phaeobacter as a Safe Probiotic Solution for Aquaculture. Appl. Environ. Microbiol. 2021, 87, e02581-20. [Google Scholar] [CrossRef] [PubMed]
- Hjelm, M.; Riaza, A.; Formoso, F.; Melchiorsen, J.; Gram, L. Seasonal Incidence of Autochthonous Antagonistic Roseobacter spp. and Vibrionaceae Strains in a Turbot Larva (Scophthalmus maximus) Rearing System. Appl. Environ. Microbiol. 2004, 70, 7288–7294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, S.; Montes, J.; Romalde, J.L.; Barja, J.L. Inhibitory Activity of Phaeobacter Strains against Aquaculture Pathogenic Bacteria. Int. Microbiol. 2009, 12, 107–114. [Google Scholar] [CrossRef]
- Slightom, R.N.; Buchan, A. Surface Colonization by Marine Roseobacters: Integrating Genotype and Phenotype. Appl. Environ. Microbiol. 2009, 75, 6027–6037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, N.L.; Nikolay, A.; Dickschat, J.S. Biosynthesis of the Antibiotic Tropodithietic Acid by the Marine Bacterium Phaeobacter inhibens. Chem. Commun. 2014, 50, 5487–5489. [Google Scholar] [CrossRef] [PubMed]
- Porsby, C.H.; Nielsen, K.F.; Gram, L. Phaeobacter and Ruegeria Species of the Roseobacter Clade Colonize Separate Niches in a Danish Turbot (Scophthalmus maximus)-Rearing Farm and Antagonize Vibrio anguillarum under Different Growth Conditions. Appl. Environ. Microbiol. 2008, 74, 7356–7364. [Google Scholar] [CrossRef] [Green Version]
- Porsby, C.H.; Gram, L. Phaeobacter inhibens as Biocontrol Agent against Vibrio vulnificus in Oyster Models. Food Microbiol. 2016, 57, 63–70. [Google Scholar] [CrossRef]
- Papandroulakis, N.; Mylonas, C.C.; Maingot, E.; Divanach, P. First Results of Greater Amberjack (Seriola dumerili) Larval Rearing in Mesocosm. Aquaculture 2005, 250, 155–161. [Google Scholar] [CrossRef]
- Zupa, R.; Fauvel, C.; Mylonas, C.C.; Pousis, C.; Santamaria, N.; Papadaki, Μ.; Fakriadis, I.; Cicirelli, V.; Mangano, S.; Passantino, L.; et al. Rearing in Captivity Affects Spermatogenesis and Sperm Quality in Greater Amberjack, Seriola dumerili (Risso, 1810). J. Anim. Sci. 2017, 95, 4085–4100. [Google Scholar] [CrossRef] [Green Version]
- Martens, T.; Heidorn, T.; Pukall, R.; Simon, M.; Tindall, B.J.; Brinkhoff, T. Reclassification of Roseobacter Gallaeciensis Ruiz-Ponte et al. 1998 as Phaeobacter gallaeciensis gen. nov., comb. nov., Description of Phaeobacter inhibens sp. nov., Reclassification of Ruegeria algicola (Lafay et al. 1995) Uchino et al. 1999 as Marinovum algicola gen. nov., comb. nov., and Emended Descriptions of the Genera Roseobacter, Ruegeria and Leisingera. Int. J. Syst. Evol. Microbiol. 2006, 56, 1293–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prol-García, M.J.; Gómez, M.; Sánchez, L.; Pintado, J. Phaeobacter Grown in Biofilters: A New Strategy for the Control of Vibrionaceae in Aquaculture. Aquac. Res. 2014, 45, 1012–1025. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Divanach, P.; Anezaki, L.; Kentouri, M. Temperature-Induced Ontogenetic Plasticity in Sea Bass (Dicentrarchus labrax). Mar. Biol. 2001, 139, 817–830. [Google Scholar] [CrossRef]
- Laggis, A.; Sfakianakis, D.G.; Divanach, P.; Kentouri, M. Ontogeny of the Body Skeleton in Seriola dumerili (Risso, 1810). Ital. J. Zool. 2010, 77, 303–315. [Google Scholar] [CrossRef]
