Portulaca oleracea L. Polysaccharide Inhibits Porcine Rotavirus In Vitro
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Preparation of POL-P
2.3. Cell Viability Assays
2.4. Western Blotting
2.5. RNA Isolation and Real-Time PCR Analysis
2.6. TCID50 Assays
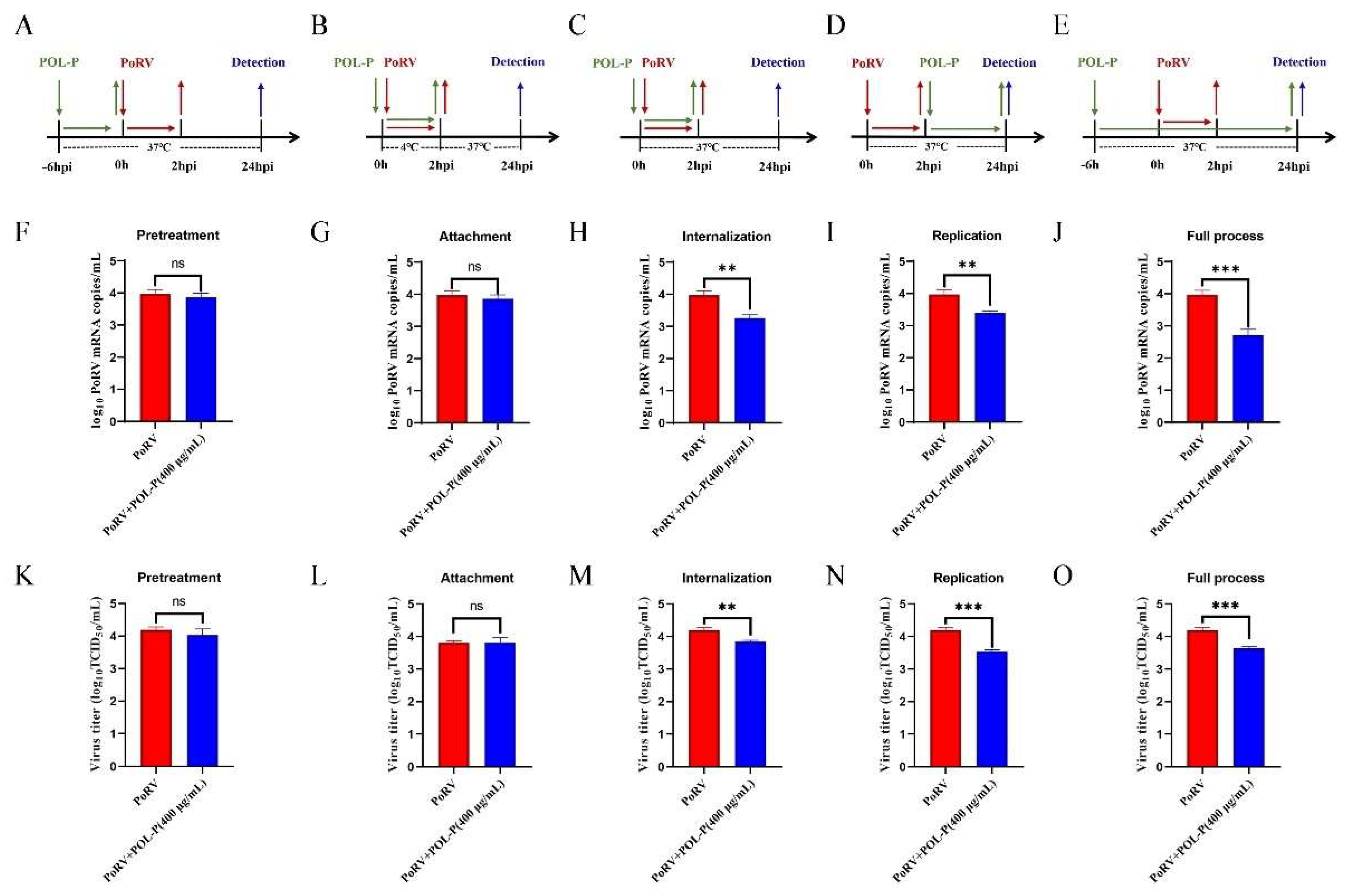
2.7. Inhibitory Effects of POL-P at Different Stages of Viral Replication
2.8. Indirect Immunofluorescence Assays
2.9. ELISA
2.10. Statistical Analysis
3. Results
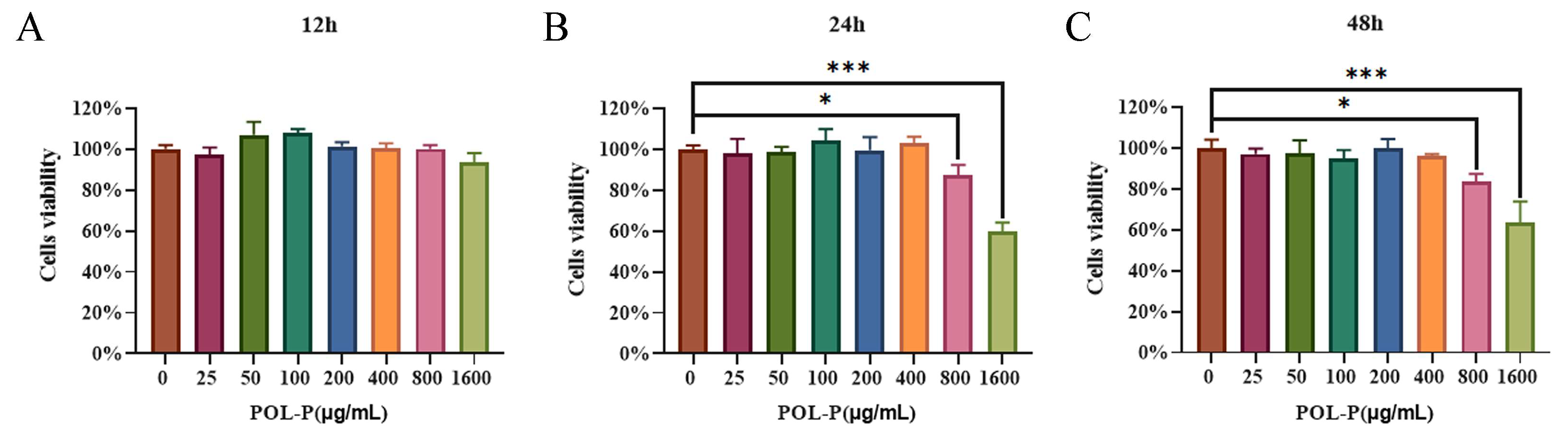
3.1. Cytotoxicity of POL-P to IPEC-J2 Cells In Vitro
3.2. Different Concentrations of POL-P Inhibit PoRV Infection In Vitro
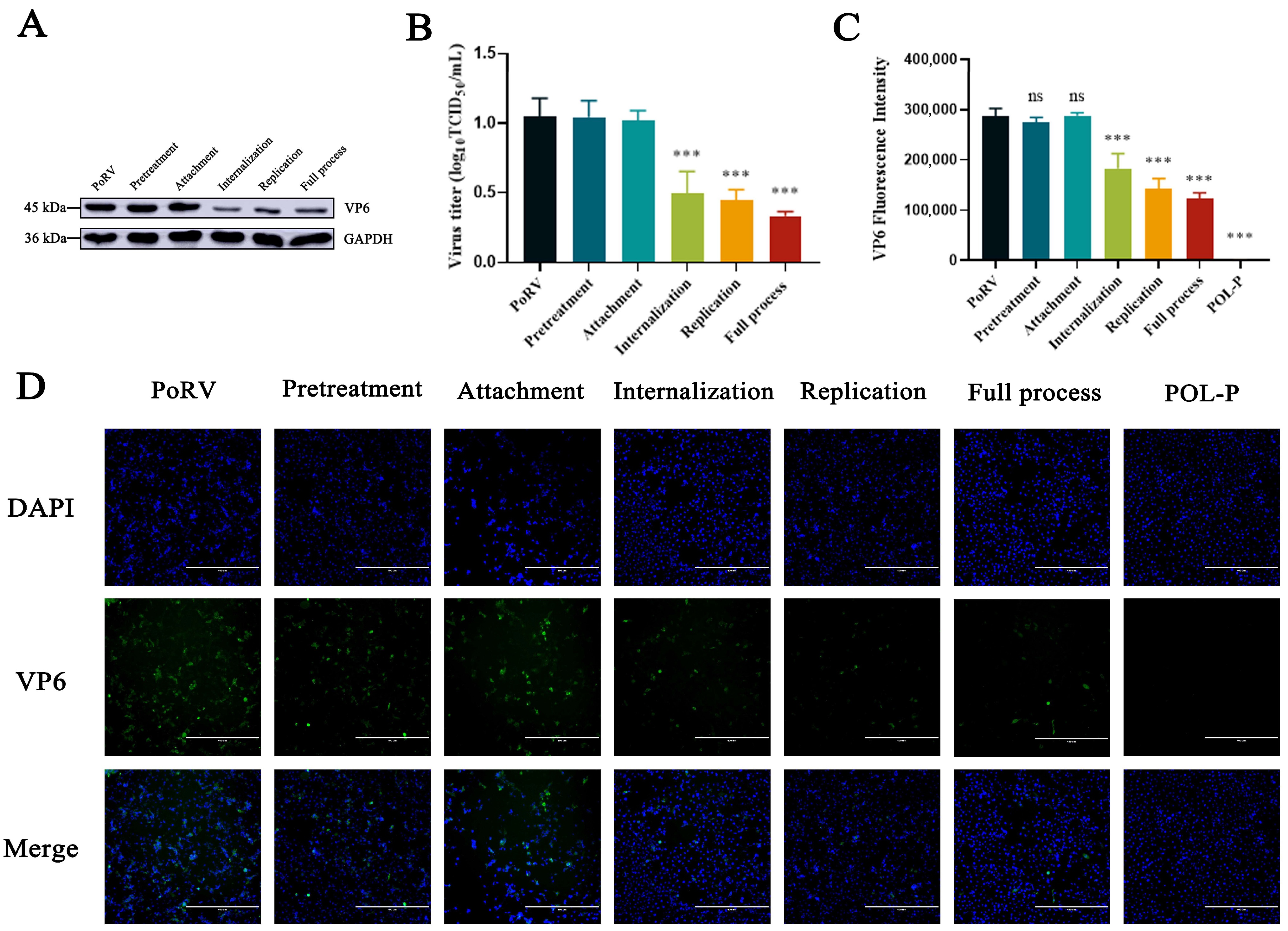
3.3. Effects of POL-P Inhibition on PoRV Life Cycle Stages
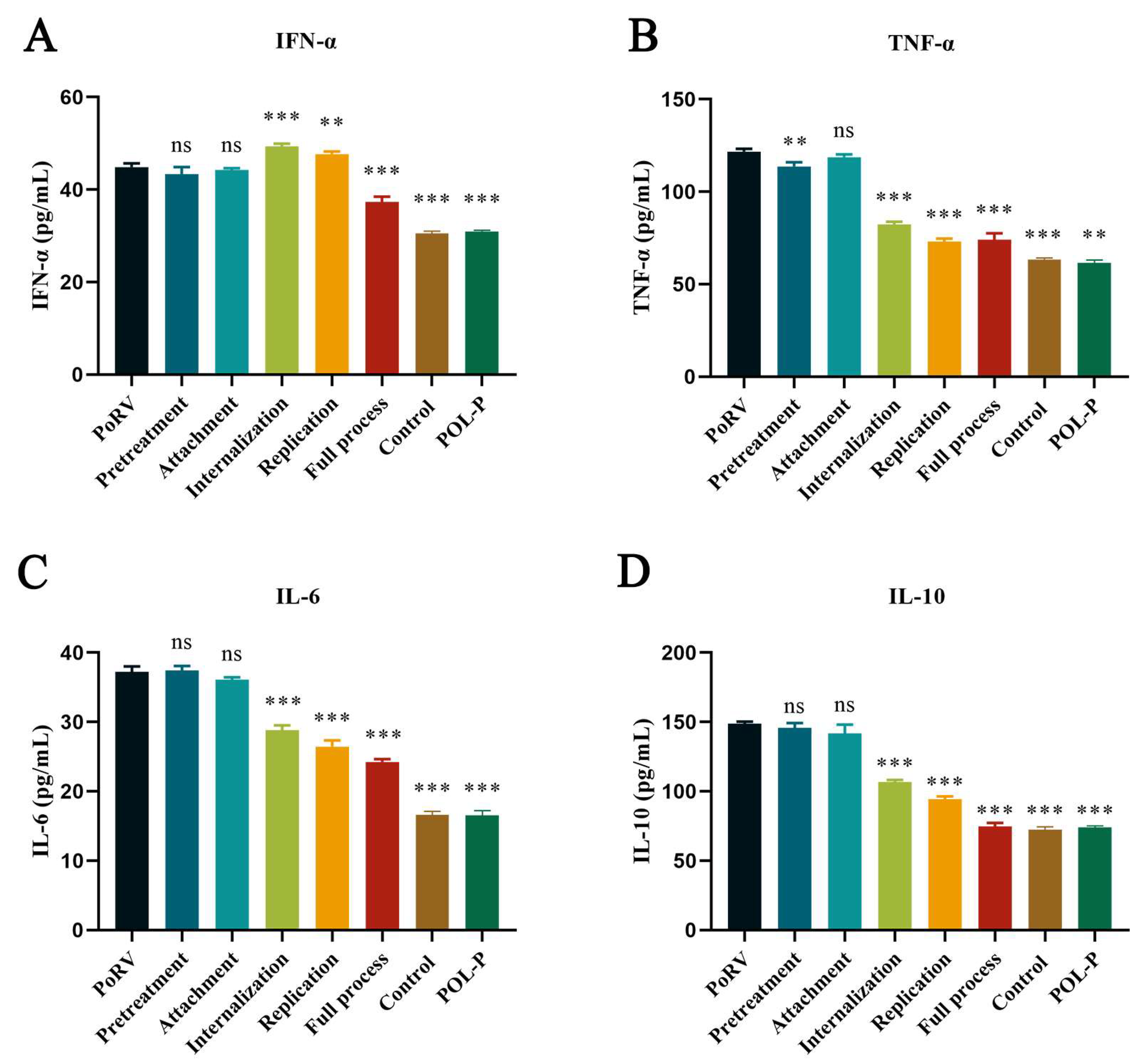
3.4. Effect of POL-P on Cytokine Release in IPEC-J2 during Different Processes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mebus, C.A.; Underdahl, N.R.; Rhodes, M.B.; Twiehaus, M.J. Further studies on neonatal calf diarrhea virus. Proc. Annu. Meet. U. S. Anim. Health Assoc. 1969, 73, 97–99. [Google Scholar] [PubMed]
- Wenske, O.; Ruckner, A.; Piehler, D.; Schwarz, B.A.; Vahlenkamp, T.W. Epidemiological analysis of porcine rotavirus A genotypes in Germany. Vet. Microbiol. 2018, 214, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, M.; Seo, J.Y.; Kim, D.S.; Kim, J.Y.; Park, J.G.; Alfajaro, M.M.; Baek, Y.B.; Cho, E.H.; Kwon, J.; Choi, J.S.; et al. Activation of PI3K, Akt, and ERK during early rotavirus infection leads to V-ATPase-dependent endosomal acidification required for uncoating. PLoS Pathog. 2018, 14, e1006820. [Google Scholar] [CrossRef] [Green Version]
- Doerksen, T.; Christensen, T.; Lu, A.; Noll, L.; Bai, J.; Henningson, J.; Palinski, R. Assessment of porcine Rotavirus-associated virome variations in pigs with enteric disease. Vet. Microbiol. 2022, 270, 109447. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Zhao, Y.; Sui, L.; Li, F.; Zhang, H.; Li, J.; Jiang, Y.; Cui, W.; Ding, G.; et al. Auxotrophic Lactobacillus Expressing Porcine Rotavirus VP4 Constructed Using CRISPR-Cas9D10A System Induces Effective Immunity in Mice. Vaccines 2022, 10, 1510. [Google Scholar] [CrossRef]
