Simple Summary
In recent years, due to industry and market preferences, local animal breeds have been exposed to genetic erosion and extinction risk. Effective strategies for their recovery and conservation are needed. Gentile di Puglia (GdP) is an autochthonous sheep breed, typical of Southern Italy, with an aptitude for wool, meat, and milk production and considerable historical and cultural value. The development of a GdP gamete and embryo cryobank could help to support the numerical reimplementation of this population. Animal germplasm conservation is mainly performed through sperm cryopreservation, whereas strategies on the female side are developed in a limited way. In this study, the dual purpose of monitoring the reproductive efficiency in one pilot GdP farm in the Apulia region and setting up a cryopreservation protocol, by vitrification, of immature cumulus-oocyte complexes (COCs) recovered from pre-pubertal lambs, followed by an analysis of their in vitro maturation potential and bioenergetic-oxidative status, was pursued. The results indicated that traditional reproductive management leads to progressive offspring reduction and that ex situ biotechnological conservation strategies, through immature oocyte vitrification and in vitro maturation, can support in situ conservation, leading to in vitro embryo production and transfer.
Abstract
Gentile di Puglia (GdP) is an autochthonous sheep breed of Southern Italy included among ovine breeds threatened by genetic erosion and extinction risk, which have been given attention by local and international institutions, thus emphasizing the need for germplasm conservation actions. In the present study, two assisted reproduction approaches, finalized for GdP conservation, were performed: (1) on-farm reproductive efficiency evaluation, expressed as pregnancy rate (PR), twin pregnancy rate (tPR), and body condition score (BCS), for three consecutive breeding cycles and (2) pre-pubertal lambs’ immature cumulus–oocyte complex (COC) retrieval, vitrification, in vitro maturation (IVM), and assessment of meiotic stage and bioenergetic-oxidative status compared with those of other Italian and European commercial breeds. PR and tPR were progressively reduced over time. In all clinical examination times, BCS was significantly lower in nonpregnant ewes compared with pregnant ones. Fresh GdP pre-pubertal lamb COCs achieved meiotic maturation and showed healthy bioenergetic–oxidative status after IVM. Vitrification reduced the oocyte maturation rate in all groups. However, mature oocytes retained their cytoplasmic maturity, expressed as a mitochondria distribution pattern and activity, indicating promising developmental competence. In conclusion, clinical- and biotechnological-assisted reproduction approaches can support conservation strategies of GdP and other local sheep breeds in Southern Italy.
1. Introduction
At a global level, many local breeds are at risk of genetic erosion and extinction due to their very localized distribution, replacement with more productive commercial breeds, and high inbreeding rates [1]. In Southern Italy, the rearing of local breeds is strongly linked to the territory for gastronomic, historical, and cultural aspects. This provides inimitable typical foods and leads to emerging and broad industry and market outcomes [2]. However, current agricultural policies tend to not effectively support rearing programs of local breeds, and farmers are turning their interest to more productive breeds, including those from other countries. This has led to a significant reduction in animal number with consequent genetic erosion and, in some cases, to the risk of extinction with a negative impact not only on typical local products that characterize and qualify the regional agro-farming activities but also and mainly on the ecosystemic services that the breeding activities provide to the communities, particularly in marginal areas.
Gentile di Puglia (GdP), literally “Gentle Apulian” because of its fine wool, is an autochthonous sheep breed of millenary origins on the Southern Italy territory, mainly bred in the area of Foggia (Tavoliere delle Puglie and Monti Dauni) [2,3]. GdP is characterized by its aptitude for wool, meat, and milk production and has been shown to be genetically adapted and resilient to the environmental conditions of marginal areas where it is normally reared [4]. In the last 50 years, due to the wool market crisis and indiscriminate crossbreeding, a significant numerical contraction has been observed in this local ovine breed [3]. Indeed, the last census of GdP population for the year 2022, carried out by ASSONAPA (Associazione Nazionale della Pastorizia; Italian Pastoral Farming Association), reported that the number of heads of this breed is very limited in Italy, consisting of approximately 4000 animals, including about 250 rams and 3500 ewes (P. Fresi, personal communication). Therefore, GdP needs to be preserved for the productive, historical, and cultural value that it represents for the Southern Italy community and worldwide biodiversity.
The conservation of genetic animal resources can be performed in situ and ex situ, depending on whether animals are kept within their natural environments or production systems [5]. As an in situ approach, pregnancy diagnosis (PD) is a key aspect of flock management. PD during early gestation allows sheep breeders to make important economic decisions. These include identifying nonpregnant or sick ewes for treatment, rebreeding or culling, and improving the nutritional plane of pregnant ones to optimize offspring weights, prevent pregnancy toxemia, and increase milk production [6]. Transabdominal B-mode ultrasound is a quick, non-invasive, and accurate reproductive technology used in small ruminants for PD, determination of fetal number and viability, and detection of pathological condition [7]. Ex situ strategies can be divided into in vivo and in vitro, depending on whether the animal germplasm is kept in the form of live animals or cryopreserved through a gene-banking strategy [5]. By setting up a cryobank, it is possible to collect and cryopreserve different kinds of cells and tissues, such as semen, oocytes, embryos, ovarian/testicular tissue, somatic, stem, and induced pluripotent stem cells, and, thanks to the advancements in reproductive biotechnologies, it is possible to obtain live animals from these cells in different times and places [1,8,9]. Gene banking of animal genetic resources is a strategic priority of the Global Plan of Action for Animal Genetic Resources, which was developed and adopted by the Food and Agriculture Organization (FAO) Member Nations (2007), and of the Sustainable Development Goals (SDGs), which were adopted by the United Nations (UN) (2015) with the goal of achievement by 2030, specifically under Target 2.5 of maintaining the biodiversity of plants and animals. In Italy, there is not yet a national animal genetic resources gene bank “https://www.eugena-erfp.net/en/ (accessed on 7 June 2023)”, and the set-up of these initiatives is still at an initial stage and localized at the regional level. In 2023, the Animal Germplasm Cryobank created by the Institute of Agricultural Biology and Biotechnology of the National Research Council (IBBA-CNR), in collaboration with the Department of Veterinary Medicine and Animal Sciences of the University of Milan (DIVAS-UNIMI), has been registered to the Italian National Registry of biodiversity for agriculture and food, as an ex situ conservation center and/or Germplasm Banks for genetic resources, through Ministerial Decree No. 207219, dated 17 April 2023. Regarding Apulia, regional law n.39 was approved in 2013 with the aim of conserving zootechnical autochthonous genetic resources that are threatened by genetic erosion or risk of extinction and for which environmental, cultural, scientific, and economic interests exist. In this context, the proposal for a cryobank of GdP genetic resources is innovative, as it meets the need to seek effective strategies for the recovery, conservation, and use of genetic resources of native animal breeds aiming to obtain sustainable products of intra- and extra-regional interest with technologies that guarantee quality, traceability, and safety.
Oocyte cryopreservation offers the possibility of storing and spreading female germplasm from endangered breeds and individuals of great value. This is a versatile tool, in combination with selected semen samples, allowing offspring to be created that will fill a current need at the time of use [9,10]. Female gametes can be retrieved from live animals or isolated ovaries after slaughter or unexpected death. Slow freezing and vitrification are the two main techniques developed for oocyte cryopreservation. However, the success rate is still challenging because of the large oocyte size, low surface-to-volume ratio, cytoskeleton structure, lipid content, and meiosis stage [9,10].
The cryopreservation of immature oocytes at the germinal vesicle (GV) stage allows the obtainment of a significant number of female gametes without hormonal stimulation or in vitro maturation (IVM) right after collection. Moreover, through the vitrification procedure, it is possible to preserve them directly in farms located in marginal areas, where equipped laboratories are too far or not available. Immature oocytes are usually cryopreserved as cumulus–oocyte complexes (COCs) because cumulus cells (CCs) are crucial for oocyte meiotic resumption and developmental competence [11]. However, IVM is challenging for cryopreserved immature COCs due to membrane damage to CCs [12] and their physical/functional detachment from the oocyte, caused by transzonal projections’ sensitivity to cryoprotectants and low temperatures [12,13]. In the last 20 years, the vitrification/warming of immature COCs from adult subjects has been applied to several domestic large (sheep [12,14,15,16,17,18,19,20,21,22], cow [23,24,25,26,27], buffalo [28,29,30], goat [31], pig [32,33,34,35], horse [36,37,38,39,40,41], donkey [42]) and small (cat [43,44,45,46,47,48], dog [49]) animal species, with various results, depending on intrinsic species-specific oocyte features. Although the results of IVM, embryo development, and blastocyst rate following immature COC vitrification are still less satisfactory compared to fresh COCs, live births have been reported in bovine [50], swine [33], equine [38,41], and domestic feline [43] species.
Immature COC vitrification from pre-pubertal animals offers additional interesting features. In Southern Italy, the high consumption of lamb meat, for traditional and cultural reasons, allows germplasm to be obtained and recovered, which would otherwise be discarded, from a significant quantity of slaughtered pre-pubertal ovaries. Moreover, the use of this kind of ovaries allows the generation interval to be reduced, the rate of genetic gain to be increased, and more COCs to be obtained than from adult ewe ovaries [51,52]. To the best of our knowledge, there is only one study focusing on immature COC vitrification using pre-pubertal lamb ovaries [53] and only one aimed at local breed conservation in the autochthonous Vietnamese Ban Pig breed [54].
According to the previous considerations, since the use of high-quality germplasm is closely linked with the tracking of productive and reproductive data, the first aim of the present study was to monitor the reproductive efficiency of a GdP farm with a traditional breeding system. Then, with the aim of developing a cryobank of GdP germplasm, in vitro reproductive potential and cryotolerance after the vitrification of pre-pubertal immature COCs from a GdP local sheep breed, assessed in terms of the maturation rate and bioenergetic-oxidative status, was investigated. Data were compared with those obtained from oocytes recovered from Italian and European commercial-breed lamb ovaries.
