Effect of Potato Vine and Leaf Mixed Silage Compared to Whole Corn Crops on Growth Performance, Apparent Digestibility, and Serum Biochemical Characteristics of Fattening Angus Bull
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ensiling of Three Silage Raw Materials
2.2. Animal Diets and Management
2.3. Sample Collection and Analysis
2.4. Statistical Analysis
3. Results
3.1. Growth Performance of Cattle
3.2. Dietary Nutrient Composition and Apparent Digestibility
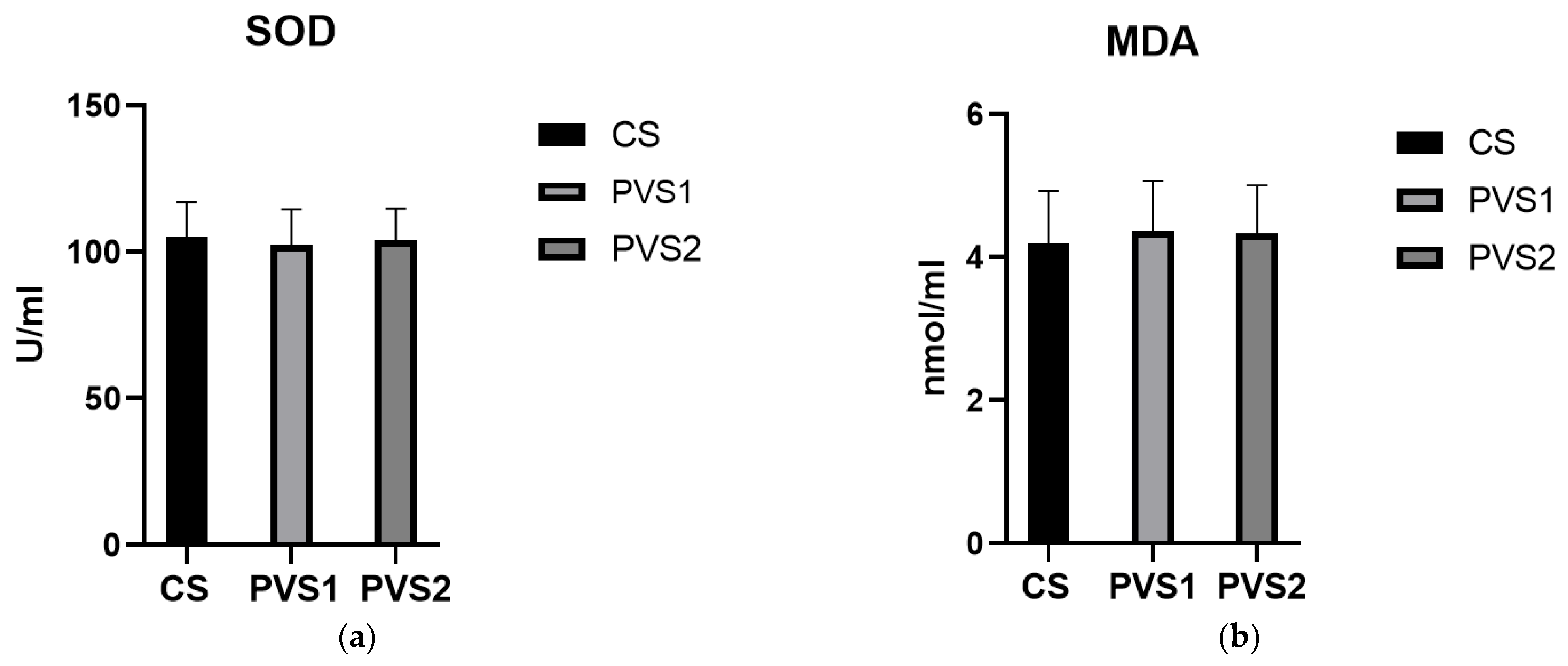
3.3. Biochemical Routine Indexes and Antioxidant Indexes of Beef Cattle Serum
4. Discussion
4.1. PVS Diets Nutritional Properties and Effects of PVS on the Growth Performance of Fattening Angus Bulls
4.2. Effect of PVS on Blood Biochemical Indicators of Angus Beef Cattle
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Gao, B.; Huang, W.; Wang, L.; Fang, X.; Xu, S.; Cui, S. Producing more potatoes with lower inputs and greenhouse gases emissions by regionalized cooperation in China. J. Clean. Prod. 2021, 299, 126883. [Google Scholar] [CrossRef]
- Abong, G. A Review of Occurrence of Glycoalkaloids in Potato and Potato Products. Curr. Res. Nutr. Food Sci. 2016, 4, 95–202. [Google Scholar]
- Akiyama, R.; Watanabe, B.; Nakayasu, M.; Lee, H.J.; Kato, J.; Umemoto, N.; Muranaka, T.; Saito, K.; Sugimoto, Y.; Mizutani, M. The biosynthetic pathway of potato solanidanes diverged from that of spirosolanes due to evolution of a dioxygenase. Nat. Commun. 2021, 12, 1300. [Google Scholar] [CrossRef]
- Dalvi, R.R.; Bowie, W.C. Toxicology of solanine: An overview. Vet. Hum. Toxicol. 1983, 25, 13–15. [Google Scholar]
- Ordóñez-Vásquez, A.; Jaramillo-Gómez, L.; Duran-Correa, C.; Escamilla-García, E.; De la Garza-Ramos, M.A.; Suárez-Obando, F. A Reliable and Reproducible Model for Assessing the Effect of Different Concentrations of α-Solanine on Rat Bone Marrow Mesenchymal Stem Cells. Bone Marrow Res. 2017, 2017, 2170306. [Google Scholar] [CrossRef] [Green Version]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato-derived products. EFSA J. 2020, 18, e06222. [Google Scholar]
- Baur, S.; Frank, O.; Hausladen, H.; Hückelhoven, R.; Hofmann, T.; Eisenreich, W.; Dawid, C. Biosynthesis of α-solanine and α-chaconine in potato leaves (Solanum tuberosum L.)—A 13CO2 study. Food Chem. 2021, 365, 130461. [Google Scholar] [CrossRef]
- Olagaray, K.E.; Brouk, M.J.; Mamedova, L.K.; Sivinski, S.E.; Liu, H.; Robert, F.; Dupuis, E.; Zachut, M.; Bradford, B.J. Dietary supplementation of Scutellaria baicalensis extract during early lactation decreases milk somatic cells and increases whole lactation milk yield in dairy cattle. PLoS ONE 2019, 14, e0210744. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, D.; Tava, A.; Galletti, S.; Tameni, M.; Varisco, G.; Costa, A.; Steidler, S. Effects of silymarin, a natural hepatoprotector, in periparturient dairy cows. J. Dairy Sci. 2004, 87, 2239–2247. [Google Scholar] [CrossRef] [Green Version]