- Santosham, M.; Steele, D. Rotavirus Vaccines—A New Hope. N. Engl. J. Med. 2017, 376, 1170–1172. [Google Scholar] [CrossRef]
- Cates, J.E.; Tate, J.E.; Parashar, U. Rotavirus vaccines: Progress and new developments. Expert Opin. Biol. Ther. 2022, 22, 423–432. [Google Scholar] [CrossRef]
- Chandler-Bostock, R.; Hancox, L.R.; Payne, H.; Iturriza-Gomara, M.; Daly, J.M.; Mellits, K.H. Diversity of group A rotavirus on a UK pig farm. Vet. Microbiol. 2015, 180, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Xue, R.; Tian, Y.; Zhang, Y.; Zhang, M.; Li, Z.; Chen, S.; Liu, Q. Diversity of group A rotavirus of porcine rotavirus in Shandong province China. Acta. Virol. 2018, 62, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Nagesha, H.S.; Brown, L.E.; Holmes, I.H. Neutralizing monoclonal antibodies against three serotypes of porcine rotavirus. J. Virol. 1989, 63, 3545–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.S.; Lee, H.J.; Shim, I.; Bae, H. Assessment of anti-depressant effect of nelumbinis semen on rats under chronic mild stress and its subchronic oral toxicity in rats and beagle dogs. BMC Complement. Altern. Med. 2012, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, L.J.; Lu, Y.; Zhang, Y.Y.; Zhu, H.Y.; Tu, P.; Li, H.; Chen, D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhong, L.; Xiao, J.; Hu, Y.; Liu, T.; Ren, Z.; Wang, Y.; Zheng, K. Ethanol extract from Artemisia argyi leaves inhibits HSV-1 infection by destroying the viral envelope. Virol. J. 2023, 20, 8. