Application of Reproductive Technologies to the Critically Endangered Baw Baw Frog, Philoria frosti
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Species
2.3. Animal Husbandry
2.4. Experiment One: Hormone-Induced Spawning
2.5. Experiment Two: Hormone-Induced Spermiation and Sperm Cryopreservation
Sperm Cryopreservation
2.6. Statistical Analyses
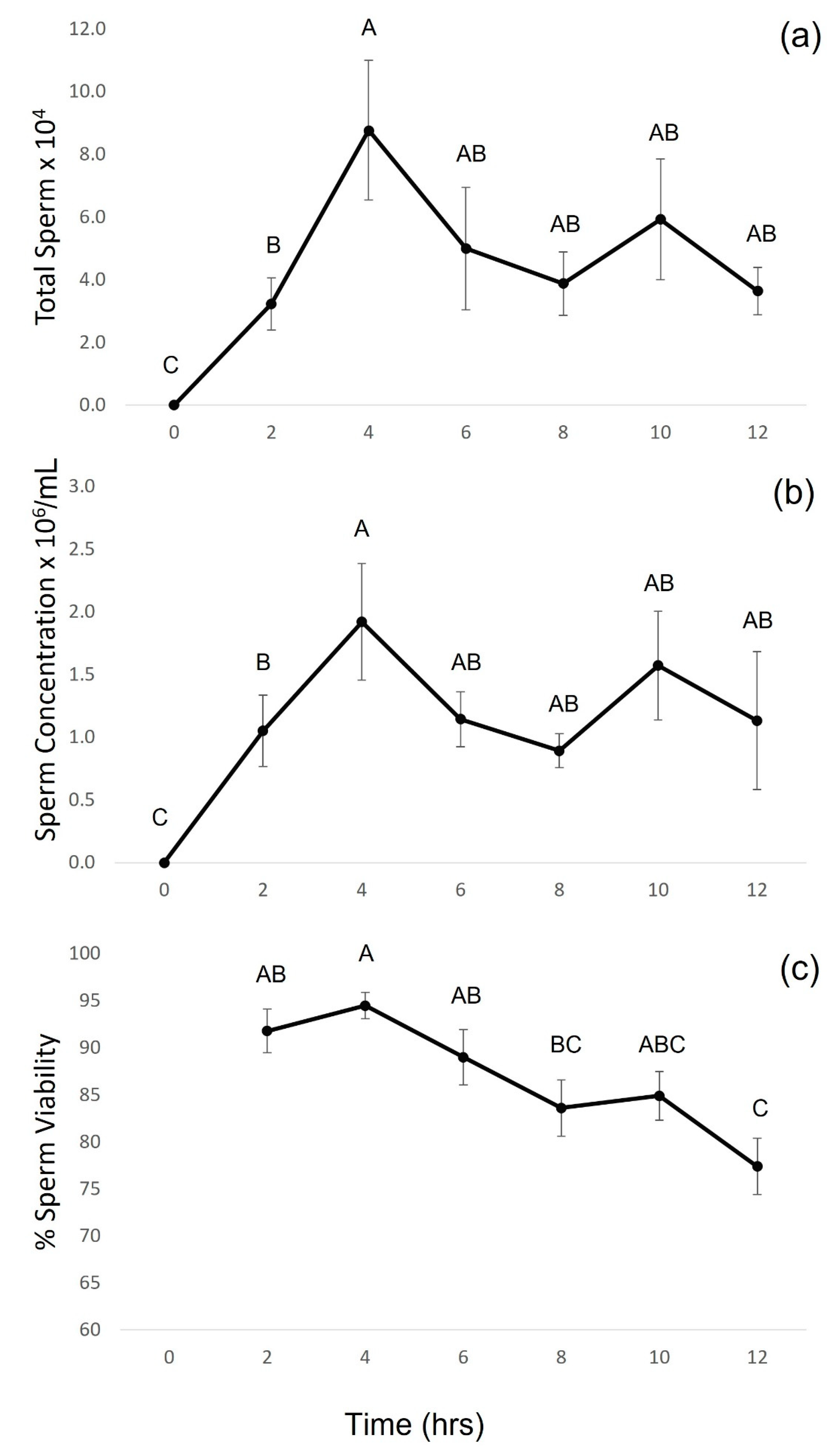
3. Results
3.1. Experiment One: Hormone-Induced Spawning
3.2. Experiment Two: Hormone-Induced Spermiation and Sperm Cryopreservation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFadden, M.S.; Gilbert, D.; Bradfield, K.; Evans, M.; Marantelli, G.; Byrne, P. The role of ex-situ amphibian conservation in Australia. In Status of Conservation and Decline of Amphibians: Australia, New Zealand, and Pacific Islands; Heatwole, H., Rowley, J., Eds.; CSIRO Publishing: Melbourne, Australia, 2018; p. 125. [Google Scholar]
- Silla, A.J.; Byrne, P.G. The role of reproductive technologies in amphibian conservation breeding programs. Annu. Rev. Anim. Biosci. 2019, 7, 499–519. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Kouba, A.J. Integrating Reproductive technologies into the conservation toolbox for the recovery of amphibian species. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A.J., Kouba, A.J., Heatwole, H., Eds.; CSIRO Publishing: Melbourne, Australia, 2022; pp. 1–9. [Google Scholar]
- Holt, W.V.; Comizzoli, P. Opportunities and limitations for reproductive science in species conservation. Annu. Rev. Anim. Biosci. 2022, 10, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Gascon, C. Amphibian Conservation Action Plan: Proceedings IUCN/SSC Amphibian Conservation Summit 2005; The World Conservation Union (IUCN): Gland, Switzerland, 2007. [Google Scholar]
- Vu, M.; Trudeau, V.L. Neuroendocrine control of spawning in amphibians and its practical applications. Gen. Comp. Endocrinol. 2016, 234, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Clulow, J.; Pomering, M.; Herbert, D.; Upton, R.; Calatayud, N.; Clulow, S.; Mahony, M.J.; Trudeau, V.L. Differential success in obtaining gametes between male and female Australian temperate frogs by hormonal induction: A review. Gen. Comp. Endocrinol. 2018, 265, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Calatayud, N.E.; Trudeau, V.L. Amphibian reproductive technologies: Approaches and welfare considerations. Conserv. Physiol. 2021, 9, coab011. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.M.; Kouba, C.K. Ultrasonography to assess female reproductive status and inform hormonally induced ovulation. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A., Kouba, A., Heatwole, H., Eds.; CSIRO Publishing: Melbourne, Australia, 2022. [Google Scholar]
- Silla, A.J.; Langhorne, C.J. Protocols for hormonally induced spermiation, and the cold storage, activation, and assessment of amphibian sperm. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A.J., Kouba, A.J., Heatwole, H., Eds.; CSIRO Publishing: Melbourne, Australia, 2022. [Google Scholar]
- Trudeau, V.L.; Raven, B.H.; Pahuja, H.K.; Narayan, E.J. Hormonal control of amphibian reproduction. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A.J., Kouba, A.J., Heatwole, H., Eds.; CSIRO Publishing: Melbourne, Australia, 2022; p. 49. [Google Scholar]
