An Ultrasound-Guided Latero-Ventral Approach to Perform the Quadratus Lumborum Block in Dog Cadavers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
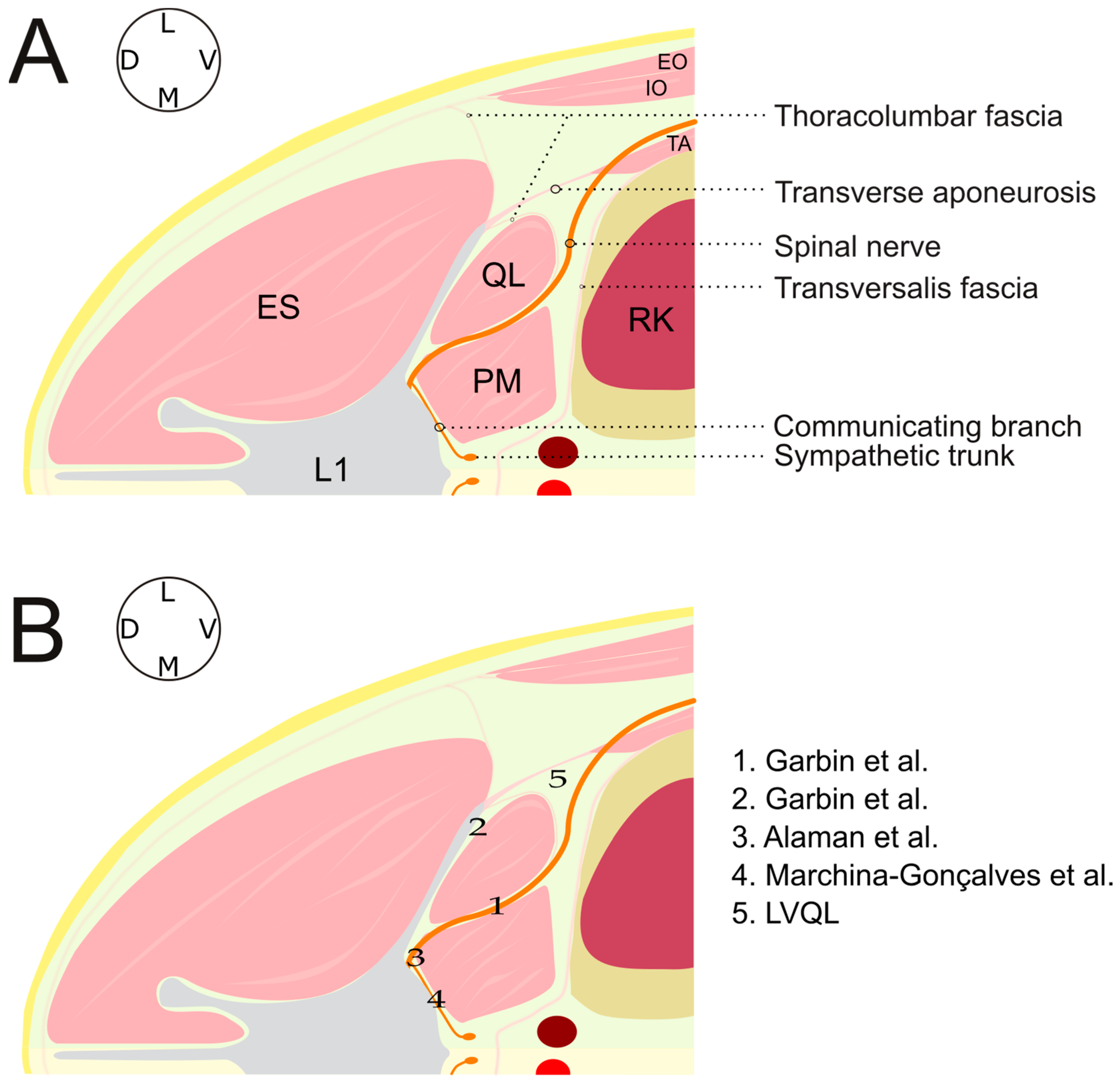
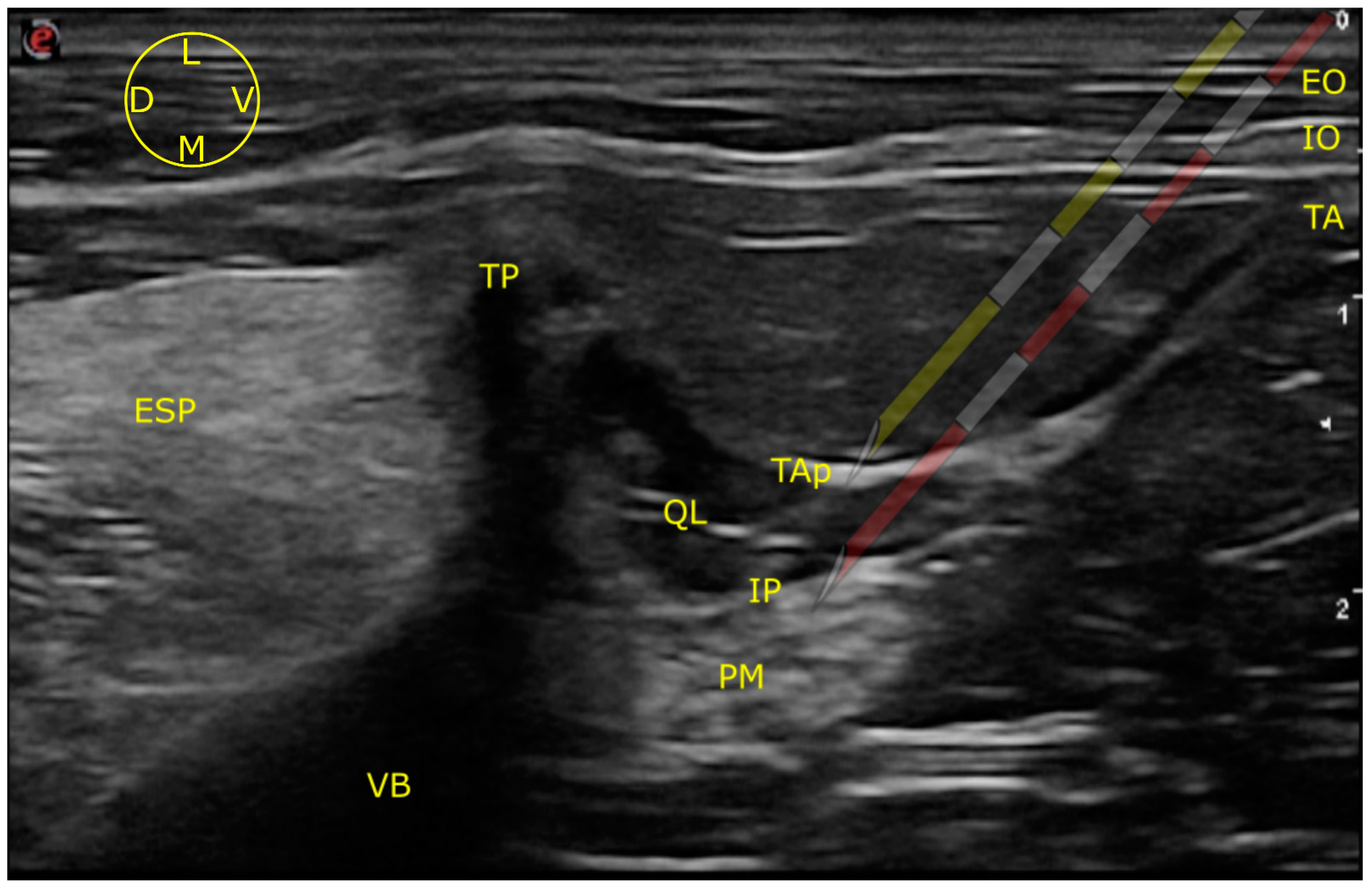
2.1. Ultrasound-Guided Technique
2.1.1. LVQL
2.1.2. IQL
2.2. Tomographic Study
2.3. Dissection Study
2.4. Statistical Analysis
3. Results
3.1. Ultrasound-Guided Injection
3.1.1. Images Quality
3.1.2. Needle Visualization
3.2. Tomographic Study
3.3. Dissection Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Elsharkawy, H.; El-Boghdadly, K.; Barrington, M. Quadratus Lumborum Block. Anesthesiology 2019, 130, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Garbin, M.; Portela, D.A.; Bertolizio, G.; Garcia-Pereira, F.; Gallastegui, A.; Otero, P.E. Description of Ultrasound-Guided Quadratus Lumborum Block Technique and Evaluation of Injectate Spread in Canine Cadavers. Vet. Anaesth. Analg. 2020, 47, 249–258. [Google Scholar] [CrossRef]
- Garbin, M.; Portela, D.A.; Bertolizio, G.; Gallastegui, A.; Otero, P.E. A Novel Ultrasound-Guided Lateral Quadratus Lumborum Block in Dogs: A Comparative Cadaveric Study of Two Approaches. Vet. Anaesth. Analg. 2020, 47, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Alaman, M.; Bonastre, C.; de Blas, I.; Gomez-Alvarez, C.M.; Laborda, A. Description of a Novel Ultrasound-Guided Approach for a Dorsal Quadratus Lumborum Block: A Canine Cadaver Study. Vet. Anaesth. Analg. 2021, 49, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Viscasillas, J.; Terrado, J.; Marti-Scharfhausen, R.; Castiñeiras, D.; Esteve, V.; Clancy, N.; Redondo, J.I. A Modified Approach for the Ultrasound-Guided Quadratus Lumborum Block in Dogs: A Cadaveric Study. Animals 2021, 11, 2945. [Google Scholar] [CrossRef]
- Marchina-Gonçalves, A.; Gil, F.; Laredo, F.G.; Soler, M.; Agut, A.; Belda, E. Evaluation of High-Volume Injections Using a Modified Dorsal Quadratus Lumborum Block Approach in Canine Cadavers. Animals 2022, 12, 18. [Google Scholar] [CrossRef]
- Viscasillas, J.; Sanchis-Mora, S.; Burillo, P.; Esteve, V.; Del Romero, A.; Lafuente, P.; Redondo, J.I. Evaluation of Quadratus Lumborum Block as Part of an Opioid-Free Anaesthesia for Canine Ovariohysterectomy. Animals 2021, 11, 3424. [Google Scholar] [CrossRef]