- Feidantsis, K.; Georgoulis, I.; Giantsis, I.A.; Michaelidis, B. Treatment with ascorbic acid normalizes the aerobic capacity, antioxidant defence, and cell death pathways in thermally stressed Mytilus galloprovincialis. Comp. Biochem. Physiol. B 2021, 255, 110611. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.E.; Somero, G.N. Interspecific Variation in Thermal Denaturation of Proteins in the Congeneric Mussels Mytilus trossulus and M. galloprovincialis: Evidence from the Heat-Shock Response and Protein Ubiquitination. Mar. Biol. 1996, 126, 65–75. [Google Scholar] [CrossRef]
- Driedzic, W.R.; Fonseca de Almeida-Val, V.M. Enzymes of Cardiac Energy Metabolism in Amazonian Teleosts and the Fresh-Water Stingray (Potamotrygon hystrix). J. Exp. Zool. 1996, 274, 327–333. [Google Scholar] [CrossRef]
- Moon, T.W.; Mommsen, T.P. Enzymes of Intermediary Metabolism in Tissue of the Little Skate, Raja erinacea. J. Exp. Zool. 1987, 244, 9–15. [Google Scholar] [CrossRef]
- Sidell, B.D.; Driedzic, W.R.; Stowe, D.B.; Johnston, I.A. Biochemical Correlations of Power Development and Metabolic Fuel Preferenda in Fish Hearts. Physiol. Zool. 1987, 60, 221–232. [Google Scholar] [CrossRef]
- Singer, T.D.; Ballantyne, J.S. Absence of Extrahepatic Lipid Oxidation in a Freshwater Elasmobranch, the Dwarf Stingray Potamotrygon magdalenae: Evidence from Enzyme Activities. J. Exp. Zool. 1989, 251, 355–360. [Google Scholar] [CrossRef]
- Zimmerman, A.M.; Lowery, M.S. Hyperplastic Development and Hypertrophic Growth of Muscle Fibers in the White Seabass (Atractoscion nobilis). J. Exp. Zool. 1999, 284, 299–308. [Google Scholar] [CrossRef]
- Higgins, P.J.; Thorpe, J.E. Hyperplasia and Hypertrophy in the Growth of Skeletal Muscle in Juvenile Atlantic Salmon, Salmo salar L. J. Fish Biol. 1990, 37, 505–519. [Google Scholar] [CrossRef]
- Rowlerson, A.; Mascarello, F.; Radaelli, G.; Veggetti, A. Differentiation and Growth of Muscle in the Fish Sparus aurata (L): II. Hyperplastic and Hypertrophic Growth of Lateral Muscle from Hatching to Adult. J. Muscle Res. Cell Motil. 1995, 16, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.N.; Björnsson, B.T.; Valdés, J.A.; Einarsdottir, I.E.; Lorca, B.; Alvarez, M.; Molina, A. IGF-I/PI3K/Akt and IGF-I/MAPK/ERK Pathways in Vivo in Skeletal Muscle Are Regulated by Nutrition and Contribute to Somatic Growth in the fine flounder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1532–R1542. [Google Scholar] [CrossRef]
- Seiliez, I.; Panserat, S.; Skiba-Cassy, S.; Fricot, A.; Vachot, C.; Kaushik, S.; Tesseraud, S. Feeding Status Regulates the Polyubiquitination Step of the Ubiquitin-Proteasome-Dependent Proteolysis in Rainbow Trout (Oncorhynchus mykiss) Muscle. J. Nutr. 2008, 138, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Seiliez, I.; Gabillard, J.-C.; Skiba-Cassy, S.; Garcia-Serrana, D.; Gutiérrez, J.; Kaushik, S.; Panserat, S.; Tesseraud, S. An in Vivo and in Vitro Assessment of TOR Signaling Cascade in Rainbow Trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R329–R335. [Google Scholar] [CrossRef] [Green Version]
- Mirghaed, A.T.; Yarahmadi, P.; Hosseinifar, S.H.; Tahmasebi, D.; Gheisvandi, N.; Ghaedi, A. The Effects Singular or Combined Administration of Fermentable Fiber and Probiotic on Mucosal Immune Parameters, Digestive Enzyme Activity, Gut Microbiota and Growth Performance of Caspian White Fish (Rutilus frisii kutum) Fingerlings. Fish Shellfish Immunol. 2018, 77, 194–199. [Google Scholar] [CrossRef]
- Zacarias-Soto, M.; Lazo, J.P.; Viana, M.T. Effect of Three Probiotics Administered through Live Feed on Digestive Enzyme Activity in California Halibut, Paralichthys californicus, Larvae. J. World Aquac. Soc. 2011, 42, 321–331. [Google Scholar] [CrossRef]
