In Silico Analysis of Honey Bee Peptides as Potential Inhibitors of Capripoxvirus DNA-Directed RNA Polymerase
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection and Retrieval of Ligand and Receptor Proteins
2.2. Phylogenetic Analysis
2.3. Homology Modeling, Refinement, and Validation
2.4. Protein–Peptide Docking
2.5. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Phylogeny of CaPVs
3.2. Homology Modeling
3.3. Protein–Peptide Docking
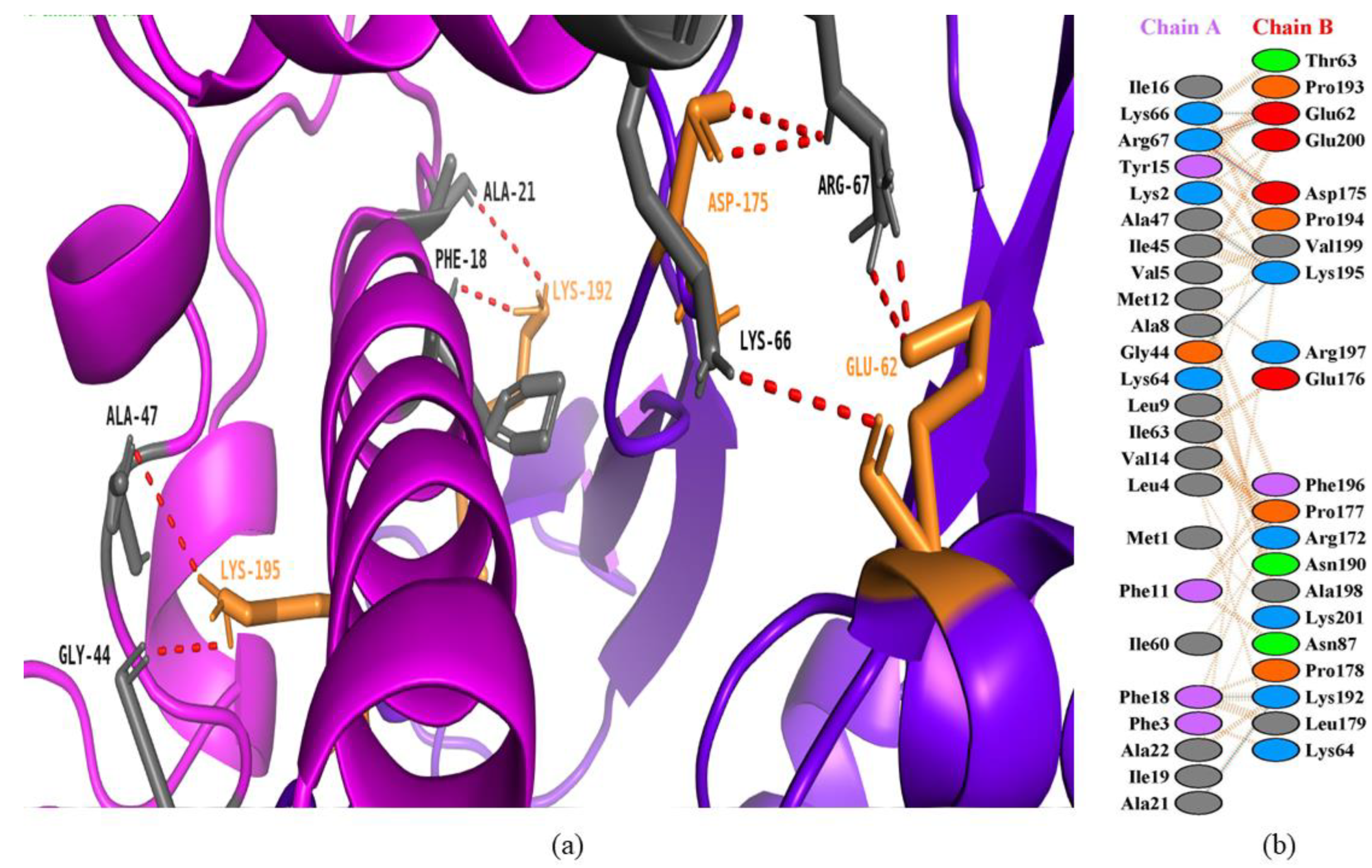
3.4. Interactions between Bee Peptides and Capripoxvirus DNA-Dependent RNA Polymerase
3.5. Molecular Dynamics (MD) Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pashupathi, M.; Anbazhagan, S.; Barkathullah, N.; Priyadarsini, S.; Panda, S.; Kumar, A. Designing and Modelling of Trivalent Chimeric Vaccine for Capripoxvirus: Lumpy Skin Disease, Sheep Pox and Goat Pox by Immunoinformatics Approach. 2022. Available online: https://www.researchsquare.com/article/rs-1437459/v1 (accessed on 30 June 2022).