2. Materials and Methods
2.1. Routine Veterinary Checkups
On-Farm Evaluation of Reproductive Efficiency
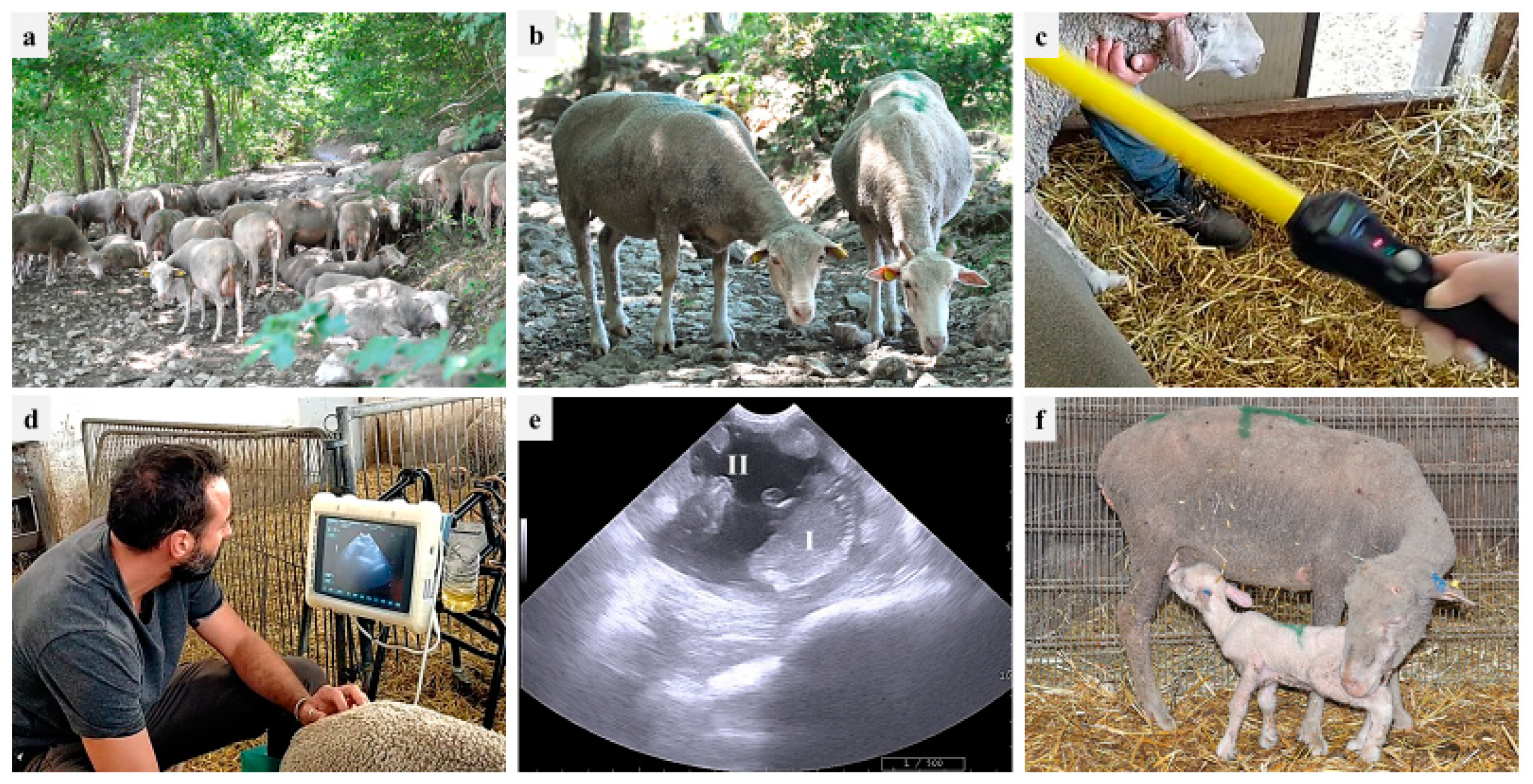
During 2021 and 2022, PDs were performed in one farm (partner of the PhD Program 2020 FSC—Piano Stralcio “R&I 2015–2017”, University of Bari Aldo Moro, Italy), located in the area of Monti Dauni (Southern Italy), a place with a historical vocation for GdP-breeding activities. The farm was established in the 20th century, and about six hundred GdP sheep, including around 20 rams, are kept through family-based traditional breeding systems (pasture plus barn-concentrate integration, if needed). The owner reported that, besides the male effect, no assisted reproductive technologies (synchronization, artificial insemination, PD) are implemented in the farm. According to traditional local consumption, 60-day-old lambs are sold three times per year: (i) Easter time, (ii) mid-August, and (iii) Christmas time. Therefore, breeding cycles are scheduled in September, January, and May, respectively. PDs were performed using an 8–10 MHz convex probe (MyLab, Esaote ultrasound device), starting from 40 days after the removal of rams from the ewe flock. The transabdominal approach was used: ewes were restrained in stanchions similar to cattle chutes with headgates, and the probe was positioned at the right inguinal level, near the base of the udder. The observations of a fluid-filled uterus with placentomes and at least one fetus were considered positive signs of pregnancy [7,55]. Figure 1 shows GdP ewes during the different stages of the PD procedure and lambs. In addition, the ewes’ body condition score (BCS) was evaluated (through the manual palpation at the lumbar vertebrae) in pregnant and nonpregnant ewes, providing a score from 0 to 5 and considering 2.75–3 as the ideal value [56].
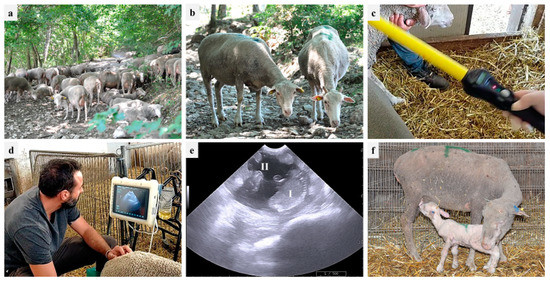
Figure 1.
On-farm reproductive monitoring in one Gentile di Puglia (GdP) flock: (a) flock in the pasture; (b) two GdP ewes; (c) ruminal bolus reading; (d) transabdominal ultrasound; (e) pregnancy diagnosis based on fetus (I) and cotyledon (II) identification; (f) suckling GdP lamb.
2.2. In Vitro Study
2.2.1. Chemicals
All chemicals for in vitro cultures and analyses were purchased from Sigma-Aldrich (Milan, Italy), unless otherwise indicated.
2.2.2. Collection of Ovaries and COC Retrieval
Ovaries from pre-pubertal lambs (less than 6 months of age) were recovered at local slaughterhouses from animals subjected to routine veterinary inspection in accordance with the specific health requirements stated in Council Directive 89/556/ECC and subsequent modifications. Ovaries were transported to the laboratory at room temperature within 4 h of collection. For COC retrieval, ovaries underwent the slicing procedure [57]. The follicular contents were released in sterile Petri dishes containing phosphate-buffered saline (PBS) and observed under a Nikon SMZ18 stereomicroscope equipped with a transparent heating stage set up at 38.5 °C (Okolab S.r.l., Napoli, Italy). Only COCs with at least three intact cumulus cell layers and homogenous cytoplasm were selected for culturing [58].
2.2.3. Vitrification and Warming Procedures
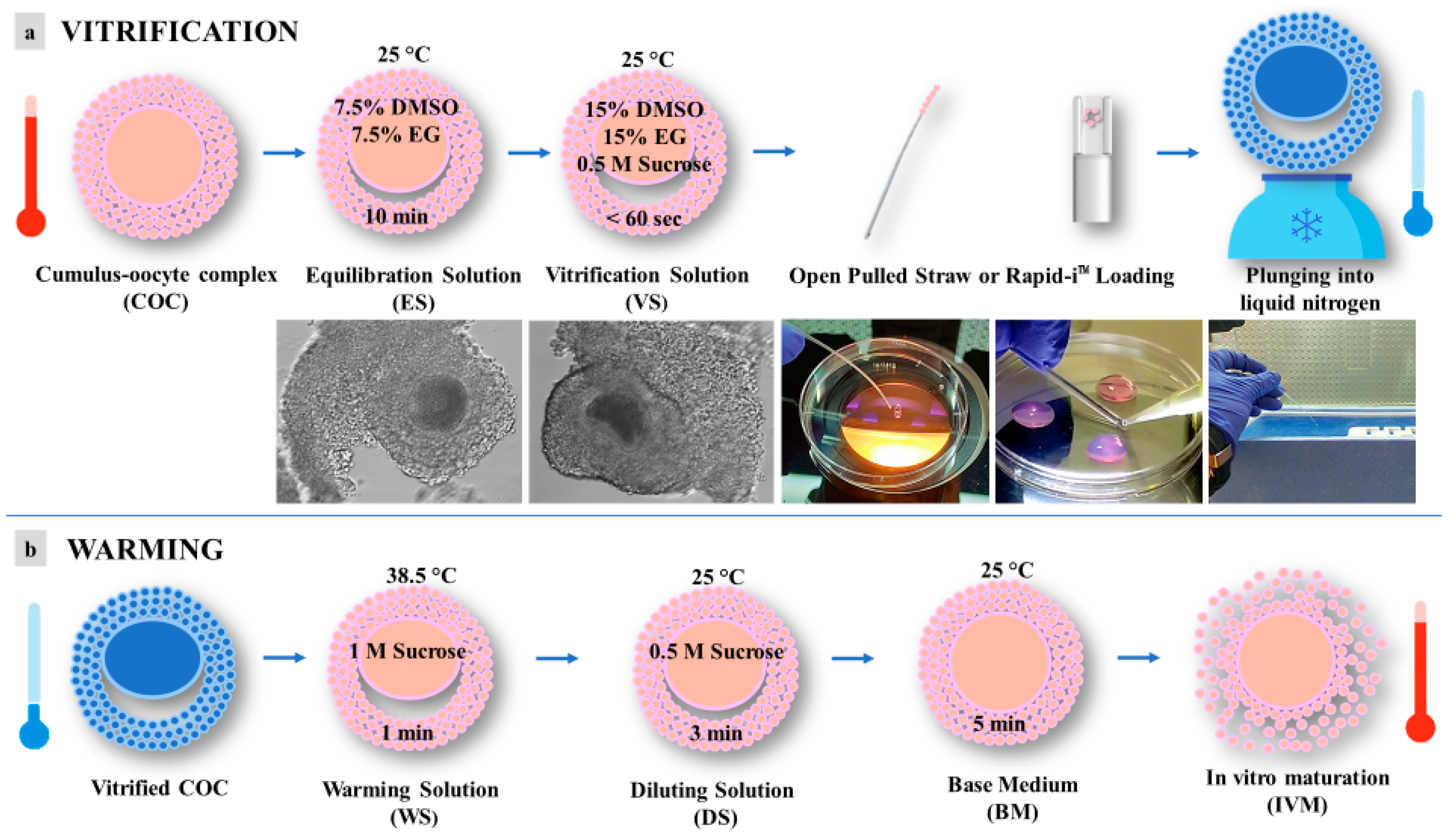
Vitrification and warming were performed according to the procedures reported by dos Santos-Neto et al., 2020 [21], with some modifications (Figure 2). In order to counteract cryoprotectant toxicity, all vitrification media were used at room temperature, except for the warming solution, which was used at 38.5 °C. Briefly, selected immature COCs, in groups of 5, were incubated for 10 min in 300 μL drops of equilibration solution (ES) containing 7.5% (v/v) ethylene glycol (EG) and 7.5% (v/v) dimethyl sulfoxide (DMSO) and dissolved in base medium (BM), containing 20% (v/v) fetal calf serum (FCS) added to Hepes-buffered TCM 199. After equilibration, the oocytes were placed into 300 μL drops of vitrification solution (VS) containing 15% (v/v) EG, 15% (v/v) DMSO, and 0.5 mol/L sucrose dissolved in BM. Oocyte vitrification was performed in two steps/drops in less than 60 s. After that, oocytes were loaded into an Open Pulled Straw (OPS) (Minitube) or onto a Rapid-i™ Kit (Vitrolife) with a minimum volume (e.g., <0.1 μL) and plunged quickly into liquid nitrogen. Warming was performed by submerging the vitrification device directly into warming solution (WS) containing 1 mol/L sucrose dissolved in BM at 38.5 °C for 1 min. Warmed oocytes were transferred to a 300 μL drop of dilution solution (DS) with BM plus sucrose 0.5 mol/L for 3 min and then washed twice in 300 μL drops of BM for 5 min.