- Mohammadi Arani, M.; Salati, A.P.; Safari, O.; Keyvanshokooh, S. Dietary Supplementation Effects of Pediococcus acidilactici as Probiotic on Growth Performance, Digestive Enzyme Activities and Immunity Response in Zebrafish (Danio rerio). Aquac. Nutr. 2019, 25, 854–861. [Google Scholar] [CrossRef]
- Hamza, A.; Fdhila, K.; Zouiten, D.; Masmoudi, A.S. Virgibacillus proomii and Bacillus mojavensis as Probiotics in Sea Bass (Dicentrarchus labrax) Larvae: Effects on Growth Performance and Digestive Enzyme Activities. Fish Physiol. Biochem. 2016, 42, 495–507. [Google Scholar] [CrossRef]
- Suzer, C.; Çoban, D.; Kamaci, H.O.; Saka, Ş.; Firat, K.; Otgucuoğlu, Ö.; Küçüksari, H. Lactobacillus Spp. Bacteria as Probiotics in Gilthead Sea Bream (Sparus aurata, L.) Larvae: Effects on Growth Performance and Digestive Enzyme Activities. Aquaculture 2008, 280, 140–145. [Google Scholar] [CrossRef]
- Assan, D.; Kuebutornye, F.K.A.; Hlordzi, V.; Chen, H.; Mraz, J.; Mustapha, U.F.; Abarike, E.D. Effects of Probiotics on Digestive Enzymes of Fish (Finfish and Shellfish); Status and Prospects: A Mini Review. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110653. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmed, I.; Fatma, S.; Peres, H. Role of Branched-Chain Amino Acids on Growth, Physiology and Metabolism of Different Fish Species: A Review. Aquac. Nutr. 2021, 27, 1270–1289. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Wang, C.; Liu, W.; Jiang, G.; Dai, Y. Evaluation of Yeast Hydrolysate as a Substitute to Dietary Fish Meal Of| Juvenile Jian Carp (Cyprinus carpio var. Jian): Protein Synthesis via TOR Pathway. Aquac. Nutr. 2021, 27, 1853–1860. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Liu, M.-Y.; Cheng, H.-H.; Huang, Y.-Y.; Dai, Y.-J.; Liu, W.-B.; Jiang, G.-Z. Replacing Fish Meal with Cottonseed Meal Protein Hydrolysate Affects Amino Acid Metabolism via AMPK/SIRT1 and TOR Signaling Pathway of Megalobrama amblycephala. Aquaculture 2019, 510, 225–233. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Vraskou, Y.; Palstra, A.P.; Planas, J.V. AMP-Activated Protein Kinase Plays an Important Evolutionary Conserved Role in the Regulation of Glucose Metabolism in Fish Skeletal Muscle Cells. PLoS ONE 2012, 7, e31219. [Google Scholar] [CrossRef] [Green Version]
- Gwak, W.-S.; Tanaka, M. Changes in RNA, DNA and Protein Contents of Laboratory-Reared Japanese Flounder Paralichthys olivaceus during Metamorphosis and Settlement. Fish Sci. 2002, 68, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Veggetti, A.; Mascarello, F.; Scapolo, P.A.; Rowlerson, A.; Carnevali, M.D.C. Muscle Growth and Myosin Isoform Transitions during Development of a Small Teleost Fish, Poecilia reticulata (Peters) (Atheriniformes, Poeciliidae): A Histochemical, Immunohistochemical, Ultrastructural and Morphometric Study. Anat. Embryol. 1993, 187, 353–361. [Google Scholar] [CrossRef]
- Pedersen, T.; Falk-Petersen, I.B. Morphological Changes during Metamorphosis in Cod (Gadus morhua L.), with Particular Reference to the Development of the Stomach and Pyloric Caeca. J. Fish Biol. 1992, 41, 449–461. [Google Scholar] [CrossRef]
- Sánchez-Amaya, M.I.; Ortiz-Delgado, J.B.; García-López, Á.; Cárdenas, S.; Sarasquete, C. Larval Ontogeny of Redbanded Seabream Pagrus auriga valenciennes, 1843 with Special Reference to the Digestive System. A Histological and Histochemical Approach. Aquaculture 2007, 263, 259–279. [Google Scholar] [CrossRef]