- Tuppurainen, E.; Venter, E.H.; Shisler, J.; Gari, G.; Mekonnen, G.; Juleff, N.; Lyons, N.; De Clercq, K.; Upton, C.; Bowden, T. Capripoxvirus diseases: Current status and opportunities for control. Transbound. Emerg. Dis. 2017, 64, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.J.; Möller, J.; Meseko, C.A.; Adole, J.A.; Tekki, I.S.; Shamaki, D.; Hoffmann, B. Molecular characterization of Capripox viruses obtained from field outbreaks in Nigeria between 2000 and 2016. Transbound. Emerg. Dis. 2019, 66, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Teffera, M.; Babiuk, S. Potential of using capripoxvirus vectored vaccines against arboviruses in sheep, goats, and cattle. Front. Vet. Sci. 2019, 6, 450. [Google Scholar] [CrossRef] [PubMed]
- Tulman, E.; Afonso, C.; Lu, Z.; Zsak, L.; Sur, J.-H.; Sandybaev, N.; Kerembekova, U.; Zaitsev, V.; Kutish, G.; Rock, D. The genomes of sheeppox and goatpox viruses. J. Virol. 2002, 76, 6054–6061. [Google Scholar] [CrossRef] [Green Version]
- Mathijs, E.; Haegeman, A.; De Clercq, K.; Van Borm, S.; Vandenbussche, F. A robust, cost-effective and widely applicable whole-genome sequencing protocol for capripoxviruses. J. Virol. Methods 2022, 301, 114464. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.; Lamien, C.E.; Diallo, A. Capripox (Lumpy Skin Disease, Sheep Pox, and Goat Pox). In Veterinary Vaccines: Principles and Applications; Metwally, S., El Idrissi, A., Viljoen, G., Eds.; 2021; pp. 383–397. [Google Scholar] [CrossRef]
- Rao, T.; Bandyopadhyay, S. A comprehensive review of goat pox and sheep pox and their diagnosis. Anim. Health Res. Rev. 2000, 1, 127–136. [Google Scholar] [CrossRef]
- Gelaye, E.; Belay, A.; Ayelet, G.; Jenberie, S.; Yami, M.; Loitsch, A.; Tuppurainen, E.; Grabherr, R.; Diallo, A.; Lamien, C.E. Capripox disease in Ethiopia: Genetic differences between field isolates and vaccine strain, and implications for vaccination failure. Antivir. Res. 2015, 119, 28–35. [Google Scholar] [CrossRef]
- McInnes, C.J.; Damon, I.K.; Smith, G.L.; McFadden, G.; Isaacs, S.N.; Roper, R.L.; Evans, D.H.; Damaso, C.R.; Carulei, O.; Wise, L.M. ICTV Virus Taxonomy Profile: Poxviridae 2023. J. Gen. Virol. 2023, 104, 001849. [Google Scholar] [CrossRef]
- Efridi, W.; Lappin, S.L. Poxviruses. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Delhon, G. Poxviridae. In Veterinary Microbiology; McVey, D.S., Kennedy, M., Chengappa, M.M., Wilkes, R., Eds.; Wiley: Online, 2022; pp. 522–532. [Google Scholar] [CrossRef]
- Grimm, C.; Hillen, H.S.; Bedenk, K.; Bartuli, J.; Neyer, S.; Zhang, Q.; Hüttenhofer, A.; Erlacher, M.; Dienemann, C.; Schlosser, A. Structural basis of poxvirus transcription: Vaccinia RNA polymerase complexes. Cell 2019, 179, 1537–1550.e19. [Google Scholar] [CrossRef]
- Pilotto, S.; Fouqueau, T.; Lukoyanova, N.; Sheppard, C.; Lucas-Staat, S.; Díaz-Santín, L.M.; Matelska, D.; Prangishvili, D.; Cheung, A.C.; Werner, F. Structural basis of RNA polymerase inhibition by viral and host factors. Nat. Commun. 2021, 12, 5523. [Google Scholar] [CrossRef]