- Hollis, G.J. National Recovery Plan for the Baw Baw Frog Philora Frosti; Department of Sustainability and Environment: Melbourne, Australia, 2011. [Google Scholar]
- Hunter, D.; Clemann, N.; Coote, D.; Gillespie, G.; Hollis, G.; Scheele, B.; Philips, A.; West, M. Frog declines and associated management response in south-eastern mainland Australia and Tasmania. In Status of Conservation and Decline of Amphibians: Australia, New Zealand and Pacific Islands; Heatwole, H., Rowley, J., Eds.; CSIRO Publishing: Melbourne, Australia, 2018; pp. 38–58. [Google Scholar]
- Gilbert, D.J.; Magrath, M.J.; Byrne, P.G. Warmer temperature and provision of natural substrate enable earlier metamorphosis in the critically endangered Baw Baw frog. Conserv. Physiol. 2020, 8, coaa030. [Google Scholar] [CrossRef] [PubMed]
- Anstis, M. Tadpoles and Frogs of Australia, 2nd ed.; New Holland: London, UK, 2018. [Google Scholar]
- Trudeau, V.L.; Schueler, F.W.; Navarro-Martin, L.; Hamilton, C.K.; Bulaeva, E.; Bennett, A.; Fletcher, W.; Taylor, L. Efficient induction of spawning of Northern leopard frogs (Lithobates pipiens) during and outside the natural breeding season. Reprod. Biol. Endocrinol. 2013, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trudeau, V.L.; Somoza, G.M.; Natale, G.S.; Pauli, B.; Wignall, J.; Jackman, P.; Doe, K.; Schueler, F.W. Hormonal induction of spawning in 4 species of frogs by coinjection with a gonadotropin-releasing hormone agonist and a dopamine antagonist. Reprod. Biol. Endocrinol. 2010, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silla, A.J.; McFadden, M.; Byrne, P.G. Hormone-induced spawning of the critically endangered northern corroboree frog Pseudophryne pengilleyi. Reprod. Fertil. Dev. 2018, 30, 1352–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silla, A.J. Effects of luteinizing hormone-releasing hormone and arginine-vasotocin on the sperm-release response of Günther’s Toadlet, Pseudophryne guentheri. Reprod. Biol. Endocrinol. 2010, 8, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silla, A.J.; Roberts, J. Investigating patterns in the spermiation response of eight Australian frogs administered human chorionic gonadotropin (hCG) and luteinizing hormone-releasing hormone (LHRHa). Gen. Comp. Endocrinol. 2012, 179, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Roberts, J.D.; Byrne, P.G. The effect of injection and topical application of hCG and GnRH agonist to induce sperm-release in the roseate frog, Geocrinia rosea. Conserv. Physiol. 2020, 8, coaa104. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; McFadden, M.S.; Byrne, P.G. Hormone-induced sperm-release in the critically endangered Booroolong frog (Litoria booroolongensis): Effects of gonadotropin-releasing hormone and human chorionic gonadotropin. Conserv. Physiol. 2019, 7, coy080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhorne, C.J. Developing Assisted Reproductive Technologies for Endangered North American Amphibians. Ph.D. Thesis, Mississippi State University, Starkville, MS, USA, 2016. Available online: https://scholarsjunction.msstate.edu/td/1373 (accessed on 10 December 2019).