- Stoldt, A.-K.; Derno, M.; Nürnberg, G.; Weitzel, J.M.; Otten, W.; Starke, A.; Wolffram, S.; Metges, C.C. Effects of a 6-wk intraduodenal supplementation with quercetin on energy metabolism and indicators of liver damage in periparturient dairy cows. J. Dairy Sci. 2015, 98, 4509–4520. [Google Scholar] [CrossRef] [Green Version]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Chay-Canul, A.J.; Miranda-Romero, L.A.; Mendoza-Martínez, G.D. Meta-analysis of flavonoids use into beef and dairy cattle diet: Performance, antioxidant status, ruminal fermentation, meat quality, and milk composition. Front. Vet. Sci. 2023, 10, 1134925. [Google Scholar] [CrossRef] [PubMed]
- Noordar, H.; Malecky, M.; Jahanian, H.; Navidshad, B. Evaluating nutritional value of processed potato vines by in vitro gas production. N. Z. J. Agric. Res. 2017, 60, 189–204. [Google Scholar] [CrossRef]
- Parfitt, D.E.; Peloquin, S.J.; Jorgensen, N.A. The nutritional-value of pressed potato vine silage. Am. Potato J. 1982, 59, 415–423. [Google Scholar] [CrossRef]
- Krizsan, S.J.; Randby, A.T. The effect of fermentation quality on the voluntary intake of grass silage by growing cattle fed silage as the sole feed. J. Anim. Sci. 2007, 85, 984–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, P.J.; Henderson, B.; Makkar, H.P. Mitigation of Greenhouse Gas Emissions in Livestock Production: A Review of Technical Options for Non-CO2 Emissions; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Adopted, I. Climate Change 2014 Synthesis Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Guo, Y.L.; Han, H.Z.; Luo, R.R.; Zhao, F.F. Determining the ensiling characteristics of potato vine silage supplemented with rice bran and corn and evaluating their ruminal fermentation potential in vitro. N. Z. J. Agric. Res. 2020, 63, 179–188. [Google Scholar] [CrossRef]
- Sugimoto, M.; Saito, W.; Ooi, M.; Sato, Y.; Saito, T. The effects of inclusion levels of urea-treated potato pulp silage in concentrate and roughage sources on finishing performance and carcass quality in cull beef cows. Anim. Sci. J. 2009, 80, 280–285. [Google Scholar] [CrossRef]
- Malecky, M.; Ghadbeigi, M.; Aliarabi, H.; Bahari, A.A.; Zaboli, K. Effect of replacing alfalfa with processed potato vines on growth performance, ruminal and total tract digestibility and blood metabolites in fattening lambs. Small Rumin. Res. 2017, 146, 13–22. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Van Keulen, J.; Young, B.A. Evaluation of Acid-Insoluble Ash as a Natural Marker in Ruminant Digestibility Studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Hilscher, F.H.; Burken, D.B.; Bittner, C.J.; Gramkow, J.L.; Bondurant, R.G.; Jolly-Breithaupt, M.L.; Watson, A.K.; MacDonald, J.C.; Klopfenstein, T.J.; Erickson, G.E. Impact of corn silage moisture at harvest on performance of growing steers with supplemental rumen undegradable protein, finishing steer performance, and nutrient digestibility by lambs. Transl. Anim. Sci. 2019, 3, 761–774. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Terry, S.A.; Basarab, J.A.; Guan, L.L.; McAllister, T.A. Strategies to improve the efficiency of beef cattle production. Can. J. Anim. Sci. 2020, 101, 19. [Google Scholar] [CrossRef]