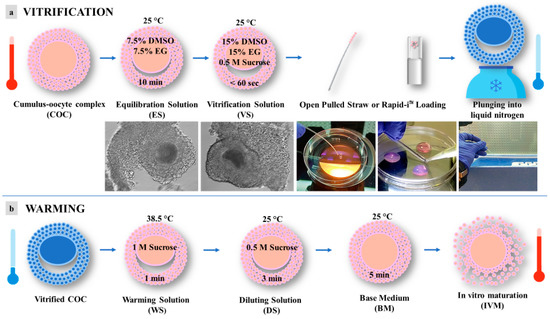
Figure 2.
Schematic representation of vitrification protocol for pre-pubertal lambs’ immature cumulus-oocyte complexes (COCs): (a) vitrification; (b) warming. DMSO = dimethyl sulfoxide; EG = ethylene glycol.
2.2.4. In Vitro Maturation (IVM)
IVM medium was prepared based on TCM-199 medium with Earle’s salts. It was buffered with 5.87 mmol/L HEPES and 33.09 mmol/L sodium bicarbonate and supplemented with 0.1 g/L L-glutamine, 2.27 mmol/L sodium pyruvate, calcium lactate pentahydrate (1.62 mmol/L Ca2+, 3.9 mmol/L Lactate), 50 μg/mL gentamicin, 20% (v/v) fetal calf serum (FCS), 10 μg/mL of porcine follicle stimulating hormone and luteinizing hormone (FSH/LH; Pluset®, Calier, Balcellona, Spain) [59], and 1 μg/mL 17β estradiol [57]. IVM medium was pre-equilibrated for 1 h under 5% CO2 in air at 38.5 °C, then loaded (400 μL/well) in a 4-well dish (Nunc Intermed, Roskilde, Denmark) and covered with pre-equilibrated lightweight paraffin oil. In each experiment, 20–25 COCs/well were added to a 4-well dish and cultured for 22–24 h at 38.5 °C under 5% CO2 in air.
2.2.5. Assessment of Cumulus Expansion and Oocyte Denuding
After IVM, COCs were recovered, and cumulus expansion was checked. COCs showing cumuli with continuous edges, consisting of cells in close contact each other, were classified as compact, whereas cumuli showing discontinuous edges following cell detachment and the production of a viscous extracellular matrix were classified as expanded. Even though the cumulus expansion does not fully ensure that maturation is achieved, the percentage of expanded COCs was recorded because it represents the response of immature COCs to the presence of gonadotropins in the culture medium. For oocyte denuding, COCs underwent cumulus cell removal by incubation in TCM-199 with 20% FCS containing 80 IU hyaluronidase/mL and aspiration in and out of finely drawn glass pipettes. Denuded oocytes were evaluated for their meiotic stage, and mature ones were used to assess bioenergetic/oxidative status.
2.2.6. Oocyte Mitochondria and ROS Staining
Oocytes were washed three times in PBS with 3% BSA and incubated for 30 min in the same medium containing 280 nmol/L MitoTracker Orange CMTM Ros (Thermo Fisher Scientific, Waltham, MA, USA) at 38.5 °C under 5% CO2 in air. After incubation with MitoTracker, oocytes were washed in PBS with 0.3% BSA and incubated for 15 min, at 38.5 °C under 5% CO2, in air in the same medium containing 10 µmol/L 2, 7—dichlorodihydrofluorescein diacetate (H2DCF-DA) to detect the dichlorofluorescein (DCF) and localize intracellular sources of ROS [60]. After incubation, oocytes were washed in PBS without BSA and fixed overnight at 4 °C in 4% paraformaldehyde (PFA) solution in PBS [61]. Particular attention was applied to avoid sample exposure to the light during staining and fixing procedures and to reduce photobleaching.
2.2.7. Oocyte Nuclear Chromatin Evaluation
To evaluate oocytes’ nuclear chromatin, after the fixation in 4% PFA in PBS, oocytes were stained with 2.5 µg/mL Hoechst 33258 in 3:1 (v/v) glycerol/PBS mounted on microscope slides with coverslips, sealed with nail polish, and kept at 4 °C in the dark until observation. Slides were examined under the epifluorescence microscope (Nikon Eclipse 600; Nikon Instruments, Firenze; ×400 magnification) equipped with a B-2A (346 nm excitation/460 nm emission) filter. Oocytes were evaluated in relation to their meiotic stage and classified as germinal vesicle (GV), metaphase to telophase I (MI to TI), and MII with the first polar body (PB) extruded [62]. Oocytes showing either multipolar meiotic spindle, irregular chromatin clumps, or the absence of chromatin were considered abnormal [63].
2.2.8. Assessment of Mitochondrial Distribution Pattern and Intracellular ROS Localization
Oocytes at the MII stage were observed at ×600 magnification in oil immersion with a Nikon C1/TE2000-U laser scanning confocal microscope (Nikon Instruments, Firenze, Italy). A 543 nm helium/neon laser and a G-2A filter were used to detect the MitoTracker Orange CMTM Ros (551 nm excitation and 576 nm emission). A 488 nm argon ion laser and a B-2A filter were used to detect DCF (495 nm excitation and 519 nm emission). Scanning was conducted with 25 optical sections from the top to the bottom of the oocytes, with a step size of 0.45 µm to allow for 3D distribution analysis. The mitochondrial distribution pattern was evaluated on the basis of previous studies: (1) finely granular, with small mitochondria aggregates spread throughout the cytoplasm, typical of immature oocytes; (2) perinuclear and subcortical (P/S) distribution of mitochondria forming large granules, which is an indicator of cytoplasmic maturity; and (3) abnormal, with irregular distribution of mitochondria [61]. Concerning intracellular ROS localization, oocytes with intracellular ROS distributed throughout the cytoplasm, together with areas/sites of mitochondria/ROS overlapping, were considered healthy.
2.2.9. Quantification of Bioenergetic/Oxidative Parameters
In each individual oocyte, MitoTracker and DCF fluorescence intensities were measured at the equatorial plane and at the excitation/emission, as described above, using the EZ-C1 Gold Version 3.70 image analysis software platform for Nikon C1 confocal microscope. A circular area was drawn in order to measure only the region including cell cytoplasm. The fluorescence intensity within the programmed scan area was recorded and plotted against the conventional pixel unit scale (0–255). Mitochondrial activity and intracellular ROS levels were recorded as MitoTracker Orange CMTM Ros and DFC fluorescence intensity in arbitrary densitometric units (ADUs). Parameters related to fluorescence intensity, such as laser energy, signal detection (gain), and pinhole size, were maintained at constant values for all measurements. The degree of mitochondria/ROS colocalization, reported as a biomarker of healthy oocytes [61,62], was quantified by the overlap coefficient between MitoTraker Orange CMTM Ros and the DCF fluorescence intensity signals.
2.2.10. Statistical Analysis
The percentage of pregnant ewes and those carrying out twin pregnancies, in the three examined periods (July 2021, November 2021, and February 2022), were compared through the Chi-Square test. Unpaired Student’s t-test was performed to compare, at each time period, the BCS between pregnant and nonpregnant ewes. One-way ANOVA (followed by Tukey’s Multiple Comparison Test) was performed to compare the BCS in pregnant and nonpregnant ewes in the three time periods.
The proportions of oocytes showing different chromatin configurations and mitochondria distribution patterns were compared between groups using the Chi-square test. Mitochondria and ROS quantification analysis was conducted on oocytes at the MII stage. Data (mean ± standard deviation (s.d.) of bioenergetic parameters) were compared using the unpaired Student’s t-test or one-way analysis of variance ANOVA followed by Tukey’s Multiple Comparison Test, according to comparison groups. Differences with p < 0.05 were statistically significant.
3. Results
3.1. On-Farm Evaluation of Reproductive Efficiency
The pregnancy rate (PR), twin pregnancy rate (tPR), and BCS of ewes reared at the farm at different evaluation times are reported in Table 1. Between months, significant reductions were found in PR and tPR. The BCS was significantly lower in nonpregnant animals compared with pregnant ones, in all time groups. Moreover, it was significantly higher in the last examined period (February 2022), as compared to the first one (July 2021), in both the pregnant and the nonpregnant groups.

Table 1.
Clinical examinations in one Gentile di Puglia (GdP) flock reared in a pilot farm in the Apulia region: pregnancy rates and body condition scores (BCS).
3.2. GdP Pre-Pubertal Lamb COCs Achieve Meiotic Maturation after IVM
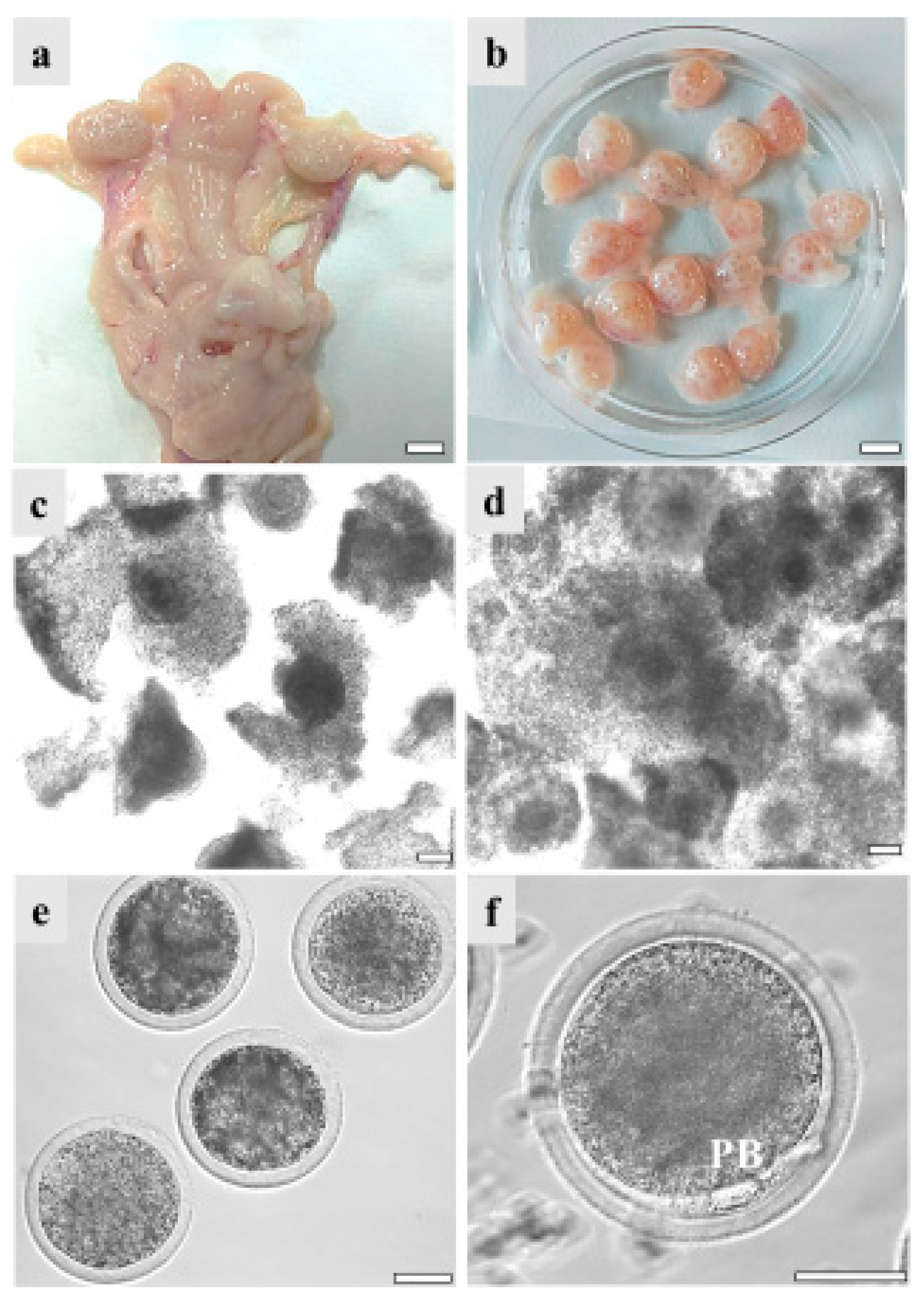
COCs recovered from the ovaries of GdP pre-pubertal lambs, reared and slaughtered in Apulia region, underwent IVM in order to evaluate their in vitro developmental potential. Data were compared with those of Italian (Comisana, Sardinian, and mixed breeds) and European (Merino and mixed breeds from Hungary and France) commercial cosmopolite sheep populations. Regardless of the sheep breed, pre-pubertal lamb ovary size ranged between about 0.5 and 1.5 cm showing follicles in different stages of growth, from 1 mm to approximately 5 mm. On the other hand, differences were found in the number of follicles and the COC recovery rate. Indeed, from GdP and Italian commercial breeds’ ovaries, it was possible to isolate around 20 good-quality COCs/ovary compared to 10 from the European ones. Pre-pubertal lamb COCs were analyzed in seven to eight independent IVM runs, followed by staining with Hoechst 33258 for nuclear chromatin evaluation. GdP COCs achieved cumulus expansion at significantly higher rates compared with other breeds (p < 0.05 and p < 0.001 with European and Italian populations, respectively; Table 2). Moreover, they achieved significantly higher maturation rates, showing the second metaphase plate and the first PB extruded, in comparison with the other sheep populations (p < 0.05 and p < 0.001 for European and Italian, respectively). Correspondingly, the percentage of oocytes that remained arrested at the GV stage was significantly reduced in GdP compared with other breeds (p < 0.001 and p < 0.00001, for European and Italian, respectively). Figure 3 shows specimens of the reproductive system of a GdP pre-pubertal lamb: a female reproductive tract with the two uterine horns, oviducts, and ovaries; ovaries showing several developing follicles isolated for COC retrieval; COCs with compact or expanded cumulus observed before and after 22–24 h IVM under inverted phase contrast microscopy; and denuded oocytes and a matured oocyte with the first PB extruded in the perivitelline space.