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, Z.; Yu, T.; Hu, H.; Zhao, Y.; Li, C.; Zhu, Q.; Wang, M.; Zhai, P.; He, L.; et al. The Antiviral Effects of Jasminin via Endogenous TNF-alpha and the Underlying TNF-alpha-Inducing Action. Molecules 2022, 27, 1598. [Google Scholar] [CrossRef]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Chen, T.; Lin, Y.; Cao, Z.; Xue, Y.; Wang, W.; Wang, X. Network pharmacology analysis and experimental study strategy reveals the potential mechanism of puerarin against rotavirus. Ann. Transl. Med. 2022, 10, 14. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Zou, Y.F.; Barsett, H.; Ho, G.T.; Inngjerdingen, K.T.; Diallo, D.; Michaelsen, T.E.; Paulsen, B.S. Immunomodulating pectins from root bark, stem bark, and leaves of the Malian medicinal tree Terminalia macroptera, structure activity relations. Carbohydr. Res. 2015, 403, 167–173. [Google Scholar] [CrossRef]
- Ming, K.; He, M.; Su, L.; Du, H.; Wang, D.; Wu, Y.; Liu, J. The inhibitory effect of phosphorylated Codonopsis pilosula polysaccharide on autophagosomes formation contributes to the inhibition of duck hepatitis A virus replication. Poult. Sci. 2020, 99, 2146–2156. [Google Scholar] [CrossRef]
- Huan, C.; Zhang, W.; Xu, Y.; Ni, B.; Gao, S. Antiviral Activity of Plantago asiatica Polysaccharide against Pseudorabies Virus In Vitro. Oxidative Med. Cell. Longev. 2022, 2022, 3570475. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Zhao, J.; Shao, Y.; Lin, Y.; Li, L.; Liu, Q.; Lu, T.; Zhang, Q. Antiviral Activity of Crude Polysaccharide Derived from Seaweed against IHNV and IPNV In Vitro. Viruses 2022, 14, 2080. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Wang, B.; Lu, Y.; Li, L.; Li, Y.; Liu, S. Evaluation of the effects of Astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 253, 109249. [Google Scholar] [CrossRef]
- Pinela, J.; Carvalho, A.M.; Ferreira, I. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. 2017, 110, 165–188. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Tao, H.; Peng, Y.; Wang, S.; Zhong, Z.; El-Seedi, H.; Dragan, S.; Zengin, G.; Cheang, W.S.; Wang, Y.; et al. The anti-inflammatory potential of Portulaca oleracea L. (purslane) extract by partial suppression on NF-kappaB and MAPK activation. Food Chem. 2019, 290, 239–245. [Google Scholar] [CrossRef]