- Silva, T.; Quigley, S.P.; Kidd, L.J.; Anderson, S.T.; McLennan, S.R.; Poppi, D.P. Effect of a high crude protein content diet during energy restriction and re-alimentation on animal performance, skeletal growth and metabolism of bone tissue in two genotypes of cattle. PLoS ONE 2021, 16, e0247718. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Lashkari, S.; Abbasi, R.; Kamangar, H. Nutrient Digestibility and Chemical Composition of Potato Vine (Solanum tuberosum) as Alternative Forage in Ruminant Diets. Agric. Commun. 2014, 2, 63–66. [Google Scholar]
- Arelovich, H.M.; Abney, C.S.; Vizcarra, J.A.; Galyean, M.L. Effects of Dietary Neutral Detergent Fiber on Intakes of Dry Matter and Net Energy by Dairy and Beef Cattle: Analysis of Published Data. Prof. Anim. Sci. 2008, 24, 375–383. [Google Scholar] [CrossRef]
- Salfer, I.J.; Morelli, M.C.; Ying, Y.; Allen, M.S.; Harvatine, K.J. The effects of source and concentration of dietary fiber, starch, and fatty acids on the daily patterns of feed intake, rumination, and rumen pH in dairy cows. J. Dairy Sci. 2018, 101, 10911–10921. [Google Scholar] [CrossRef] [Green Version]
- Niu, M.; Ying, Y.; Bartell, P.A.; Harvatine, K.J. The effects of feeding rations that differ in fiber and fermentable starch within a day on milk production and the daily rhythm of feed intake and plasma hormones and metabolites in dairy cows. J. Dairy Sci. 2017, 100, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Cortese, M.; Segato, S.; Andrighetto, I.; Ughelini, N.; Chinello, M.; Schiavon, E.; Marchesini, G. The Effects of Decreasing Dietary Crude Protein on the Growth Performance, Feed Efficiency and Meat Quality of Finishing Charolais Bulls. Animals 2019, 9, 906. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.I.M.; Wassie, S.E.; Korir, D.; Merbold, L.; Goopy, J.P.; Butterbach-Bahl, K.; Dickhoefer, U.; Schlecht, E. Supplementing Tropical Cattle for Improved Nutrient Utilization and Reduced Enteric Methane Emissions. Animals 2019, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Aziz Ur Rahman, M.; Yang, H.; Shao, T.; Qiu, Q.; Su, H.; Cao, B. Effect of increased dietary crude protein levels on production performance, nitrogen utilisation, blood metabolites and ruminal fermentation of Holstein bulls. Asian-Australas. J. Anim. Sci. 2018, 31, 1643–1653. [Google Scholar] [CrossRef] [Green Version]
- Chantiratikul, A.; Chumpawadee, S.; Kanchanamayoon, W.; Chantiratikul, P. Effect of dietary protein on nutrient digestibility and nitrogen metabolism in Thai-Indigenous heifers. J. Anim. Vet. Adv. 2009, 8, 297–300. [Google Scholar]
