3. Results
Female sugar gliders have a pouch in the ventral part of the abdomen. Its opening is placed in the midline, caudal to the
umbilicus. The pouch follows the longitudinal axis of the animal; it has two lateral pockets and contains four
mamae (nipples or teats): two in each pocket, one cranial and one caudal. The mucosa that lines the inner wall of the pouch has secretory glands, which are macroscopically visible (
Figure 1).
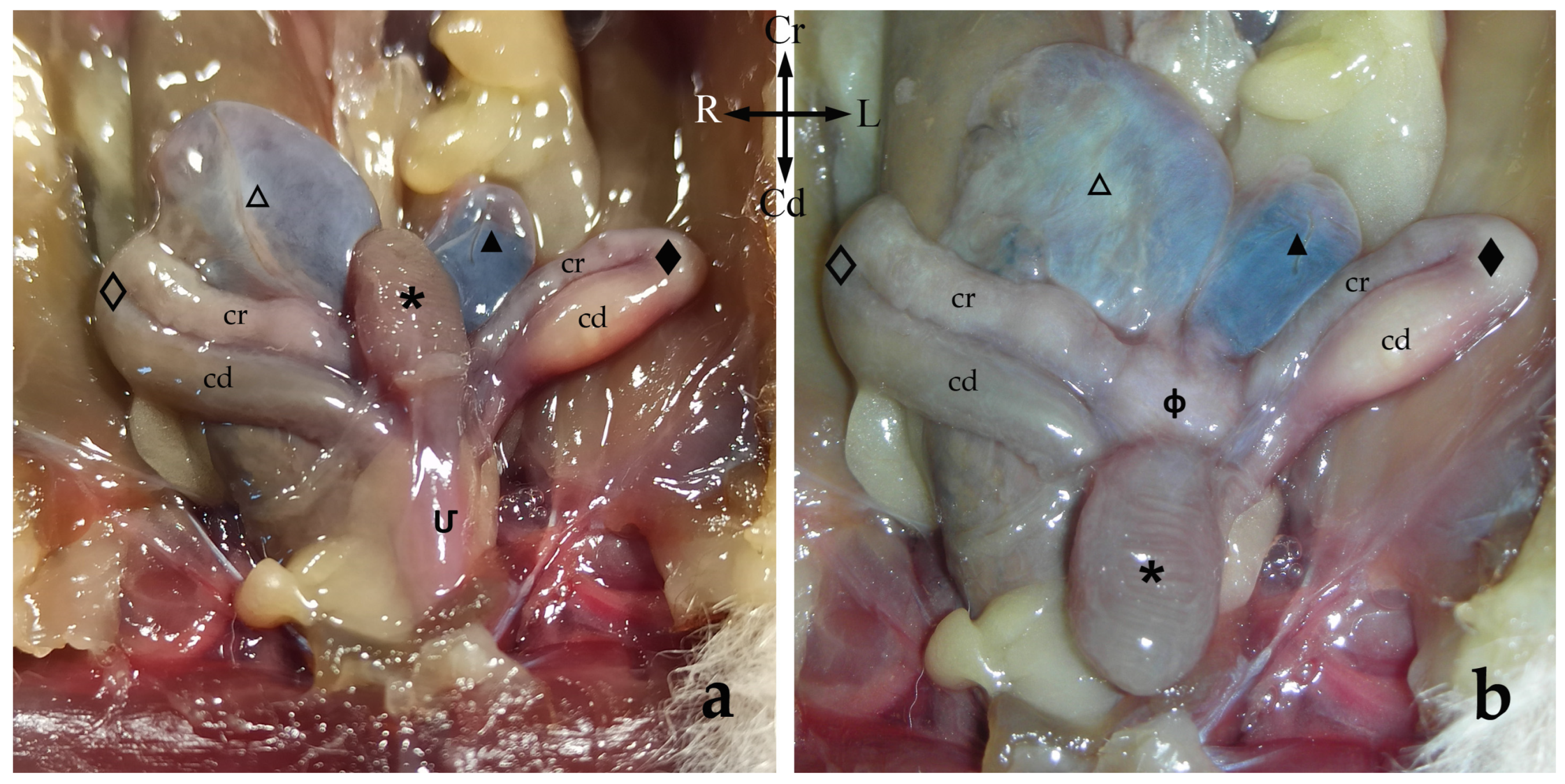
The female genitalia are in the caudal half of the abdominal cavity, and they are related dorsally to the rectum and ventrally to the urethra and the urinary bladder. The sugar glider female reproductive organs include paired ovaries, paired uterine tubes, paired uteri and a vaginal complex that includes paired lateral
vaginae (
Figure 2 and
Figure 3a).