- Namazi, F.; Khodakaram Tafti, A. Lumpy skin disease, an emerging transboundary viral disease: A review. Vet. Med. Sci. 2021, 7, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Tasioudi, K.; Antoniou, S.; Iliadou, P.; Sachpatzidis, A.; Plevraki, E.; Agianniotaki, E.; Fouki, C.; Mangana-Vougiouka, O.; Chondrokouki, E.; Dile, C. Emergence of lumpy skin disease in Greece, 2015. Transbound. Emerg. Dis. 2016, 63, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Şevik, M.; Doğan, M. Epidemiological and molecular studies on lumpy skin disease outbreaks in Turkey during 2014–2015. Transbound. Emerg. Dis. 2017, 64, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, G.; Chadha, J.; Khullar, L.; Chhibber, S.; Harjai, K. Lumpy Skin Disease: A Comprehensive Review on Virus Biology, Pathogenesis, and Sudden Global Emergence. 2023. Preprints. Available online: https://www.preprints.org/manuscript/202302.0074/v3 (accessed on 30 June 2022).
- Monama, M.Z.; Olotu, F.; Tastan Bishop, Ö. Investigation of Multi-Subunit Mycobacterium tuberculosis DNA-Directed RNA Polymerase and Its Rifampicin Resistant Mutants. Int. J. Mol. Sci. 2023, 24, 3313. [Google Scholar] [CrossRef]
- Ko, S.J.; Park, E.; Asandei, A.; Choi, J.-Y.; Lee, S.-C.; Seo, C.H.; Luchian, T.; Park, Y. Bee venom-derived antimicrobial peptide melectin has broad-spectrum potency, cell selectivity, and salt-resistant properties. Sci. Rep. 2020, 10, 10145. [Google Scholar] [CrossRef]
- Trumbeckaite, S.; Dauksiene, J.; Bernatoniene, J.; Janulis, V. Knowledge, attitudes, and usage of apitherapy for disease prevention and treatment among undergraduate pharmacy students in Lithuania. Evid.-Based Complement. Altern. Med. 2015, 2015, 172502. [Google Scholar] [CrossRef] [Green Version]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [Green Version]
- McMenamin, A.J.; Daughenbaugh, K.F.; Parekh, F.; Pizzorno, M.C.; Flenniken, M.L. Honey bee and bumble bee antiviral defense. Viruses 2018, 10, 395. [Google Scholar] [CrossRef] [Green Version]
- Consortium, U. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, G.; Mahrosh, H.S.; Arif, R. Sequence and structural characterization of toll-like receptor 6 from human and related species. BioMed Res. Int. 2021, 2021, 5545183. [Google Scholar] [CrossRef]
- Li, W.; Cowley, A.; Uludag, M.; Gur, T.; McWilliam, H.; Squizzato, S.; Park, Y.M.; Buso, N.; Lopez, R. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. 2015, 43, W580–W584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.; Su, H.; Wang, W.; Ye, L.; Wei, H.; Peng, Z.; Anishchenko, I.; Baker, D.; Yang, J. The trRosetta server for fast and accurate protein structure prediction. Nat. Protoc. 2021, 16, 5634–5651. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef]
- Ko, J.; Park, H.; Heo, L.; Seok, C. GalaxyWEB server for protein structure prediction and refinement. Nucleic Acids Res. 2012, 40, W294–W297. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Rullmann, J.A.C.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of protein models with three-dimensional profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef]
- Porollo, A.; Meller, J.; Cai, W.; Hong, H. Computational methods for prediction of protein–protein interaction sites. Protein-Protein Interact.-Comput. Exp. Tools 2012, 472, 3–26. [Google Scholar]