Table 2.
Nuclear and cytoplasmic parameters of Gentile di Puglia (GdP) pre-pubertal lamb oocytes after in vitro maturation (IVM) compared with commercial sheep breeds.
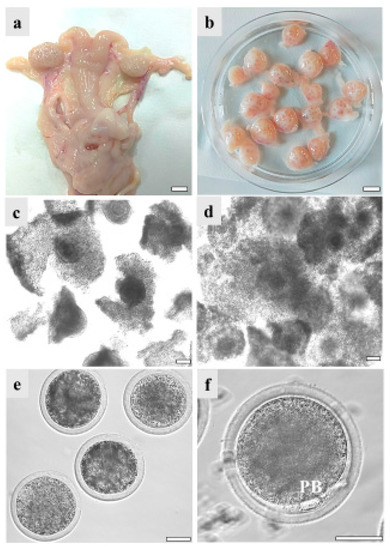
Figure 3.
GdP female reproductive system specimens: (a) female reproductive tract with the two uterine horns, oviducts, and ovaries. Scale bar represents 1 cm; (b) ovaries isolated for cumulus–oocyte complex (COC) retrieval. Scale bar represents 1 cm; (c) COCs with compact or (d) expanded cumuli observed under inverted-phase contrast microscopy before and after 22–24 h IVM. Scale bars represent 40 μm; (e) denuded oocytes. Scale bar represents 40 μm. (f) Matured oocyte with the first polar body (PB) extruded. Scale bar represents 40 μm.
3.3. GdP Pre-Pubertal Lamb MII Oocytes Show Healthy Bioenergetic/Oxidative Status after IVM
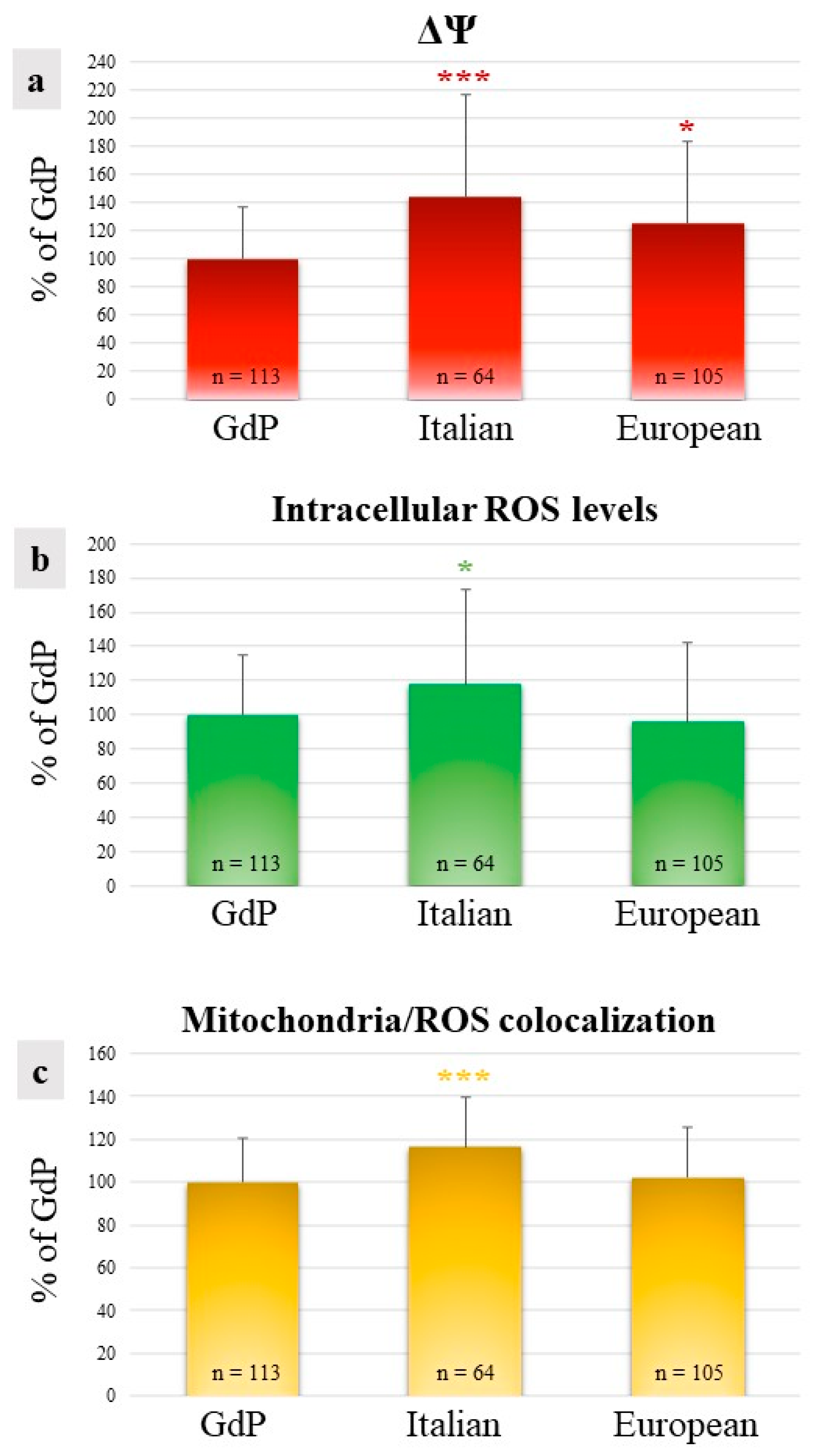
In GdP MII oocytes obtained after IVM, qualitative and quantitative parameters of the bioenergetic/oxidative status were analyzed as a measure of oocytes’ cytoplasmic maturity and competence to undergo fertilization and development. The percentages of MII oocytes showing a heterogeneous perinuclear and subcortical mitochondrial distribution pattern (P/S) did not vary among sheep population, indicating that a good rate of GdP oocytes, like those of other sheep populations, reached cytoplasmic maturity in the culture system used (Table 2). The mitochondrial membrane potential (ΔΨ) in GdP MII oocytes was significantly lower in comparison to other sheep populations (p < 0.05 and p < 0.001 for European and Italian, respectively; Figure 4a). Intracellular ROS levels and the overlap coefficient, indicating mitochondria/ROS colocalization, were lower in GdP MII oocytes compared with Italian ones (p < 0.05 and p < 0.001 for ROS levels and overlap coefficient, respectively; Figure 4b,c) whereas no differences were found compared with oocytes of the European sheep population.
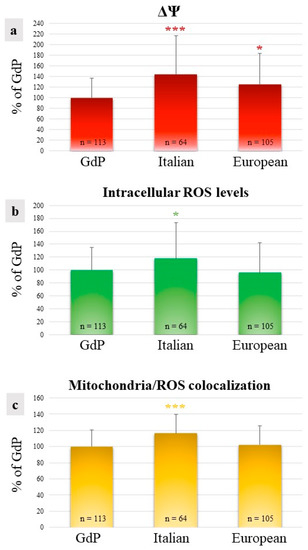
Figure 4.
Quantification data of (a) mitochondrial membrane potential (ΔΨ), (b) intracellular reactive oxygen species (ROS) levels, and (c) mitochondria/ROS colocalization in GdP MII oocytes compared with Italian and European ones. Values are presented as percentages of the signal of GdP samples. Means ± SD of fluorescence intensity of the MitoTracker Orange CMTM Ros, DCF, and overlap coefficient are presented. The numbers of analyzed oocytes per sheep population are indicated at the bottom of each bar. One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test: * p < 0.05; *** p < 0.001.
3.4. Vitrification of GdP Pre-Pubertal Lambs’ Immature COCs Reduce Their Meiotic Maturation after IVM
With the aim of developing a germplasm cryobank, the effects of vitrification on GdP immature COCs were evaluated. Indeed, in biodiversity-conservation programs, the development of cryopreservation strategies using immature COCs represents the only method for female germplasm rescue when activities are performed in areas remote from an equipped reproductive biotechnology laboratory. GdP and other pre-pubertal lamb COCs, in six to ten independent runs, were cryopreserved by vitrification. Vitrified COCs were subsequently warmed and subjected to IVM. After culture, cumulus expansion and oocyte maturation rates were compared between the fresh and vitrified samples of each population group (GdP, Italian, and European). In all three sheep populations, the cumulus-expansion rate was significantly reduced upon vitrification (p < 0.00001 for all groups, between fresh and vitrified/warmed samples; Table 3). Figure 5 shows GdP COCs after vitrification/warming as observed before (Figure 5a,c) and after (Figure 5b,d) IVM. At warming, the majority of COCs displayed complete compact and multilayered cumulus. After IVM, the majority of GdP vitrified/warmed COCs underwent regular cumulus expansion even though some of them still showed compact or partially/completely removed cumulus. Moreover, cumulus cells maintained the integrity of their cytoplasmic protrusions, which are very important structures for cell-to-cell and cell-to-oocyte communications. In addition, for all sheep population groups, vitrified/warmed (V/W) COCs showed a significantly reduced maturation rate, and corresponding increased rates of oocytes remained at the GV stage (p < 0.00001; Table 3).