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; AlMotwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, Antiviral, and In-Vitro Cytotoxicity and Mosquitocidal Activities of Portulaca oleracea-Based Green Synthesis of Selenium Nanoparticles. J. Funct. Biomater. 2022, 13, 157. [Google Scholar] [CrossRef]
- He, Y.; Long, H.; Zou, C.; Yang, W.; Jiang, L.; Xiao, Z.; Li, Q.; Long, S. Anti-nociceptive effect of Portulaca oleracea L. ethanol extracts attenuated zymosan-induced mouse joint inflammation via inhibition of Nrf2 expression. Innate. Immun. 2021, 27, 230–239. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, C.; Wang, W.; Tang, G.; Dong, L.; Zhou, J.; Zhong, Z. Ethanol extracts from Portulaca oleracea L. attenuated ischemia/reperfusion induced rat neural injury through inhibition of HMGB1 induced inflammation. Am. J. Transl. Res. 2016, 8, 5016–5024. [Google Scholar]
- Liu, Y.; Zhao, L.; Xie, Y.; Chen, Z.; Yang, S.; Yin, B.; Li, G.; Guo, H.; Lin, S.; Wu, J. Antiviral activity of portulaca oleracea L. extracts against porcine epidemic diarrhea virus by partial suppression on myd88/NF-kappab activation in vitro. Microb. Pathog. 2021, 154, 104832. [Google Scholar] [CrossRef]
- Li, Y.H.; Lai, C.Y.; Su, M.C.; Cheng, J.C.; Chang, Y.S. Antiviral activity of Portulaca oleracea L. against influenza A viruses. J. Ethnopharmacol. 2019, 241, 112013. [Google Scholar] [CrossRef]
- Jia, G.; Shao, X.; Zhao, R.; Zhang, T.; Zhou, X.; Yang, Y.; Li, T.; Chen, Z.; Liu, Y. Portulaca oleracea L. polysaccharides enhance the immune efficacy of dendritic cell vaccine for breast cancer. Food Funct. 2021, 12, 4046–4059. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Shi, D.; Xing, X.; Qi, S.; Yang, D.; Zhang, J.; Han, Y.; Zhu, Q.; Sun, H.; Wang, X.; et al. Coronavirus Porcine Epidemic Diarrhea Virus Nucleocapsid Protein Interacts with p53 To Induce Cell Cycle Arrest in S-Phase and Promotes Viral Replication. J. Virol. 2021, 95, e0018721. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Hao, J.; Niu, Y.Y.; Tian, M.; Yang, X.; Zhu, C.H.; Ding, X.L.; Liu, X.H.; Zhang, H.R.; Liu, C.; et al. Network pharmacology dissection of multiscale mechanisms of herbal medicines in stage IV gastric adenocarcinoma treatment. Medicine 2016, 95, e4389. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Zhang, D.; Wang, K.; Duan, X.; Zhang, X. A Network Pharmacology Approach to Uncover the Multiple Mechanisms of Hedyotis diffusa Willd. on Colorectal Cancer. Evid. Based Complement. Alternat. Med. 2018, 2018, 6517034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puluhulawa, L.E.; Joni, I.M.; Mohammed, A.F.A.; Arima, H.; Wathoni, N. The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications. Molecules 2021, 26, 3362. [Google Scholar] [CrossRef]
- Du, S.; Han, B.; Li, K.; Zhang, X.; Sha, X.; Gao, L. Lycium barbarum Polysaccharides Protect Rat Corneal Epithelial Cells against Ultraviolet B-Induced Apoptosis by Attenuating the Mitochondrial Pathway and Inhibiting JNK Phosphorylation. Biomed. Res. Int. 2017, 2017, 5806832. [Google Scholar] [CrossRef] [Green Version]
- Huan, C.; Xu, Y.; Zhang, W.; Ni, B.; Gao, S. Glycyrrhiza Polysaccharide Inhibits Pseudorabies Virus Infection by Interfering with Virus Attachment and Internalization. Viruses 2022, 14, 1772. [Google Scholar] [CrossRef]
- Huan, C.; Yao, J.; Xu, W.; Zhang, W.; Zhou, Z.; Pan, H.; Gao, S. Huaier Polysaccharide Interrupts PRV Infection via Reducing Virus Adsorption and Entry. Viruses 2022, 14, 745. [Google Scholar] [CrossRef]
- Yan, X.Y.; Wang, Y.; Xiong, L.F.; Jian, J.C.; Wu, Z.H. Phylogenetic analysis of newly isolated grass carp reovirus. Springerplus 2014, 3, 190. [Google Scholar] [CrossRef] [Green Version]