- Yúfera, M.; Ortiz-Delgado, J.B.; Hoffman, T.; Siguero, I.; Urup, B.; Sarasquete, C. Organogenesis of Digestive System, Visual System and Other Structures in Atlantic Bluefin Tuna (Thunnus thynnus) Larvae Reared with Copepods in Mesocosm System. Aquaculture 2014, 426–427, 126–137. [Google Scholar] [CrossRef]
- Panteli, N.; Demertzioglou, M.; Feidantsis, K.; Karapanagiotis, S.; Tsele, N.; Tsakoniti, K.; Gkagkavouzis, K.; Mylonas, C.C.; Kormas, K.A.; Mente, E.; et al. Advances in Understanding the Mitogenic, Metabolic, and Cell Death Signaling in Teleost Development: The Case of Greater Amberjack (Seriola dumerili, Risso 1810). Fish Physiol. Biochem. 2022, 48, 1665–1684. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Sofia, E.; Shakil, A.; Haque, N.F.; Khan, M.N.A.; Ikeda, D.; Kinoshita, S.; Abol-Munafi, A.B. Host Gut-Derived Probiotic Bacteria Promote Hypertrophic Muscle Progression and Upregulate Growth-Related Gene Expression of Slow-Growing Malaysian Mahseer Tor tambroides. Aquac. Rep. 2018, 9, 37–45. [Google Scholar] [CrossRef]
- Takii, K.; Seoka, M.; Takaoka, O.; Furuta, S.; Nakamura, M.; Kumai, H. Chemical Composition, RNA and DNA Contents, and Alkaline Phosphatase Activity with Growth of Striped Jack Larvae through Juveniles. Fish Sci. 1994, 60, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Weston, A.D.; Sampaio, A.V.; Ridgeway, A.G.; Underhill, T.M. Inhibition of P38 MAPK Signaling Promotes Late Stages of Myogenesis. J. Cell Sci. 2003, 116, 2885–2893. [Google Scholar] [CrossRef] [Green Version]
- Rollo, A.; Sulpizio, R.; Nardi, M.; Silvi, S.; Orpianesi, C.; Caggiano, M.; Cresci, A.; Carnevali, O. Live Microbial Feed Supplement in Aquaculture for Improvement of Stress Tolerance. Fish Physiol. Biochem. 2006, 32, 167–177. [Google Scholar] [CrossRef]
- Makled, S.O.; Hamdan, A.M.; El-Sayed, A.-F.M.; Hafez, E.E. Evaluation of Marine Psychrophile, Psychrobacter namhaensis SO89, as a Probiotic in Nile Tilapia (Oreochromis niloticus) Diets. Fish Shellfish Immunol. 2017, 61, 194–200. [Google Scholar] [CrossRef]
- Mohapatra, S.; Chakraborty, T.; Prusty, A.K.; PaniPrasad, K.; Mohanta, K.N. Beneficial Effects of Dietary Probiotics Mixture on Hemato-Immunology and Cell Apoptosis of Labeo rohita Fingerlings Reared at Higher Water Temperatures. PLoS ONE 2014, 9, e100929. [Google Scholar] [CrossRef]
- Sanders, B.M. Stress Proteins in Aquatic Organisms: An Environmental Perspective. Crit. Rev. Toxicol. 1993, 23, 49–75. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y.S. Ontogeny of Thyroid Hormones, Cortisol, Hsp70 and Hsp90 during Silver Sea Bream Larval Development. Life Sci. 2003, 72, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Chung-Davidson, Y.-W.; Yeh, C.-Y.; Bussy, U.; Li, K.; Davidson, P.J.; Nanlohy, K.G.; Brown, C.T.; Whyard, S.; Li, W. Hsp90 and Hepatobiliary Transformation during Sea Lamprey Metamorphosis. BMC Dev. Biol. 2015, 15, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baolong, B.; Guimei, Y.; Daming, R. Apoptosis in the metamorphosis of Japanese flounder Paralichthys olivaceus. Acta Zool. Sin. 2006, 52, 355–361. [Google Scholar]
- Sun, M.; Wei, F.; Li, H.; Xu, J.; Chen, X.; Gong, X.; Tian, Y.; Chen, S.; Bao, B. Distortion of Frontal Bones Results from Cell Apoptosis by the Mechanical Force from the Up-Migrating Eye during Metamorphosis in Paralichthys olivaceus. Mech. Dev. 2015, 136, 87–98. [Google Scholar] [CrossRef]
- Gao, L.; Huang, Y.; Sun, M.; Bao, B. The Role of Autophagy on Eye Migration during the Metamorphosis of Paralichthys olivaceus. Cells Dev. 2022, 169, 203751. [Google Scholar] [CrossRef]