Table 3.
Nuclear and cytoplasmic parameters of Gentile di Puglia (GdP) pre-pubertal lamb vitrified/warmed immature cumulus–oocyte complexes (COCs) after IVM compared with commercial sheep breeds.
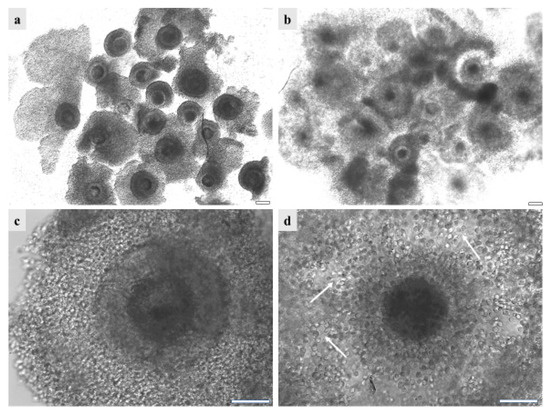
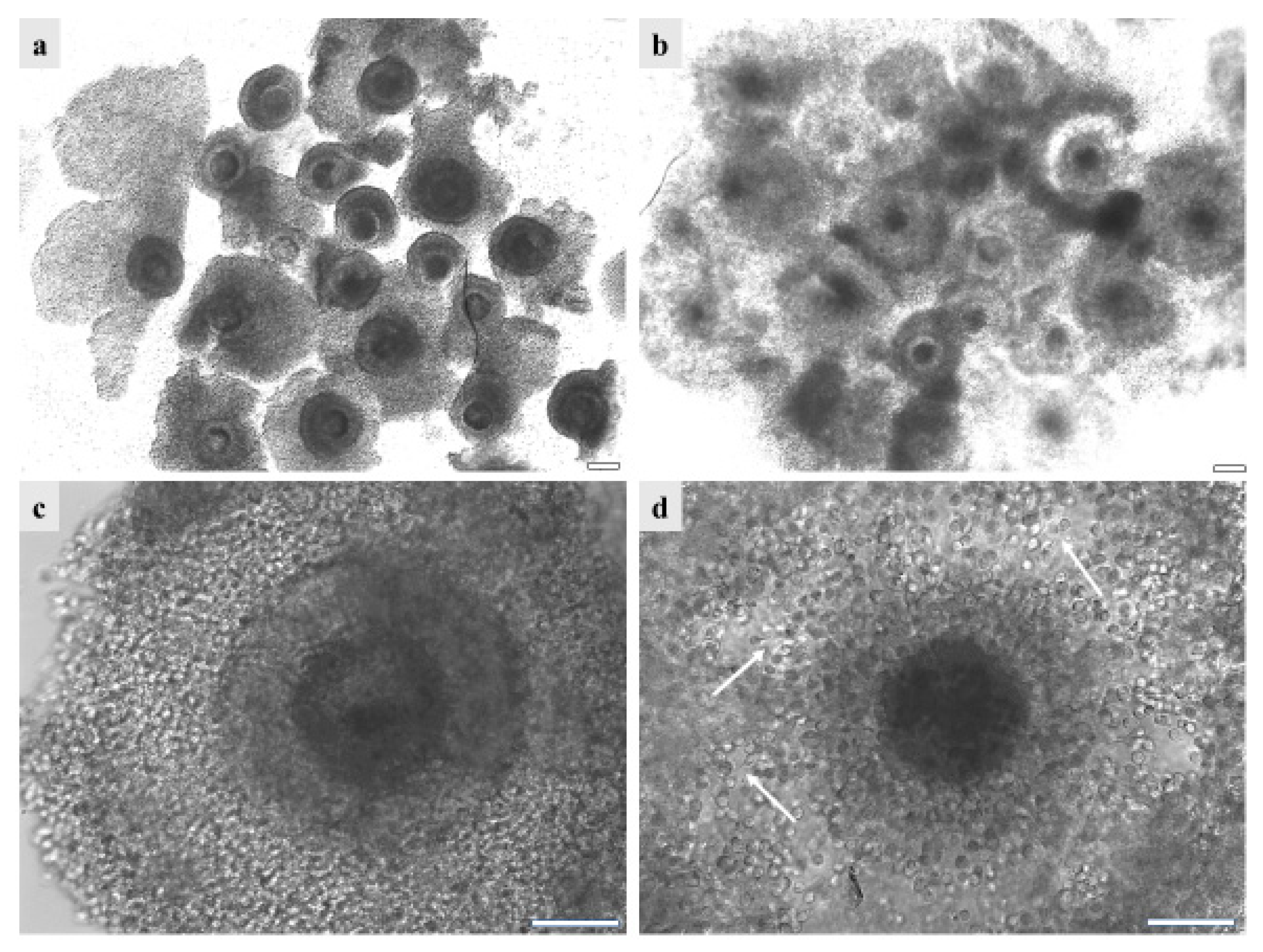
Figure 5.
GdP COCs after vitrification/warming as observed (a,c) before and (b,d) after IVM, (a,b) in groups or (c,d) individually. It can be seen that, before IVM, the majority of GdPs’ vitrified/warmed COCs show complete compact and multilayered cumulus. After IVM, the majority of GdP vitrified/warmed COCs show regular cumulus expansion, with individually visible cumulus cells and their cytoplasmic protrusions. Scale bars represent 40 µm. The arrows indicate the intact cytoplasmic protusions of cumulus cells, after COC vitrification/warming and IVM.
3.5. Vitrification of GdP Pre-Pubertal Lambs’ Immature COCs Does Not Affect Their Bioenergetic/Oxidative Status after IVM
The rate of GdP MII oocytes showing P/S mitochondrial distribution patterns did not differ between fresh and vitrified oocytes, indicating that oocyte cytoplasmic maturation was maintained after COC vitrification. This was also observed in oocytes of European breeds but not in Italian ones, in which this percentage was significantly lower (p < 0.05; Table 3). In COCs of all three sheep populations, bioenergetic/oxidative quantification parameters did not vary based on the vitrification procedure as no differences were observed for mitochondria activity, ROS levels, or overlap coefficient between fresh and vitrified oocytes. Figure 6 shows representative photomicrographs of GdP MII oocytes obtained after IVM of fresh and vitrified/warmed COCs and observed for nuclear chromatin, mitochondria pattern and activity, intracellular ROS localization and levels, and mitochondria/ROS colocalization (Figure 6a) and quantification analysis of the effects of vitrification/warming on oocyte bioenergetic/oxidative status (Figure 6b–d).
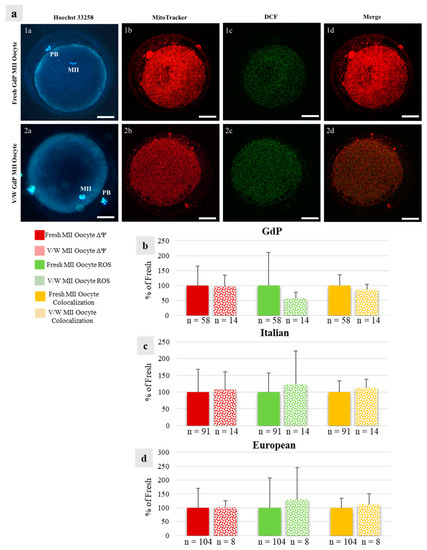
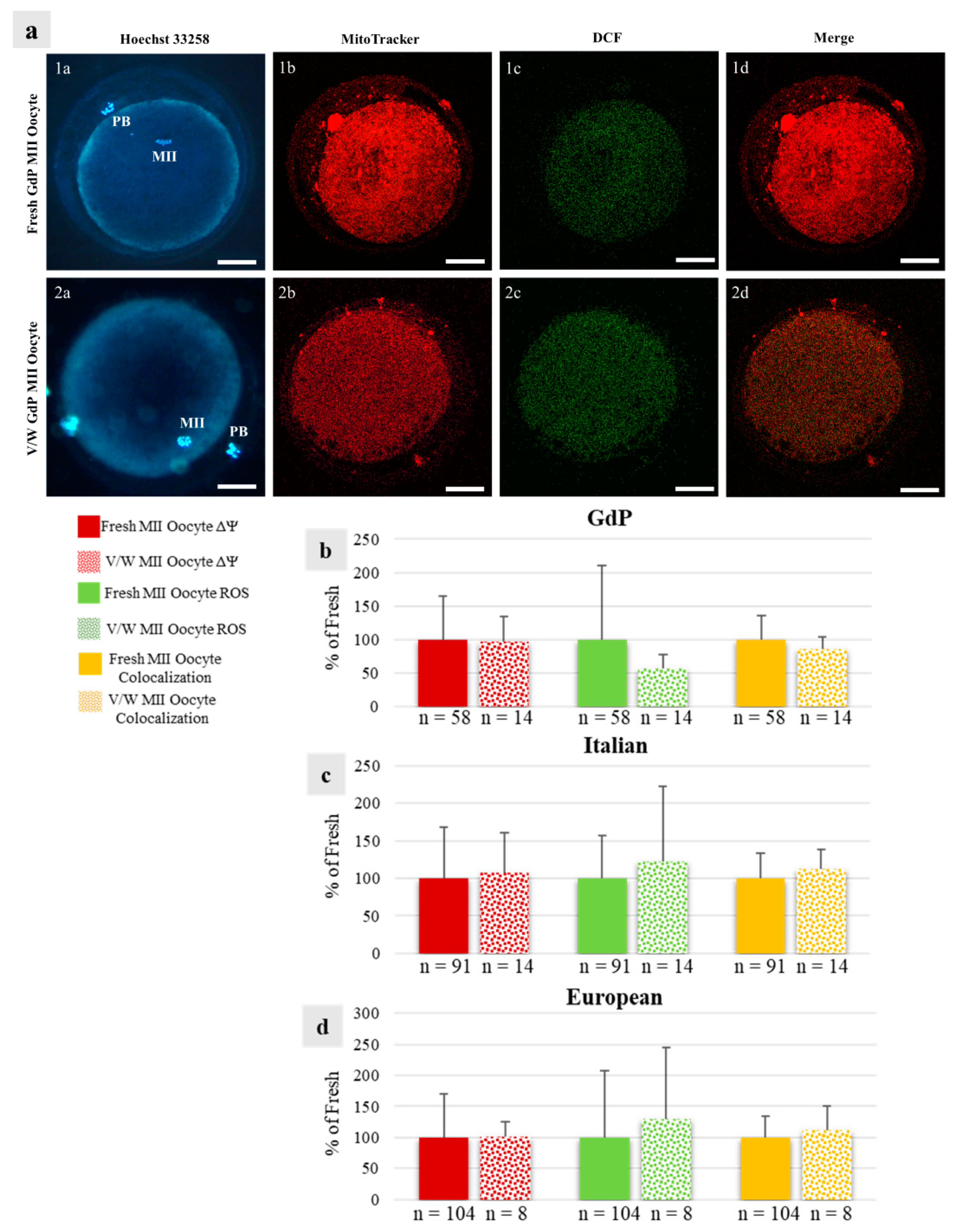
Figure 6.