The ovaries are close and dorsolateral to their ipsilateral uterus, near the junction between the uterus and the uterine tube. They have a brownish colour and ellipsoidal form and are flattened dorsoventrally. In this study, all the females were pubescent, so the gonad’s surface was irregular due to the projection of follicles and/or corpora lutea. Thus, all functional ovarian structures are visible. Each ovary is attached to the abdominal roof by a long ligament known as the
mesovarium. The cranial free border of the
mesovarium is a strong band called the suspensory ligament of the ovary (
ligamentum suspensorium ovarii) (
Figure 4a). The
mesovarium also supports the proper ligament of the ovary (
ligamentum ovarii propium), a band of connective tissue covered with peritoneum, which extends from the caudal pole of the gonad (
extremitas uterina ovarii) to the adjacent tip of the uterus. As the peritoneal folds do not cover the dorsal surface of the ovary, an
ovarian bursa cannot be described in this species (
Figure 4b).
Uterine tubes or oviducts (
tubae uterinae) are long and flexuous. They begin next to the corresponding ovary and lead to the uterus on the same side. The free cranial extremity of the tube is funnel-shaped and has a very thin wall. It is called the infundibulum (
infundibulum tubae uterinae) and is close to the ipsilateral ovary. The rest of the oviduct is tubular and very convoluted. It is divided into two parts: the proximal and narrower one, usually called the
ampulla (
ampulla tubae uterinae), and the most coiled part, which is the
isthmus (
isthmus tubae uterinae). Each tuba is supported by a serous fold called the
mesosalpinx, a lateral fold released from the
mesovarium. The
mesosalpinx and
mesovarium enclose a pouch that contains the tuba but not the ovary. This fact, together with the proximity of the ovary and the uterus, sometimes makes it difficult to visualize the tubes (
Figure 4b).
The paired uteri are elongated and fusiform-shaped, with their major axis running obliquely to the corporal axis. Sugar glider uteri lack horns (
cornua uteri), but they do have a body (
corpus uteri) and a neck (
cervix uteri). A ligament joins the medial surfaces of the caudal part of both uterine bodies. Although they are independent structures, both uteri appear to be fused caudally since they are surrounded by connective tissue (
Figure 4). The caudal part of each uterus narrows and they run parallel to each other on each side of the median line forming the uterine neck or cervix, which projects onto a papilla into the vaginal complex. The cervix lumen is almost occluded by mucosal folds, almost hiding the orifice that communicates the interior of the vaginal complex with each uterus (
ostium uteri externum) (
Figure 2 and
Figure 5).