- Hall, P.; Cash, J. What is the real function of the liver ‘function’ tests? Ulst. Med. J. 2012, 81, 30–36. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: San Diego, CA, USA, 2008; Volume 493, pp. 889–895. [Google Scholar]
- Zaitsev, S.; Bogolyubova, N.; Zhang, X.; Brenig, B. Biochemical parameters, dynamic tensiometry and circulating nucleic acids for cattle blood analysis: A review. PeerJ 2020, 8, e8997. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, B.; Shu, S.; Zhang, H.; Xu, C.; Wu, L.; Xia, C. Critical thresholds of liver function parameters for ketosis prediction in dairy cows using receiver operating characteristic (ROC) analysis. Vet. Q. 2015, 35, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mair, B.; Drillich, M.; Klein-Jöbstl, D.; Kanz, P.; Borchardt, S.; Meyer, L.; Schwendenwein, I.; Iwersen, M. Glucose concentration in capillary blood of dairy cows obtained by a minimally invasive lancet technique and determined with three different hand-held devices. BMC Vet. Res. 2016, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef]
- Alberghina, D.; Giannetto, C.; Vazzana, I.; Ferrantelli, V.; Piccione, G. Reference intervals for total protein concentration, serum protein fractions, and albumin/globulin ratios in clinically healthy dairy cows. J. Vet. Diagn. Investig. 2011, 23, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tshuma, T.; Fosgate, G.T.; Hamman, R.; Holm, D.E. Effect of different levels of dietary nitrogen supplementation on the relative blood urea nitrogen concentration of beef cows. Trop. Anim. Health Prod. 2019, 51, 1883–1891. [Google Scholar] [CrossRef]
- Castillo, C.; Benedito, J.L.; Vázquez, P.; Pereira, V.; Méndez, J.; Sotillo, J.; Hernández, J. Effects of supplementation with plant extract product containing carvacrol, cinnamaldehyde and capsaicin on serum metabolites and enzymes during the finishing phase of feedlot-fed bull calves. Anim. Feed. Sci. Technol. 2012, 171, 246–250. [Google Scholar] [CrossRef]
- Gleghorn, J.F.; Elam, N.A.; Galyean, M.L.; Duff, G.C.; Cole, N.A.; Rivera, J.D. Effects of crude protein concentration and degradability on performance, carcass characteristics, and serum urea nitrogen concentrations in finishing beef steers. J. Anim. Sci. 2004, 82, 2705–2717. [Google Scholar] [CrossRef]
- Pilachai, R.; Schonewille, J.T.; Thamrongyoswittayakul, C.; Aiumlamai, S.; Wachirapakorn, C.; Everts, H.; Hendriks, W.H. The effects of high levels of rumen degradable protein on rumen pH and histamine concentrations in dairy cows. J. Anim. Physiol. Anim. Nutr. 2012, 96, 206–213. [Google Scholar] [CrossRef]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weekes, T.L.; Luimes, P.H.; Cant, J.P. Responses to Amino Acid Imbalances and Deficiencies in Lactating Dairy Cows. J. Dairy Sci. 2006, 89, 2177–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omphalius, C.; Lapierre, H.; Guinard-Flament, J.; Lamberton, P.; Bahloul, L.; Lemosquet, S. Amino acid efficiencies of utilization vary by different mechanisms in response to energy and protein supplies in dairy cows: Study at mammary-gland and whole-body levels. J. Dairy Sci. 2019, 102, 9883–9901. [Google Scholar] [CrossRef] [PubMed]