- Roth, T.L.; Bush, L.M.; Wildt, D.E.; Weiss, R.B. Scimitar-Horned Oryx (Oryx dammah) Spermatozoa Are Functionally Competent in a Heterologous Bovine In Vitro Fertilization System after Cryopreservation on Dry Ice, in a Dry Shipper, or over Liquid Nitrogen Vapor1. Biol. Reprod. 1999, 60, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godome, T.; Sintondji, S.W.; Azon, M.T.C.; Tossavi, C.E.; Ouattara, N.I.; Fiogbe, E.D. Artificial Reproduction and Embryogeny of the Tiger Frog Hoplobatrachus occipitalis (Günther 1858). Proc. Zool. Soc. 2021, 74, 43–51. [Google Scholar] [CrossRef]
- do Nascimento, N.F.; da Silva, R.C.; Valentin, F.N.; Paes, M.d.C.F.; De Stefani, M.V.; Nakaghi, L.S.O. Efficacy of buserelin acetate combined with a dopamine antagonist for spawning induction in the bullfrog (Lithobates catesbeianus). Aquac. Res. 2015, 46, 3093–3096. [Google Scholar] [CrossRef]
- Vu, M.; Weiler, B.; Trudeau, V.L. Time-and dose-related effects of a gonadotropin-releasing hormone agonist and dopamine antagonist on reproduction in the northern leopard frog (Lithobates pipiens). Gen. Comp. Endocrinol. 2017, 254, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bronson, E.; Guy, E.L.; Murphy, K.J.; Barrett, K.; Kouba, A.J.; Poole, V.; Kouba, C.K. Influence of oviposition-inducing hormone on spawning and mortality in the endangered Panamanian golden frog (Atelopus zeteki). BMC Zool. 2021, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Della Togna, G.; Trudeau, V.L.; Gratwicke, B.; Evans, M.; Augustine, L.; Chia, H.; Bronikowski, E.J.; Murphy, J.B.; Comizzoli, P. Effects of hormonal stimulation on the concentration and quality of excreted spermatozoa in the critically endangered Panamanian golden frog (Atelopus zeteki). Theriogenology 2017, 91, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastas, Z.M.; Byrne, P.G.; O’Brien, J.K.; Hobbs, R.J.; Upton, R.; Silla, A.J. The increasing role of short-term sperm storage and cryopreservation for conserving threatened amphibian species. Animals 2023, 13, 2094. [Google Scholar] [CrossRef]

| Response Variable | Hormone Treatment | p-Value | ||
|---|---|---|---|---|
| 0 µg/g GnRHa (Control) | 0.5 µg/g GnRHa | 0.5 µg/g GnRHa + 10 µg/g MET | ||
| Pairs ovipositing (%) | 2/6 (33%) A | 4/7 (57%) A | 5/7 (71%) A | |
| Total number of eggs | 50 ± 0 A | 55 ± 8.6 A | 46 ± 2.5 A | 0.522 |
| Percent fertilisation | 45 ± 45 A | 36 ± 13.9 A | 48 ± 13.8 A | 0.876 |
| Fresh | Post-Thaw | |
|---|---|---|
| Sample size | 22 | 4 |
| Percent viability (live/dead) | 89.8 ± 1.5 | 58.7 ± 6.6 |
| Percent total motility | 84.2 ± 1.8 | 17.4 ± 5.4 |
| Percent progressive motility | 53.5 ± 3.3 | 2.4 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silla, A.J.; Hobbs, R.J.; Gilbert, D.J.; Goodall, D.; Parrott, M.L.; Lee, A.; O’Brien, J.K.; Byrne, P.G. Application of Reproductive Technologies to the Critically Endangered Baw Baw Frog, Philoria frosti. Animals 2023, 13, 2232. https://doi.org/10.3390/ani13132232
Silla AJ, Hobbs RJ, Gilbert DJ, Goodall D, Parrott ML, Lee A, O’Brien JK, Byrne PG. Application of Reproductive Technologies to the Critically Endangered Baw Baw Frog, Philoria frosti. Animals. 2023; 13(13):2232. https://doi.org/10.3390/ani13132232
Chicago/Turabian StyleSilla, Aimee J., Rebecca J. Hobbs, Deon J. Gilbert, Damian Goodall, Marissa L. Parrott, Adam Lee, Justine K. O’Brien, and Phillip G. Byrne. 2023. "Application of Reproductive Technologies to the Critically Endangered Baw Baw Frog, Philoria frosti" Animals 13, no. 13: 2232. https://doi.org/10.3390/ani13132232