- Salinas, I.; Meseguer, J.; Esteban, M.Á. Antiproliferative Effects and Apoptosis Induction by Probiotic Cytoplasmic Extracts in Fish Cell Lines. Vet. Microbiol. 2008, 126, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Gioacchini, G.; Valle, L.D.; Benato, F.; Fimia, G.M.; Nardacci, R.; Ciccosanti, F.; Piacentini, M.; Borini, A.; Carnevali, O.; Gioacchini, G.; et al. Interplay between Autophagy and Apoptosis in the Development of Danio rerio Follicles and the Effects of a Probiotic. Reprod. Fertil. Dev. 2013, 25, 1115–1125. [Google Scholar] [CrossRef]
- Geffen, A.J.; van der Veer, H.W.; Nash, R.D.M. The Cost of Metamorphosis in Flatfishes. J. Sea Res. 2007, 58, 35–45. [Google Scholar] [CrossRef]
- Yi, C.-C.; Liu, C.-H.; Chuang, K.-P.; Chang, Y.-T.; Hu, S.-Y. A Potential Probiotic Chromobacterium aquaticum with Bacteriocin-like Activity Enhances the Expression of Indicator Genes Associated with Nutrient Metabolism, Growth Performance and Innate Immunity against Pathogen Infections in Zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 93, 124–134. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [Green Version]
- Johnston, I.A.; Cole, N.J.; Abercromby, M.; Vieira, V.L.A. Embryonic Temperature Modulates Muscle Growth Characteristics in Larval and Juvenile Herring. J. Exp. Biol. 1998, 201, 623–646. [Google Scholar] [CrossRef] [PubMed]
- López-Albors, O.; Abdel, I.; Periago, M.J.; Ayala, M.D.; Alcázar, A.G.; Graciá, C.M.; Nathanailides, C.; Vázquez, J.M. Temperature Influence on the White Muscle Growth Dynamics of the Sea Bass Dicentrarchus labrax, L. Flesh Quality Implications at Commercial Size. Aquaculture 2008, 277, 39–51. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panteli, N.; Feidantsis, K.; Demertzioglou, M.; Paralika, V.; Karapanagiotis, S.; Mylonas, C.C.; Kormas, K.A.; Mente, E.; Makridis, P.; Antonopoulou, E. The Probiotic Phaeobacter inhibens Provokes Hypertrophic Growth via Activation of the IGF-1/Akt Pathway during the Process of Metamorphosis of Greater Amberjack (Seriola dumerili, Risso 1810). Animals 2023, 13, 2154. https://doi.org/10.3390/ani13132154
Panteli N, Feidantsis K, Demertzioglou M, Paralika V, Karapanagiotis S, Mylonas CC, Kormas KA, Mente E, Makridis P, Antonopoulou E. The Probiotic Phaeobacter inhibens Provokes Hypertrophic Growth via Activation of the IGF-1/Akt Pathway during the Process of Metamorphosis of Greater Amberjack (Seriola dumerili, Risso 1810). Animals. 2023; 13(13):2154. https://doi.org/10.3390/ani13132154
Chicago/Turabian StylePanteli, Nikolas, Konstantinos Feidantsis, Maria Demertzioglou, Vasiliki Paralika, Stelios Karapanagiotis, Constantinos C. Mylonas, Konstantinos Ar. Kormas, Eleni Mente, Pavlos Makridis, and Efthimia Antonopoulou. 2023. "The Probiotic Phaeobacter inhibens Provokes Hypertrophic Growth via Activation of the IGF-1/Akt Pathway during the Process of Metamorphosis of Greater Amberjack (Seriola dumerili, Risso 1810)" Animals 13, no. 13: 2154. https://doi.org/10.3390/ani13132154
APA StylePanteli, N., Feidantsis, K., Demertzioglou, M., Paralika, V., Karapanagiotis, S., Mylonas, C. C., Kormas, K. A., Mente, E., Makridis, P., & Antonopoulou, E. (2023). The Probiotic Phaeobacter inhibens Provokes Hypertrophic Growth via Activation of the IGF-1/Akt Pathway during the Process of Metamorphosis of Greater Amberjack (Seriola dumerili, Risso 1810). Animals, 13(13), 2154. https://doi.org/10.3390/ani13132154