- Satter, L.D.; Slyter, L.L. Effect of ammonia concentration of rumen microbial protein production in vitro. Br. J. Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, A.S.H.; Bessa, R.J.B.; Pires, V.M.R.; Rolo, E.A.; Pinto, R.M.A.; Andrade Fontes, C.M.G.; Prates, J.A.M. Is hepatic lipid metabolism of beef cattle influenced by breed and dietary silage level? BMC Vet. Res. 2014, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Garverick, H.A.; Harris, M.N.; Vogel-Bluel, R.; Sampson, J.D.; Bader, J.; Lamberson, W.R.; Spain, J.N.; Lucy, M.C.; Youngquist, R.S. Concentrations of nonesterified fatty acids and glucose in blood of periparturient dairy cows are indicative of pregnancy success at first insemination. J. Dairy Sci. 2013, 96, 181–188. [Google Scholar] [CrossRef]
- Rizos, C.V.; Elisaf, M.S.; Liberopoulos, E.N. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc. Med. J. 2011, 5, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Juniper, D.T. Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition. Animals 2019, 9, 462. [Google Scholar] [CrossRef] [Green Version]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox Biology in Transition Periods of Dairy Cattle: Role in the Health of Periparturient and Neonatal Animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, L. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef] [Green Version]


| Item | Treatment | ||
|---|---|---|---|
| CS 1 | PVS1 | PVS2 | |
| pH value | 4.48 | 4.69 | 4.87 |
| Nutritive values, %DM 2 | |||
| Dry matter | 30.07 | 25.59 | 35.71 |
| CP | 6.91 | 7.84 | 8.45 |
| EE | 2.46 | 3.30 | 3.09 |
| NDF | 44.04 | 43.42 | 41.79 |
| ADF | 24.45 | 30.00 | 27.48 |
| Ash | 4.94 | 10.42 | 18.48 |
| Starch | 31.81 | 19.95 | 24.15 |
| Metabolic energy 3, MJ/kg | 11.08 | 10.51 | 9.38 |
| Item% | Treatment | ||
|---|---|---|---|
| CS | PVS1 | PVS2 | |
| Flaked corn | 21.85 | 21.59 | 22.08 |
| Soybean meal | 5.59 | 5.36 | 5.79 |
| Bran | 3.73 | 3.57 | 3.86 |
| Cotton meal | 2.98 | 2.86 | 3.09 |
| 5% Premix 1 | 2.98 | 2.86 | 3.09 |
| Dried distiller’s grains with soluble (DDGS) | 2.98 | 2.86 | 3.09 |
| Rapeseed meal | 2.01 | 1.93 | 2.08 |
| Probiotics 2 | 1.34 | 1.29 | 1.39 |
| Fermented feed 3 | 4.97 | 4.76 | 5.14 |
| Straw | 2.48 | 2.38 | 2.57 |
| Rice stalks | 6.21 | 5.95 | 6.43 |
| Silage | 42.89 | 45.25 | 40.83 |
| Nutritive values, % DM | |||
| Dry matter | 57.2 | 54.4 | 62.5 |
| Crude protein | 11.24 | 13.17 | 13.6 |
| Ether extract | 7.87 | 5.75 | 5.53 |
| Neutral detergent fiber | 28.35 | 28.76 | 27.7 |
| Acid detergent fiber | 17.00 | 17.00 | 15.41 |
| Ash | 8.51 | 9.11 | 10.81 |
| Ca 4, % | 0.41 | 0.39 | 0.42 |