- Blanco, R. Tap Block under Ultrasound Guidance: The Description of a “No Pops” Technique. Reg. Anesth. Pain Med. 2007, 32 (Suppl. S1), 130. [Google Scholar] [CrossRef]
- Schroeder, C.A.; Snyder, L.B.C.; Tearney, C.C.; Baker-Herman, T.L.; Schroeder, K.M. Ultrasound-Guided Transversus Abdominis Plane Block in the Dog: An Anatomical Evaluation. Vet. Anaesth. Analg. 2011, 38, 267–271. [Google Scholar] [CrossRef]
- Bruggink, S.M.; Schroeder, K.M.; Baker-Herman, T.L.; Schroeder, C.A. Weight-Based Volume of Injection Influences Cranial to Caudal Spread of Local Anesthetic Solution in Ultrasound-Guided Transversus Abdominis Plane Blocks in Canine Cadavers. Vet. Surg. 2012, 41, 455–457. [Google Scholar] [CrossRef]
- Drozdzynska, M.; Monticelli, P.; Neilson, D.; Viscasillas, J. Ultrasound-Guided Subcostal Oblique Transversus Abdominis Plane Block in Canine Cadavers. Vet. Anaesth. Analg. 2017, 44, 183–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoff, A.; Laborda-Vidal, P.; Mortier, J.; Amengual, M.; Rioja, E. Comparison of the Spread of Two Different Volumes of Contrast Medium When Performing Ultrasound-Guided Transversus Abdominis Plane Injection in Dog Cadavers. J. Small Anim. Pract. 2017, 58, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.K.; Bauquier, S.H.; Carter, J.E.; Whittem, T.; Beths, T. Two-Point Ultrasound-Guided Transversus Abdominis Plane Injection in Canine Cadavers—A Pilot Study. Vet. Anaesth. Analg. 2018, 45, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Portela, D.A.; Thomson, A.; Otero, P.E. Comparison between Two Approaches for the Transversus Abdominis Plane Block in Canine Cadavers. Vet. Anaesth. Analg. 2021, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.E.; Romano, M.; Zaccagnini, A.S.; Fuensalida, S.E.; Verdier, N.; Sanchez, F.; Portela, D.A. Transversus Abdominis Plane Block in Cat Cadavers: Anatomical Description and Comparison of Injectate Spread Using Two- and Three-Point Approaches. Vet. Anaesth. Analg. 2021, 48, 432–441. [Google Scholar] [CrossRef]
- Portela, D.A.; Fuensalida, S.E.; Otero, P.E. Bloqueo Del Cuadrado Lumbar. In Anestesia Regional en Animales de Compañía, 1st ed.; Inter-Médica: Buenos Aires, República Argentina, 2017; pp. 255–261. [Google Scholar]