- Honorato, R.V.; Koukos, P.I.; Jiménez-García, B.; Tsaregorodtsev, A.; Verlato, M.; Giachetti, A.; Rosato, A.; Bonvin, A.M. Structural biology in the clouds: The WeNMR-EOSC ecosystem. Front. Mol. Biosci. 2021, 8, 729513. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr 2002, 40, 82–92. [Google Scholar]
- Laskowski, R.A. PDBsum new things. Nucleic Acids Res. 2009, 37, D355–D359. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. In Evolving Genes and Protein; Elsevier: Amsterdam, The Netherlands, 1965; pp. 97–166. [Google Scholar]
- Sumana, K.; Revanaiah, Y.; Shivachandra, S.B.; Mothay, D.; Apsana, R.; Saminathan, M.; Basavaraj, S.; Reddy, G.M. Molecular phylogeny of Capripoxviruses based on major immunodominant protein (P32) reveals circulation of host specific sheeppox and goatpox viruses in small ruminants of India. Infect. Genet. Evol. 2020, 85, 104472. [Google Scholar] [CrossRef]
- Karimpour Somedel, A.; Varshoiee, H.; AGHAIYPOUR, K.; Hedayati, Z.; Aghaebrahimian, M. Phylogenetic analysis of Iran capripoxvirus isolates during 2013–2016. Vet. Res. Biol. Prod. 2019, 32, 2–16. [Google Scholar]
- Saidi, A.; Elmottaki, M.; Sadikaoui, A. Molecular characterization of Capripoxvirus isolates from Moroccan sheep. Open Vet. Sci. 2023, 4, 20220117. [Google Scholar] [CrossRef]
- Miller, E.R.; Fowler, M.E. Fowler’s Zoo and Wild Animal Medicine Current Therapy; Elsevier Health Sciences: London, UK, 2011; Volume 7. [Google Scholar]
- Limon, G.; Gamawa, A.A.; Ahmed, A.I.; Lyons, N.A.; Beard, P.M. Epidemiological characteristics and economic impact of lumpy skin disease, sheeppox and goatpox among subsistence farmers in northeast Nigeria. Front. Vet. Sci. 2020, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Mercier, A.; Arsevska, E.; Bournez, L.; Bronner, A.; Calavas, D.; Cauchard, J.; Falala, S.; Caufour, P.; Tisseuil, C.; Lefrançois, T. Spread rate of lumpy skin disease in the Balkans, 2015–2016. Transbound. Emerg. Dis. 2018, 65, 240–243. [Google Scholar] [CrossRef]
- Mustafa, G.; Majid, M.; Ghaffar, A.; Yameen, M.; Samad, H.A.; Mahrosh, H.S. Screening and molecular docking of selected phytochemicals against NS5B polymerase of hepatitis C virus. Pak. J. Pharm. Sci. 2020, 33, 2317–2322. [Google Scholar] [PubMed]
- Azeem, M.; Mustafa, G.; Mahrosh, H.S. Virtual screening of phytochemicals by targeting multiple proteins of severe acute respiratory syndrome coronavirus 2: Molecular docking and molecular dynamics simulation studies. Int. J. Immunopathol. Pharmacol. 2022, 36, 03946320221142793. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.H.; Seo, H.S. Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef] [Green Version]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the SC’06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; pp. 84–es. [Google Scholar]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrand, P.W.; Rose, A.S.; Tiemann, J.K. Bringing molecular dynamics simulation data into view. Trends Biochem. Sci. 2019, 44, 902–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, M.A.; Iqbal, M.N.; Saddick, S.; Ali, I.; Khan, F.S.; Kanwal, S.; Ahmed, D.; Ibrahim, M.; Afzal, U.; Awais, M. Identification of lead compounds against Scm (fms10) in Enterococcus faecium using computer aided drug designing. Life 2021, 11, 77. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, B.Y.; Yoon, H.J.; Choi, Y.S.; Jin, B.R. Secapin, a bee venom peptide, exhibits anti-fibrinolytic, anti-elastolytic, and anti-microbial activities. Dev. Comp. Immunol. 2016, 63, 27–35. [Google Scholar] [CrossRef]