Effects of immature COC vitrification on bioenergetic/oxidative parameters in GdP MII oocytes: (a) Photomicrographs showing representative images of one fresh (lane 1) and one vitrified/warmed (V/W) (lane 2) GdP oocyte. Corresponding epifluorescence images showing nuclear chromatin configuration (column a: Hoechst 33258) and confocal images showing the mitochondrial distribution pattern and activity (column b: MitoTracker Orange), intracellular ROS localization and levels (column c: H2DCF-DA), and mitochondria/ROS colocalization (column d: Merge). Confocal images were taken at the oocyte equatorial plane. Scale bars represent 40 µm. (b–d) Quantification data of mitochondrial activity (ΔΨ), intracellular reactive oxygen species (ROS) levels, and mitochondria/ROS colocalization in GdP MII oocytes, obtained after IVM of fresh and V/W COCs, compared with Italian and European ones. Within each sheep population, values of V/W oocytes are presented as a percentage of the signal of fresh samples. Means ± SD of fluorescence intensity of MitoTracker Orange CMTM Ros, DCF, and overlap coefficient are presented, respectively. The numbers of analyzed oocytes per sheep population are indicated at the bottom of each bar. Unpaired Student’s t-test: not significant.
4. Discussion
Local breeds are important for the area in which they are reared, combining their adaptation and resilience to the territory with the production of unique and inimitable typical products [4]. Moreover, autochthonous breeds represent the historical and cultural heritage of their territory and inhabitants [1]. However, these breeds are facing numeric reduction and are at risk of extinction. Too often, the reason for such a reduction is claimed to be low productivity. However, most of the time, it is the lack of selection programs and the traditional breeding system that impair the obtainment of higher production. The need to retrieve, preserve, and enhance local breeds goes through the application of genetic improvement and assisted reproductive technologies such as ultrasonography, livestock precision farming tools, and biotechnological approaches.
Ultrasonography (US) is a fast (less than one minute for ewes is needed), non-invasive tool that helps in the identification of reproductive failure with large advances compared with the traditional breeding system, where breeders notice the nonpregnant animal only at lambing. Therefore, US could help in the evaluation and optimization of animals’ reproductive performances, thus supporting animal production in marginal areas. Observed PRs are far from the ideal situation, where more than 85% of animals should be pregnant. Due to the problem of retrieving data about sheep reared under a traditional system, it is difficult to explain the reason for such low PRs. Some of the ewes could have been too old for breeding or were inserted into the mating group without a complete recovering after parturition. The use of livestock precision farming tools, such as a real-time database (e.g., SementusaTech®) would allow farmers to keep track and easily monitor the reproductive efficiency of each head of the flock using a smartphone [64]. The nutritional and health management of animals and BCS evaluation are key aspects of reproductive efficiency; hence, the lower BCS in nonpregnant ewes was an expected result. However, the overall improvement in the BCS from July to February, not followed by an improvement in the PRs or tPR, might indicate that other factors are affecting the reproductive efficiency of ewes.
The IVM culture of GdP COCs highlighted successful meiosis resumption and showed that nuclear and cytoplasmic maturation were acquired. Indeed, GdP COCs showed good cumulus expansion and maturation rates compared to the oocytes of commercial breeds from Italy or Europe. Moreover, no statistically significant difference was observed in the rate of matured oocytes exhibiting a P/S mitochondrial distribution pattern, indicating that in our culture, most MII oocytes reached cytoplasmic maturation, regardless of the sheep’s breed and origin. Interestingly, oocyte ΔΨ, ROS levels, and he overlap coefficient were shown to be lower in GdPs, usually reared under pasture-based farming systems, compared to commercial sheep breeds, mainly managed under semi-intensive practices with feeding based on industrial fodder and concentrates [4]. Some studies have investigated the correlation between oocyte bioenergetic–oxidative status and unbalanced nutritional intakes in animal models, showing that high-fat, high-fat/high-sugar, or low0protein dietary regimes could increase ROS production and alter oocytes’ mitochondrial membrane potential [65,66,67,68,69]. In conclusion, GdPs’ pre-pubertal lamb COCs showed promising in vitro reproductive potential. Further studies will be needed to evaluate how bioenergetic–oxidative status could influence embryos’ developmental competence for GdP oocytes.
In order to counteract genetic erosion and the possible extinction of this local breed of millenary tradition in the territory of Southern Italy, it is necessary to carry out and implement conservation strategies. According to the DAD-IS database by FAO, cryopreserved semen samples from GdP rams are available; however, there is a total absence of oocytes and embryos. Therefore, this study represents the first attempt to cryopreserve GdP oocytes, using female pre-pubertal lambs as donors, with the aim of developing a germplasm cryobank of this local sheep breed. Immature COC vitrification in endangered species conservation programs represents a versatile and strategic tool, allowing female germplasm to be recovered in large quantities, directly in farms and slaughterhouses located in marginal areas, where these animals are normally bred. Our data are in agreement with most previous studies in adult sheep in which it was found that the vitrification of immature COCs significantly affected cumulus expansion [14,15] and the oocyte maturation rate [12,14,15,17,20,70]. This result was associated with a statistically significant increase in the rate of oocytes arrested at the GV stage [12,14,15,20], regardless of the procedure used (conventional, direct, or on a solid surface) and the device (conventional straw, open pulled straw, cryotop, or cryoloop). Other studies refer to comparable, or even low, rates of matured oocytes between V/W and fresh COCs [22,71,72]. In line with these observations, we found no differences in the maturation rate after using OPS or Rapid-i vitrification devices, both in GdP samples and those of all examined ovine breeds. To date, only one study has reported on pre-pubertal lambs [53] in which the results were similar to those obtained in the present study. To the best of our knowledge, other studies in pre-pubertal subjects are reported only in pigs to date. In this species, V/W immature COCs from pre-pubertal gilts were reported to be significantly affected in their maturation rate after vitrification [33,73], even though later studies reported the fertilization, embryo development [74], and generation of live piglets [33] using V/W immature COCs. Finally, there is only one study on the vitrification of immature COCs applied to local breeds. This study was performed in the Vietnamese Ban Pig, and there was no statistically significant difference in the maturation rate observed after vitrification between the Ban Pig and commercially slaughtered hybrid pigs [54]. Vitrification is known to induce oxidative stress through ROS overproduction, membrane lipid peroxidation, amino acid and nucleic acid oxidation, gene expression alteration, and mitochondrial damage, resulting in cell apoptosis [75]. In our study, although the maturation rate was low after IVM of V/W immature COCs, compared with their fresh counterparts, the cytoplasmic maturation of MII oocytes, expressed as the P/S mitochondrial distribution pattern rate and mitochondria activity, ROS levels, and overlap coefficient quantifications, resulted in preserved COCs comparable to fresh ones. To the best of our knowledge, this is the first study in sheep evaluating cytoplasmic maturation, expressed as bioenergetic/oxidative status, in mature oocytes after the IVM of vitrified immature COCs. Further studies are needed to improve the vitrification procedure and/or IVM conditions with the aim of developing a cryobank of the local sheep breed GdP.
5. Conclusions
In conclusion, this study represents the first attempt to establish collaborations with Apulian farms that are located in marginal areas and still use the traditional management of reproductive activity for the autochthonous breed GdP, to push them to modern practices of assisted reproduction and in situ and ex situ conservation strategies. The proposal to monitor the reproductive efficiency on the farm provides the benefits of maintaining high levels of fertility and reproductive health in a GdP flock, and the proposal to develop a cryobank of female germplasm could contribute to maintaining and preserving GdP genetic diversity. Indeed, the present study showed that the ovaries of pre-pubertal lambs slaughtered for food purposes can be used to recover oocytes without interfering with productive and reproductive activities on the farm. After animal genotyping and oocyte vitrification, these cells can be used with fresh or cryopreserved GdP sperm for the in vitro production of embryos. Such strategies, in association with collecting, freezing, and using semen, could allow farmers to plan the maintenance or expansion of the number of animals with a controlled and reducible environmental impact. Flock monitoring and reproductive biotechnologies could also constitute the basis of genetic-improvement strategies in this local breed for its valuable products [76,77]. The following final objectives of the project were reached: (1) the realization of a GdP germplasm cryobank with a sustainable cost that is useful to contain its genetic erosion; (2) the training of reproductive clinicians and biotechnologists in the Apulia region capable of operating in technological, regulatory, and managerial aspects in the conservation of native livestock germplasm; and (3) the enhancement of Apulian livestock farms rearing the local sheep breed GdP.
Author Contributions
Conceptualization, L.T., D.M., G.M.L., E.C. and M.E.D.; Data curation, L.T., D.M. and A.M.; Formal analysis, L.T.; Funding acquisition, G.M.L., E.C. and M.E.D.; Investigation, L.T., D.M. and A.M.; Methodology, L.T., D.M., A.M., N.A.M. and S.C.; Project administration, M.E.D.; Resources, G.M.L. and M.E.D.; Supervision, S.C., G.M.L., E.C. and M.E.D.; Validation, L.T.; Visualization, L.T., D.M., N.A.M. and M.E.D.; Writing—original draft, L.T., D.M. and M.E.D.; Writing—review and editing, L.T., D.M., N.A.M., S.C., G.M.L., E.C. and M.E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: 1. Piano Stralcio “Ricerca e innovazione 2015–2017”—Asse “Capitale Umano” del Fondo per lo Sviluppo e la Coesione (FSC), a PhD program with restricted theme, internal areas/marginalized areas, a.a. 2020/2021, XXXVI cycle, the PhD Program “Functional and Applied Genomics and Proteomics”, University of Bari “Aldo Moro” (“DOT1302781”—Grant n.1), 2. “Zootechnical Biodiversity for Meat: Recovery and Enhancement (Acronym: BioZooCare)” Rural Development Program (PSR) PSR PUGLIA 2014–2022 Article 28 of Regulation (EU) no. 1305/2013, 3. “Biodiversity and enhancement of local sheep and goat genotypes with a prevalent aptitude for milk production (Acronym: LOCAL)” Rural Development Program PSR PUGLIA 2014-2022 Article 28 of Regulation (EU) no. 1305/2013.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the non-invasive methods (ultrasound scans) performed on the animals, as part of routine herd fertility checks (exemption Prot.n. 3037-III/13 CESA DiMeV UniBa).