- Portela, D.A.; Verdier, N.; Otero, P.E. Regional Anesthetic Techniques for the Pelvic Limb and Abdominal Wall in Small Animals: A Review of the Literature and Technique Description. Vet. J. 2018, 238, 27–40. [Google Scholar] [CrossRef]
- Gupta, A.; Sondekoppam, R.; Kalagara, H. Quadratus Lumborum Block: A Technical Review. Curr. Anesthesiol. Rep. 2019, 9, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Portela, D.A.; Fuensalida, S.E.; Otero, P.E. Bloqueo de Los Nervios Espinales de Tórax y Abdomen. In Anestesia Regional en Animales de Compañía, 1st ed.; Inter-Médica: Buenos Aires, República Argentina, 2017; p. 221. [Google Scholar]
- Belda, E.; Laredo, F.G.; Gil, F.; Soler, M.; Murciano, J.; Ayala, M.D.; Gómez, S.; Castells, M.T.; Escobar, M.; Agut, A. Ultrasound-Guided Administration of Lidocaine into the Sciatic Nerve in a Porcine Model: Correlation between the Ultrasonographic Evolution of the Lesions, Locomotor Function and Histological Findings. Vet. J. 2014, 200, 170–174. [Google Scholar] [CrossRef]
- Laredo, F.G.; Belda, E.; Soler, M.; Gil, F.; Murciano, J.; Sánchez-Campillo, J.; Agut, A. Short-Term Effects of Deliberate Subparaneural or Subepineural Injections with Saline Solution or Bupivacaine 0.75% in the Sciatic Nerve of Rabbits. Front. Vet. Sci. 2020, 7, 217. [Google Scholar] [CrossRef]
- Evans, H.E.; de Lahunta, A. Spinal Nerves. In Miller’s Anatomy of the Dog, 4th ed.; Elsevier: St. Louis, MO, USA, 2013; pp. 611–657. [Google Scholar]
- Wikner, M. Unexpected Motor Weakness Following Quadratus Lumborum Block for Gynaecological Laparoscopy. Anaesthesia 2017, 72, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.M.; Jimenez, C.P.; Monticelli, P.; Plested, M.; Viscasillas, J. Assessment of an Ultrasound-Guided Technique for Catheterization of the Caudal Thoracic Paravertebral Space in Dog Cadavers. Open Vet. J. 2019, 9, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portela, D.A.; Fuensalida, S.E.; Otero, P.E. Bloqueo de Los Nervios de La Pared Abdominal En El Plano Del Músculo Transverso Abdominal. In Anestesia Regional en Animales de Compañía; Inter-Médica: Buenos Aires, República Argentina, 2017; pp. 248–254. [Google Scholar]