- James, V.L.; Lambden, P.R.; Caul, E.O.; Clarke, I.N. Enzyme-linked immunosorbent assay based on recombinant human group C rotavirus inner capsid protein (VP6) To detect human group C rotaviruses in fecal samples. J. Clin. Microbiol. 1998, 36, 3178–3181. [Google Scholar] [CrossRef] [Green Version]
- Gaffney, K.J.; Urban, T.A.; Lucena, M.; Anwer, F.; Dean, R.M.; Gerds, A.T.; Hamilton, B.K.; Jagadeesh, D.; Kalaycio, M.E.; Khouri, J.; et al. Toxicity analysis of busulfan pharmacokinetic therapeutic dose monitoring. J. Oncol. Pharm. Pract. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Y.; Peng, P.; Liu, Y.; Huang, M.; Ma, Y.; Xue, C.; Cao, Y. Aloe extract inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet. Microbiol. 2020, 249, 108849. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Sha, Z.; Wang, H.; Miao, Y.; Niu, X.; Chen, R.; Huang, J.; Huang, H.; Wei, K.; Zhu, R. Taishan Pinus massoniana pollen polysaccharide inhibits H9N2 subtype influenza virus infection both in vitro and in vivo. Vet. Microbiol. 2020, 248, 108803. [Google Scholar] [CrossRef] [PubMed]
- Chepngeno, J.; Takanashi, S.; Diaz, A.; Michael, H.; Paim, F.C.; Rahe, M.C.; Hayes, J.R.; Baker, C.; Marthaler, D.; Saif, L.J.; et al. Comparative Sequence Analysis of Historic and Current Porcine Rotavirus C Strains and Their Pathogenesis in 3-Day-Old and 3-Week-Old Piglets. Front. Microbiol. 2020, 11, 780. [Google Scholar] [CrossRef]
- Yim, S.K.; Kim, K.; Kim, I.H.; Chun, S.H.; Oh, T.H.; Kim, J.U.; Kim, J.W.; Jung, W.H.; Moon, H.S.; Ku, B.S.; et al. Inhibition of SARS-CoV-2 Virus Entry by the Crude Polysaccharides of Seaweeds and Abalone Viscera In Vitro. Mar. Drugs. 2021, 19, 219. [Google Scholar] [CrossRef]