Informed Consent Statement
Written informed consent about the use of fertility check data was obtained from the owner of the animals.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Francesco D’Innocenzio, “Masseria Salecchia” Azienda agrituristica, Bovino (Fg), Italy for his availability to collaborate at this project. We thank Pancrazio Fresi for providing us the updated number of GdP sheep in Italy registered to ASSONAPA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Woelders, H.; Windig, J.; Hiemstra, S.J. How developments in cryobiology, reproductive technologies and conservation genomics could shape gene banking strategies for (farm) animals. Reprod. Domest. Anim. 2012, 47 (Suppl. 4), 264–273. [Google Scholar] [CrossRef] [PubMed]
- Ciani, E.; Crepaldi, P.; Nicoloso, L.; Lasagna, E.; Sarti, F.M.; Moioli, B.; Napolitano, F.; Carta, A.; Usai, G.; D’Andrea, M.; et al. Genome-wide analysis of Italian sheep diversity reveals a strong geographic pattern and cryptic relationships between breeds. Anim. Genet. 2014, 45, 256–266. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Albenzio, M.; Sevi, A.; Ciampolini, R.; Cecchi, F.; Ciani, E.; Muscio, A. Genetic variability of the Gentile di Puglia sheep breed based on microsatellite polymorphism. J. Anim. Sci. 2009, 87, 1205–1209. [Google Scholar] [CrossRef]
- Sardaro, R.; La Sala, P. New value to wool: Innovative garments for preservation of sheep landraces in Italy. Animals 2021, 11, 731. [Google Scholar] [CrossRef]
- Cryoconservation of animal genetic resources. In FAO Animal Production and Health Guidelines No. 12; FAO: Rome, Italy, 2012; pp. 3–5.
- Jones, A.K.; Gately, R.E.; McFadden, K.K.; Zinn, S.A.; Govoni, K.E.; Reed, S.A. Transabdominal ultrasound for detection of pregnancy, fetal and placental landmarks, and fetal age before Day 45 of gestation in the sheep. Theriogenology 2016, 85, 939–945. [Google Scholar] [CrossRef]
- Noakes, D.; Parkinson, T.; England, G. Veterinary Reproduction and Obstetrics, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 78–114. [Google Scholar]
- Mujitaba, M.A.; Vass, N.; Bodó, S. The recent state of cryopreservation techniques for ex-situ gene conservation and breeding purposes in small ruminants: A review. Acta Agrar. Debr. 2020, 1, 81–87. [Google Scholar] [CrossRef]
- Boes, J.; Boettcher, P.; Honkatukia, M. Innovations in cryoconservation of animal genetic resources-Practical guide. In FAO Animal Production and Health Guidelines, No. 33; FAO: Rome, Italy, 2023; pp. 41–67. [Google Scholar]
- Prentice, J.R.; Anzar, M. Cryopreservation of mammalian oocyte for conservation of animal genetics. Vet. Med. Int. 2010, 2011, 146405. [Google Scholar] [CrossRef]
- Turathum, B.; Gao, E.M.; Chian, R.C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef]
- Bogliolo, L.; Ariu, F.; Fois, S.; Rosati, I.; Zedda, M.T.; Leoni, G.; Succu, S.; Pau, S.; Ledda, S. Morphological and biochemical analysis of immature ovine oocytes vitrified with or without cumulus cells. Theriogenology 2007, 68, 1138–1149. [Google Scholar] [CrossRef]
- Colombo, M.; Alkali, I.M.; Luvoni, G.C. Microenvironment factors promoting the quality of vitrified cat oocytes. Theriogenology 2023, 196, 275–283. [Google Scholar] [CrossRef]
- Moawad, A.R.; Fisher, P.; Zhu, J.; Choi, I.; Polgar, Z.; Dinnyes, A.; Campbell, K.H.S. In vitro fertilization of ovine oocytes vitrified by solid surface vitrification at germinal vesicle stage. Cryobiology 2012, 65, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.R.; Zhu, J.; Choi, I.; Amarnath, D.; Chen, W.; Campbell, K.H. Production of good-quality blastocyst embryos following IVF of ovine oocytes vitrified at the germinal vesicle stage using a cryoloop. Reprod. Fertil. Dev. 2013, 25, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, A.; Taheri, F.; Nazari, H.; Norbakhsh-Nia, M.; Ahmadi, E.; Heidari, B. Developmental competence of ovine oocyte following vitrification: Effect of oocyte developmental stage, cumulus cells, cytoskeleton stabiliser, FBS concentration, and equilibration time. Zygote 2014, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.B.; Wu, G.Q.; Wang, Y.J.; Ma, Y.; Lv, C.R.; Hong, Q.H. Meiotic maturation and developmental capability of ovine oocytes at germinal vesicle stage following vitrification using different cryodevices. Cryobiology 2016, 72, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.M.; Borjian Boroujeni, S.; Sarvari, A.; Heidari, B.; Akhondi, M.M.; Zarnani, A.H.; Shirazi, A. The effect of media supplementation with angiotensin on developmental competence of ovine embryos derived from vitrified-warmed oocytes. Avicenna J. Med. Biotechnol. 2016, 8, 139–144. [Google Scholar]
- Moawad, A.R.; Choi, I.; Zhu, J.; El-Wishy, A.B.A.; Amarnath, D.; Chen, W.; Campbell, K.H.S. Caffeine and oocyte vitrification: Sheep as an animal model. Int. J. Vet. Sci. Med. 2018, 6, S41–S48. [Google Scholar] [CrossRef]
- Ahmadi, E.; Shirazi, A.; Shams-Esfandabadi, N.; Nazari, H. Antioxidants and glycine can improve the developmental competence of vitrified/warmed ovine immature oocytes. Reprod. Domest. Anim. 2019, 54, 595–603. [Google Scholar] [CrossRef]
- Dos Santos-Neto, P.C.; Vilariño, M.; Cuadro, F.; Barrera, N.; Crispo, M.; Menchaca, A. Cumulus cells during in vitro fertilization and oocyte vitrification in sheep: Remove, maintain or add? Cryobiology 2020, 92, 161–167. [Google Scholar] [CrossRef]
- Davoodian, N.; Kadivar, A.; Ahmadi, E.; Nazari, H.; Mehrban, H. Quercetin effect on the efficiency of ovine oocyte vitrification at GV stage. Theriogenology 2021, 174, 53–59. [Google Scholar] [CrossRef]
- Diez, C.; Duque, P.; Gómez, E.; Hidalgo, C.O.; Tamargo, C.; Rodríguez, A.; Fernández, L.; de la Varga, S.; Fernández, A.; Facal, N.; et al. Bovine oocyte vitrification before or after meiotic arrest: Effects on ultrastructure and developmental ability. Theriogenology 2005, 64, 317–333. [Google Scholar] [CrossRef]
- Yamada, C.; Feitosa, W.B.; Simões, R.; Nicacio, A.C.; Mendes, C.M.; Assumpção, M.E.; Visintin, J.A. Vitrification with glutamine improves maturation rate of vitrified/warmed immature bovine oocytes. Reprod. Domest. Anim. 2011, 46, 173–176. [Google Scholar] [CrossRef]
- Ezoe, K.; Yabuuchi, A.; Tani, T.; Mori, C.; Miki, T.; Takayama, Y.; Beyhan, Z.; Kato, Y.; Okuno, T.; Kobayashi, T.; et al. Developmental Competence of Vitrified-Warmed Bovine Oocytes at the Germinal-Vesicle Stage is Improved by Cyclic Adenosine Monophosphate Modulators during In Vitro Maturation. PLoS ONE 2015, 10, e0126801. [Google Scholar] [CrossRef]
- Sprícigo, J.F.; Sena Netto, S.B.; Muterlle, C.V.; Rodrigues Sde, A.; Leme, L.O.; Guimarães, A.L.; Caixeta, F.M.; Franco, M.M.; Pivato, I.; Dode, M.A. Intrafollicular transfer of fresh and vitrified immature bovine oocytes. Theriogenology 2016, 86, 2054–2062. [Google Scholar] [CrossRef]
- Alfoteisy, B.; Singh, J.; Anzar, M. Natural honey acts as a nonpermeating cryoprotectant for promoting bovine oocyte vitrification. PLoS ONE 2020, 15, e0238573. [Google Scholar] [CrossRef]
- Wani, N.A.; Maurya, S.N.; Misra, A.K.; Saxena, V.B.; Lakhchaura, B.D. Effect of cryoprotectants and their concentration on in vitro development of vitrified-warmed immature oocytes in buffalo (Bubalus bubalis). Theriogenology 2004, 61, 831–842. [Google Scholar] [CrossRef]
- Mahesh, Y.U.; Gibence, H.R.W.; Shivaji, S.; Rao, B.S. Effect of different cryo-devices on in vitro maturation and development of vitrified-warmed immature buffalo oocytes. Cryobiology 2017, 75, 106–116. [Google Scholar] [CrossRef]
- El-Shalofy, A.S.; Ismail, S.T.; Badawy, A.A.B.; Darwish, G.M.; Badr, M.R.; Moawad, A.R. Effect of disaccharide inclusion in vitrification and warming solutions on developmental competence of vitrified/warmed germinal vesicle stage buffalo oocytes. Cryo Lett. 2020, 41, 351–357. [Google Scholar]
- Rao, B.S.; Mahesh, Y.U.; Charan, K.V.; Suman, K.; Sekhar, N.; Shivaji, S. Effect of vitrification on meiotic maturation and expression of genes in immature goat cumulus oocyte complexes. Cryobiology 2012, 64, 176–184. [Google Scholar] [CrossRef]
- Fujihira, T.; Kishida, R.; Fukui, Y. Developmental capacity of vitrified immature porcine oocytes following ICSI: Effects of cytochalasin B and cryoprotectants. Cryobiology 2004, 49, 286–290. [Google Scholar] [CrossRef]
- Somfai, T.; Yoshioka, K.; Tanihara, F.; Kaneko, H.; Noguchi, J.; Kashiwazaki, N.; Nagai, T.; Kikuchi, K. Generation of live piglets from cryopreserved oocytes for the first time using a defined system for in vitro embryo production. PLoS ONE 2014, 9, e97731. [Google Scholar] [CrossRef]
- Casillas, F.; Ducolomb, Y.; Lemus, A.E.; Cuello, C.; Betancourt, M. Porcine embryo production following in vitro fertilization and intracytoplasmic sperm injection from vitrified immature oocytes matured with a granulosa cell co-culture system. Cryobiology 2015, 71, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Nohalez, A.; Martinez, C.A.; Gil, M.A.; Almiñana, C.; Roca, J.; Martinez, E.A.; Cuello, C. Effects of two combinations of cryoprotectants on the in vitro developmental capacity of vitrified immature porcine oocytes. Theriogenology 2015, 84, 545–552. [Google Scholar] [CrossRef]
- Tharasanit, T.; Colleoni, S.; Lazzari, G.; Colenbrander, B.; Galli, C.; Stout, T.A. Effect of cumulus morphology and maturation stage on the cryopreservability of equine oocytes. Reproduction 2006, 132, 759–769. [Google Scholar] [CrossRef]
- Tharasanit, T.; Colleoni, S.; Galli, C.; Colenbrander, B.; Stout, T.A. Protective effects of the cumulus-corona radiata complex during vitrification of horse oocytes. Reproduction 2009, 137, 391–401. [Google Scholar] [CrossRef]
- Ortiz-Escribano, N.; Bogado Pascottini, O.; Woelders, H.; Vandenberghe, L.; De Schauwer, C.; Govaere, J.; Van den Abbeel, E.; Vullers, T.; Ververs, C.; Roels, K.; et al. An improved vitrification protocol for equine immature oocytes, resulting in a first live foal. Equine Vet. J. 2018, 50, 391–397. [Google Scholar] [CrossRef]