- Evans, H.E.; de Lahunta, A. The Autonomic Nervous System. In Miller’s Anatomy of the Dog, 4th ed.; Elsevier: St. Louis, MO, USA, 2013; pp. 575–588. [Google Scholar]
- Portela, D.A.; Fuensalida, S.E.; Otero, P.E. Bloqueo Del Nervio Genitofemoral. In Anestesia Regional en Animales de Compañía, 1st ed.; Inter-Médica: Buenos Aires, República Argentina, 2017; pp. 216–218. [Google Scholar]
- Liu, P.L.; Feldman, H.S.; Giasi, R.; Patterson, M.K.; Covino, B.G. Comparative CNS Toxicity of Lidocaine, Etidocaine, Bupivacaine, and Tetracaine in Awake Dogs Following Rapid Intravenous Administration. Anesth. Analg. 1983, 62, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Feldman, H.S.; Covino, B.M.; Giasi, R. Acute Cardiovascular Toxicity of Intravenous Amide Local Anesthetics in Anesthetized Ventilated Dogs. Anesth. Analg. 1982, 61, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Groban, L.; Deal, D.D.; Vernon, J.C.; James, R.L.; Butterworth, J. Cardiac Resuscitation after Incremental Overdosage with Lidocaine, Bupivacaine, Levobupivacaine, and Ropivacaine in Anesthetized Dogs. Anesth. Analg. 2001, 92, 37–43. [Google Scholar] [CrossRef]
- Feldman, H.S.; Arthur, G.R.; Covino, B.G. Comparative Systemic Toxicity of Convulsant and Supraconvulsant Doses of Intravenous Ropivacaine, Bupivacaine, and Lidocaine in the Conscious Dog. Anesth. Analg. 1989, 69, 794–801. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchina-Gonçalves, A.; Laredo, F.G.; Gil, F.; Soler, M.; Agut, A.; Redondo, J.I.; Belda, E. An Ultrasound-Guided Latero-Ventral Approach to Perform the Quadratus Lumborum Block in Dog Cadavers. Animals 2023, 13, 2214. https://doi.org/10.3390/ani13132214
Marchina-Gonçalves A, Laredo FG, Gil F, Soler M, Agut A, Redondo JI, Belda E. An Ultrasound-Guided Latero-Ventral Approach to Perform the Quadratus Lumborum Block in Dog Cadavers. Animals. 2023; 13(13):2214. https://doi.org/10.3390/ani13132214
Chicago/Turabian StyleMarchina-Gonçalves, André, Francisco G. Laredo, Francisco Gil, Marta Soler, Amalia Agut, José Ignacio Redondo, and Eliseo Belda. 2023. "An Ultrasound-Guided Latero-Ventral Approach to Perform the Quadratus Lumborum Block in Dog Cadavers" Animals 13, no. 13: 2214. https://doi.org/10.3390/ani13132214