- Tang, F.; Huang, G.; Lin, L.; Yin, H.; Shao, L.; Xu, R.; Cui, X. Anti-HBV Activities of Polysaccharides from Thais clavigera (Kuster) by In Vitro and In Vivo Study. Mar. Drugs. 2021, 19, 195. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Zhou, H.; Zeng, L.; Chen, T.; Chen, Q.; Zhou, B.; Wang, Y.; Chen, Q.; Hu, P.; et al. Radix isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro. Molecules 2017, 22, 116. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, S.; Zhang, L.; Niu, G.; Zhang, X.; Yang, L.; Ji, W.; Ren, L. Coinfection of Porcine Circovirus 2 and Pseudorabies Virus Enhances Immunosuppression and Inflammation through NF-kappaB, JAK/STAT, MAPK, and NLRP3 Pathways. Int. J. Mol. Sci. 2022, 23, 4469. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; You, X.; Pei, C.; Wang, Z.; Jiao, S.; Zhao, X.; Lin, X.; Lu, Y.; Jin, C.; et al. Novel Insights Into the Sulfated Glucuronic Acid-Based Anti-SARS-CoV-2 Mechanism of Exopolysaccharides From Halophilic Archaeon Haloarcula hispanica. Front. Chem. 2022, 10, 871509. [Google Scholar] [CrossRef]
- Yan, L.M.; Lau, S.P.N.; Poh, C.M.; Chan, V.S.F.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Heterosubtypic Protection Induced by a Live Attenuated Influenza Virus Vaccine Expressing Galactose-alpha-1,3-Galactose Epitopes in Infected Cells. mBio 2020, 11, 10–128. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Li, Y.; Li, T.; Cao, J.; Guan, Z.; Xu, T.; Jia, G.; Ma, G.; Zhao, R. Portulaca oleracea L. Polysaccharide Inhibits Porcine Rotavirus In Vitro. Animals 2023, 13, 2306. https://doi.org/10.3390/ani13142306
Zhou X, Li Y, Li T, Cao J, Guan Z, Xu T, Jia G, Ma G, Zhao R. Portulaca oleracea L. Polysaccharide Inhibits Porcine Rotavirus In Vitro. Animals. 2023; 13(14):2306. https://doi.org/10.3390/ani13142306
Chicago/Turabian StyleZhou, Xiechen, Yan Li, Tao Li, Junyang Cao, Zijian Guan, Tianlong Xu, Guiyan Jia, Gaopeng Ma, and Rui Zhao. 2023. "Portulaca oleracea L. Polysaccharide Inhibits Porcine Rotavirus In Vitro" Animals 13, no. 14: 2306. https://doi.org/10.3390/ani13142306