- Ducheyne, K.D.; Rizzo, M.; Daels, P.F.; Stout, T.A.E.; de Ruijter-Villani, M. Vitrifying immature equine oocytes impairs their ability to correctly align the chromosomes on the MII spindle. Reprod. Fertil. Dev. 2019, 31, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Angel, D.; Canesin, H.S.; Brom-de-Luna, J.G.; Morado, S.; Dalvit, G.; Gomez, D.; Posada, N.; Pascottini, O.B.; Urrego, R.; Hinrichs, K.; et al. Embryo development after vitrification of immature and in vitro-matured equine oocytes. Cryobiology 2020, 92, 251–254. [Google Scholar] [CrossRef]
- Clérico, G.; Taminelli, G.; Veronesi, J.C.; Polola, J.; Pagura, N.; Pinto, C.; Sansinena, M. Mitochondrial function, blastocyst development and live foals born after ICSI of immature vitrified/warmed equine oocytes matured with or without melatonin. Theriogenology 2021, 160, 40–49. [Google Scholar] [CrossRef]
- Douet, C.; Reigner, F.; Barrière, P.; Blard, T.; Deleuze, S.; Goudet, G. First attempts for vitrification of immature oocytes in donkey (Equus asinus): Comparison of two vitrification methods. Theriogenology 2019, 126, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Tharasanit, T.; Manee-In, S.; Buarpung, S.; Chatdarong, K.; Lohachit, C.; Techakumphu, M. Successful pregnancy following transfer of feline embryos derived from vitrified immature cat oocytes using ‘stepwise’ cryoprotectant exposure technique. Theriogenology 2011, 76, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Turathum, B.; Roytrakul, S.; Changsangfa, C.; Sroyraya, M.; Tanasawet, S.; Kitiyanant, Y.; Saikhun, K. Missing and overexpressing proteins in domestic cat oocytes following vitrification and in vitro maturation as revealed by proteomic analysis. Biol. Res. 2018, 51, 27. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Morselli, M.G.; Tavares, M.R.; Apparicio, M.; Luvoni, G.C. Developmental competence of domestic cat vitrified cocytes in 3D enriched culture conditions. Animals 2019, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Luvoni, G.C. Minimum volume vitrification of immature feline oocytes. J. Vis. Exp. 2020, 160, e61523. [Google Scholar] [CrossRef]
- Colombo, M.; Morselli, M.G.; Apparicio, M.; Luvoni, G.C. Granulosa cells in three-dimensional culture: A follicle-like structure for domestic cat vitrified oocytes. Reprod. Domest. Anim. 2020, 55 (Suppl. 2), 74–80. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Zahmel, J.; Jänsch, S.; Jewgenow, K.; Luvoni, G.C. Inhibition of apoptotic pathways improves DNA integrity but not developmental competence of domestic cat immature vitrified oocytes. Front. Vet. Sci. 2020, 7, 588334. [Google Scholar] [CrossRef]
- Turathum, B.; Saikhun, K.; Sangsuwan, P.; Kitiyanant, Y. Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reprod. Biol. Endocrinol. 2010, 8, 70. [Google Scholar] [CrossRef]
- Vieira, A.D.; Forell, F.; Feltrin, C.; Rodrigues, J.L. Calves born after direct transfer of vitrified bovine in vitro-produced blastocysts derived from vitrified immature oocytes. Reprod. Domest. Anim. 2008, 43, 314–318. [Google Scholar] [CrossRef]
- Morton, K.M. Developmental capabilities of embryos produced in vitro from prepubertal lamb oocytes. Reprod. Domest. Anim. 2008, 43 (Suppl. 2), 137–143. [Google Scholar] [CrossRef]
- Izquierdo, D.; Catalá, M.G.; Paramio, M.T. Small ruminants: Prepubertal oocyte donors. Methods Mol. Biol. 2019, 2006, 155–163. [Google Scholar] [CrossRef]
- Silvestre, M.A.; Yániz, J.; Salvador, I.; Santolaria, P.; López-Gatius, F. Vitrification of pre-pubertal ovine cumulus-oocyte complexes: Effect of cytochalasin B pre-treatment. Anim. Reprod. Sci. 2006, 93, 176–182. [Google Scholar] [CrossRef]
- Somfai, T.; Nguyen, V.K.; Vu, H.T.T.; Nguyen, H.L.T.; Quan, H.X.; Viet Linh, N.; Phan, S.L.; Pham, L.D.; Cuc, N.T.K.; Kikuchi, K. Cryopreservation of immature oocytes of the indigeneous Vietnamese Ban Pig. Anim. Sci. J. 2019, 90, 840–848. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Pallares, P.; Vazquez, M.I. Ultrasonographic imaging in small ruminant reproduction. Reprod. Domest. Anim. 2010, 45 (Suppl. 2), 9–20. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Maloney, S.K.; Blache, D. Review of sheep body condition score in relation to production characteristics. N. Z. J. Agric. Res. 2014, 57, 38–64. [Google Scholar] [CrossRef]
- Martino, N.A.; Marzano, G.; Mangiacotti, M.; Miedico, O.; Sardanelli, A.M.; Gnoni, A.; Lacalandra, G.M.; Chiaravalle, A.E.; Ciani, E.; Bogliolo, L.; et al. Exposure to cadmium during in vitro maturation at environmental nanomolar levels impairs oocyte fertilization through oxidative damage: A large animal model study. Reprod. Toxicol. 2017, 69, 132–145. [Google Scholar] [CrossRef]
- Mastrorocco, A.; Cacopardo, L.; Lamanna, D.; Temerario, L.; Brunetti, G.; Carluccio, A.; Robbe, D.; Dell’Aquila, M.E. Bioengineering approaches to improve in vitro performance of prepubertal lamb oocytes. Cells 2021, 10, 1458. [Google Scholar] [CrossRef]
- de Oliveira Santos, M.V.; de Queiroz Neta, L.B.; Avezedo Borges, A.; Fernandes Pereira, A. Influence of commercially available follicle stimulating hormone on the in vitro maturation of bovine oocytes. Semin. Ciênc. Agrár. 2017, 38, 1393–1402. [Google Scholar] [CrossRef]
- Yang, H.W.; Hwang, K.J.; Kwon, H.C.; Kim, H.S.; Choi, K.W.; Oh, K.S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 1998, 13, 998–1002. [Google Scholar] [CrossRef]
- Martino, N.A.; Ariu, F.; Bebbere, D.; Uranio, M.F.; Chirico, A.; Marzano, G.; Sardanelli, A.M.; Cardinali, A.; Minervini, F.; Bogliolo, L.; et al. Supplementation with nanomolar concentrations of verbascoside during in vitro maturation improves embryo development by protecting the oocyte against oxidative stress: A large animal model study. Reprod. Toxicol. 2016, 65, 204–211. [Google Scholar] [CrossRef]
- Mastrorocco, A.; Martino, N.A.; Marzano, G.; Lacalandra, G.M.; Ciani, E.; Roelen, B.A.J.; Dell’Aquila, M.E.; Minervini, F. The mycotoxin beauvericin induces oocyte mitochondrial dysfunction and affects embryo development in the juvenile sheep. Mol. Reprod. Dev. 2019, 86, 1430–1443. [Google Scholar] [CrossRef]
- Mastrorocco, A.; Cacopardo, L.; Martino, N.A.; Fanelli, D.; Camillo, F.; Ciani, E.; Roelen, B.A.J.; Ahluwalia, A.; Dell’Aquila, M.E. One-step automated bioprinting-based method for cumulus-oocyte complex microencapsulation for 3D in vitro maturation. PLoS ONE 2020, 15, e0238812. [Google Scholar] [CrossRef]
- Abruzzo, N.; Marino, G.; Falsone, L.; Marino, D.A.; Brianti, E.; Boi, R.; Chiofalo, V.; Argiolas, G. Improvement of reproductive performances with a combined strategy (Sementusa®) in sheep farms in Sicily. Large Anim. Rev. 2014, 20, 209–213. [Google Scholar]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef]
- Wu, L.L.; Russell, D.L.; Wong, S.L.; Chen, M.; Tsai, T.S.; St John, J.C.; Norman, R.J.; Febbraio, M.A.; Carroll, J.; Robker, R.L. Mitochondrial dysfunction in oocytes of obese mothers: Transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015, 142, 681–691. [Google Scholar] [CrossRef]
- Boudoures, A.L.; Saben, J.; Drury, A.; Scheaffer, S.; Modi, Z.; Zhang, W.; Moley, K.H. Obesity-exposed oocytes accumulate and transmit damaged mitochondria due to an inability to activate mitophagy. Dev. Biol. 2017, 426, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Andreas, E.; Reid, M.; Zhang, W.; Moley, K.H. The effect of maternal high-fat/high-sugar diet on offspring oocytes and early embryo development. Mol. Hum. Reprod. 2019, 25, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Fabozzi, G.; Iussig, B.; Cimadomo, D.; Vaiarelli, A.; Maggiulli, R.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. The impact of unbalanced maternal nutritional intakes on oocyte mitochondrial activity: Implications for reproductive function. Antioxidants 2021, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Reyez, F.; Ducolomb, Y.; Romo, S.; Casas, E.; Salazar, Z.; Betancourt, M. Viability, maturation and embryo development in vitro of immature porcine and ovine oocytes vitrified in different devices. Cryobiology 2012, 64, 261–266. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Valojerdi, M.R.; Eftekhari-Yazdi, P.; Baharvand, H.; Farrokhi, A. IVM and gene expression of sheep cumulus-oocyte complexes following different methods of vitrification. Reprod. Biomed. Online 2010, 20, 26–34. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Valojerdi, M.R.; Eftekhari-Yazdi, P.; Baharvand, H. In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J. Assist. Reprod. Genet. 2010, 27, 239–246. [Google Scholar] [CrossRef]
- Somfai, T.; Noguchi, J.; Kaneko, H.; Nakai, M.; Ozawa, M.; Kashiwazaki, N.; Egerszegi, I.; Rátky, J.; Nagai, T.; Kikuchi, K. Production of good-quality porcine blastocysts by in vitro fertilization of follicular oocytes vitrified at the germinal vesicle stage. Theriogenology 2010, 73, 147–156. [Google Scholar] [CrossRef]
- Casillas, F.; Betancourt, M.; Cuello, C.; Ducolomb, Y.; López, A.; Juárez-Rojas, L.; Retana-Márquez, S. An efficiency comparison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porc. Health Manag. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Otero, Y.; Yeste, M.; Damato, A.; Giaretta, E. Cryopreservation and oxidative stress in porcine oocytes. Res. Vet. Sci. 2021, 135, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Temerario, L.; D’Onghia, A.; Landi, V.; Monaco, D.; Lacalandra, G.M.; D’Innocenzio, F.; Mangini, G.; De Palo, P.; Dell’Aquila, M.E.; Ciani, E. Resuming wool: A strategy for active conservation of an Italian Merino-type sheep breed. In Proceedings of the 8th European Symposium on South American Camelids & 4th European Meeting on Fibre Animals, Bolzano, Italy, 26–29 September 2022. [Google Scholar]
- Landi, V.; Molina, G.; De Palo, P.; Topputi, R.; Grande, S.; Mangini, G.; D’Onghia, A.; Sarti, F.M.; Temerario, L.; Pilla, F.; et al. Wool quality assessment as a tool for Gentile di Puglia promotion. In Proceedings of the IEEE International Workshop on Measurements and Applications in Veterinary and Animal Science (MeAVeAs), Napoli, Italy, 26–28 April 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).