| P 4, % | 0.15 | 0.14 | 0.15 |
| Starch | 31.81 | 19.95 | 24.57 |
| Metabolic energy, MJ/kg 5 | 12.17 | 11.83 | 11.37 |
| CS | PVS1 | PVS2 | SEM | p-Value | |
|---|---|---|---|---|---|
| Initial weight, kg | 400.36 | 400.27 | 404.73 | 7.06 | 0.960 |
| Final weight, kg | 528.82 | 500.82 | 506.00 | 8.69 | 0.388 |
| ADG, kg/day | 1.44 a | 1.13 b | 1.14 b | 0.52 | 0.014 |
| DMI, kg/day | 9.09 | 9.43 | 9.07 | 0.10 | 0.296 |
| FCR | 6.59 b | 8.64 a | 8.38 a | 0.33 | 0.022 |
| CS | PVS1 | PVS2 | SEM | p-Value | |
|---|---|---|---|---|---|
| Apparent digestibility% | |||||
| DM, % | 69.55 | 70.53 | 69.32 | 0.35 | 0.377 |
| CP, % | 53.48 b | 70.18 a | 71.41 a | 3.47 | 0.028 |
| EE, % | 68.09 | 62.26 | 61.57 | 1.78 | 0.294 |
| NDF, % | 49.75 | 60.68 | 59.18 | 2.73 | 0.225 |
| ADF, % | 45.50 | 53.56 | 50.40 | 2.24 | 0.383 |
| CS | PVS1 | PVS2 | SEM | p-Value | |
|---|---|---|---|---|---|
| Biochemical Index | |||||
| ALT | 25.87 | 23.28 | 25.16 | 1.09 | 0.619 |
| AST | 90.64 | 103.05 | 94.75 | 3.87 | 0.423 |
| TP | 54.93 | 58.06 | 56.80 | 1.29 | 0.623 |
| ALP | 144.24 | 167.43 | 206.73 | 14.25 | 0.198 |
| CHO | 3.18 b | 3.54 b | 4.30 a | 0.12 | <0.001 |
| TG | 0.20 | 0.22 | 0.18 | 0.01 | 0.085 |
| HDL-C | 0.97 c | 1.01 b | 1.19 a | 0.03 | 0.010 |
| LDL-C | 0.90 b | 0.96 b | 1.13 a | 0.03 | 0.003 |
| BUN | 2.88 b | 4.36 a | 4.31 a | 0.16 | <0.001 |
| NEFA | 0.23 b | 0.29 a | 0.36 a | 0.07 | 0.036 |
| GLU | 1.79 | 1.61 | 1.66 | 0.44 | 0.602 |
| CREA | 87.83 | 93.33 | 92.50 | 2.55 | 0.652 |
| ALB | 29.46 | 31.50 | 32.13 | 0.58 | 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Zhang, S.; Li, Y.; Shi, C.; Qiu, X.; Cao, B.; He, Y.; Su, H. Effect of Potato Vine and Leaf Mixed Silage Compared to Whole Corn Crops on Growth Performance, Apparent Digestibility, and Serum Biochemical Characteristics of Fattening Angus Bull. Animals 2023, 13, 2284. https://doi.org/10.3390/ani13142284
Deng J, Zhang S, Li Y, Shi C, Qiu X, Cao B, He Y, Su H. Effect of Potato Vine and Leaf Mixed Silage Compared to Whole Corn Crops on Growth Performance, Apparent Digestibility, and Serum Biochemical Characteristics of Fattening Angus Bull. Animals. 2023; 13(14):2284. https://doi.org/10.3390/ani13142284
Chicago/Turabian StyleDeng, Jiajie, Siyu Zhang, Yingqi Li, Changxiao Shi, Xinjun Qiu, Binghai Cao, Yang He, and Huawei Su. 2023. "Effect of Potato Vine and Leaf Mixed Silage Compared to Whole Corn Crops on Growth Performance, Apparent Digestibility, and Serum Biochemical Characteristics of Fattening Angus Bull" Animals 13, no. 14: 2284. https://doi.org/10.3390/ani13142284
APA StyleDeng, J., Zhang, S., Li, Y., Shi, C., Qiu, X., Cao, B., He, Y., & Su, H. (2023). Effect of Potato Vine and Leaf Mixed Silage Compared to Whole Corn Crops on Growth Performance, Apparent Digestibility, and Serum Biochemical Characteristics of Fattening Angus Bull. Animals, 13(14), 2284. https://doi.org/10.3390/ani13142284