- Muzammal, M.; Khan, M.A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Farid, A. In Silico Analysis of Honeybee Venom Protein Interaction with Wild Type and Mutant (A82V+ P375S) Ebola Virus Spike Protein. Biologics 2022, 2, 45–55. [Google Scholar] [CrossRef]
- Burranboina, K.K.; Kumar, K.; Reddy, G.M.; Yogisharadhya, R.; Prashantha, C.; Dhulappa, A. GC-MS analysis, molecular Docking and pharmacokinetic studies of various bioactive compounds from methanolic leaf extracts of Leucas aspera (L) against anti-Capripox viral activity. Chem. Data Collect. 2022, 39, 100873. [Google Scholar] [CrossRef]
- Kar, P.P.; Araveti, P.B.; Kuriakose, A.; Srivastava, A. Design of a multi-epitope protein as a subunit vaccine against lumpy skin disease using an immunoinformatics approach. Sci. Rep. 2022, 12, 19411. [Google Scholar] [CrossRef]
- Enayathullah, M.G.; Parekh, Y.; Banu, S.; Ram, S.; Nagaraj, R.; Kumar, B.K.; Idris, M.M. Gramicidin S and melittin: Potential anti-viral therapeutic peptides to treat SARS-CoV-2 infection. Sci. Rep. 2022, 12, 3446. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Delgado, O.; Espinoza-Culupú, A.O.; López-López, E. Antimicrobial Activity of Apis mellifera Bee Venom Collected in Northern Peru. Antibiotics 2023, 12, 779. [Google Scholar] [CrossRef] [PubMed]


| Peptide | Source | DNA-Directed RNA Polymerase | ||
|---|---|---|---|---|
| Sheeppox Virus | Goatpox Virus | Lumpy Skin Disease Virus | ||
| Melittin | Apis mellifera (Honey bee) | −21.1 +/− 9.2 | −106.9 +/− 7.2 | −36.8 +/− 5.8 |
| Apamin | Apis mellifera (Honey bee) | −44.3 +/− 2.7 | −78.2 +/− 2.3 | −25.8 +/− 12.1 |
| Melectin | Melecta albifrons (Cuckoo bee) | −39.1 +/− 6.1 | −86.0 +/− 7.2 | 12.7 +/− 4.2 |
| Xal-2 | Xylocopa appendiculata circumvolans (Japanese carpenter bee) | −22.0 +/− 5.0 | −79.5 +/− 5.6 | 34.4 +/− 4.8 |
| Secapin-1 | Apis mellifera (Honey bee) | −48.7 +/− 3.3 | −101.4 +/− 11.3 | −4.5 +/− 12.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, G.; Mahrosh, H.S.; Salman, M.; Ali, M.; Arif, R.; Ahmed, S.; Ebaid, H. In Silico Analysis of Honey Bee Peptides as Potential Inhibitors of Capripoxvirus DNA-Directed RNA Polymerase. Animals 2023, 13, 2281. https://doi.org/10.3390/ani13142281
Mustafa G, Mahrosh HS, Salman M, Ali M, Arif R, Ahmed S, Ebaid H. In Silico Analysis of Honey Bee Peptides as Potential Inhibitors of Capripoxvirus DNA-Directed RNA Polymerase. Animals. 2023; 13(14):2281. https://doi.org/10.3390/ani13142281
Chicago/Turabian StyleMustafa, Ghulam, Hafiza Salaha Mahrosh, Mahwish Salman, Muhammad Ali, Rawaba Arif, Sibtain Ahmed, and Hossam Ebaid. 2023. "In Silico Analysis of Honey Bee Peptides as Potential Inhibitors of Capripoxvirus DNA-Directed RNA Polymerase" Animals 13, no. 14: 2281. https://doi.org/10.3390/ani13142281