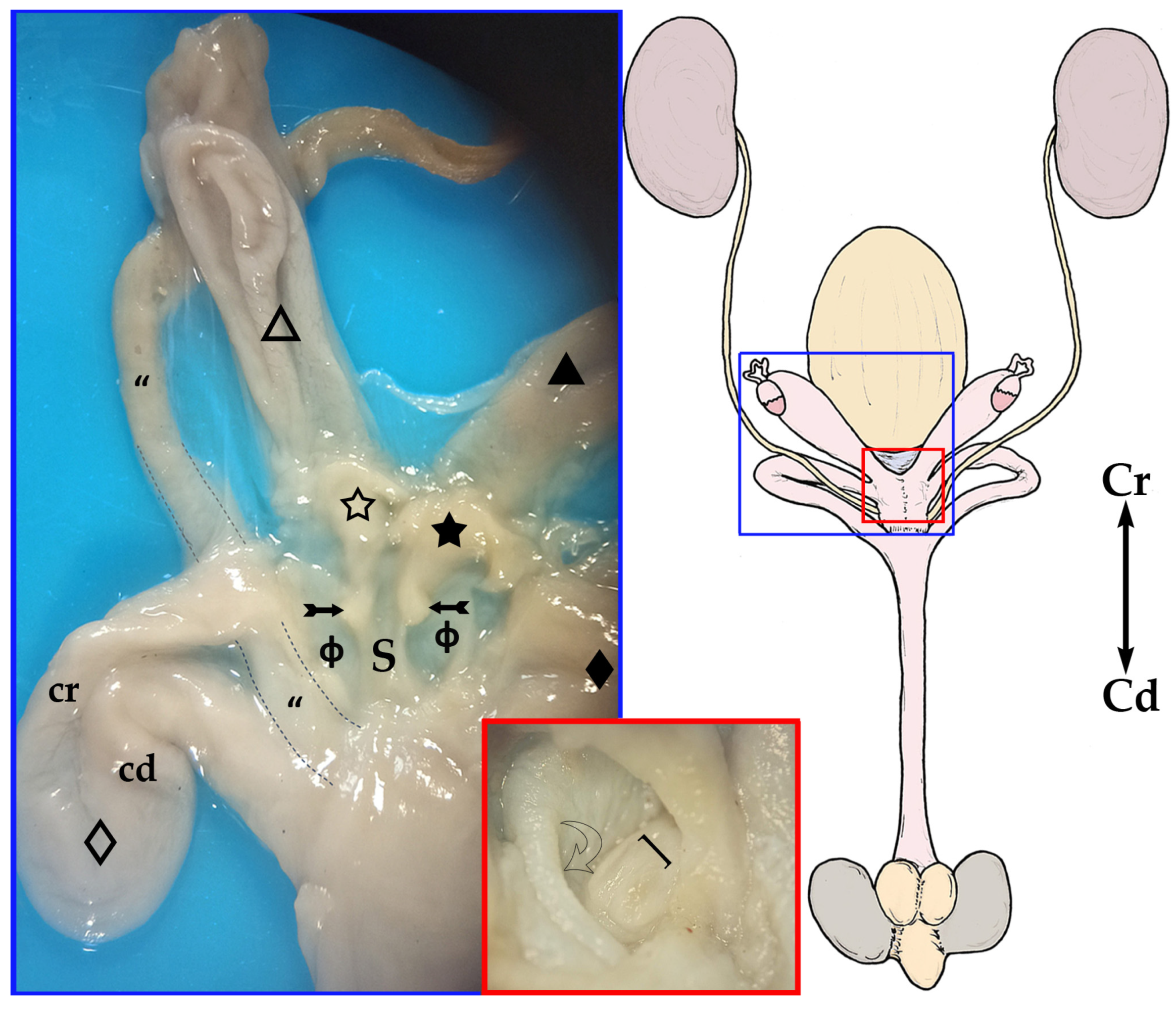
The vaginal apparatus or vaginal complex includes two lateral
vaginae and a median vagina, also called the ‘birth canal’. Lateral
vaginae are long and curved in a U-shape. They are placed caudal to the uteri and form two loops located on either side of the midline. Each loop has two ends, one cranial and another caudal, very close to each other and located on the same plane and joined by a peritoneal fold. The cranial end of both lateral
vaginae partially fuse, forming an expansion named the vaginal sinus (formerly called the ‘anterior vaginal expansion’ or ‘cul-de-sac’) (
Figure 2,
Figure 3b and
Figure 5). It is divided by a longitudinal
septum into two parts, one for each vagina, where the corresponding uterine cervix opens. This separation is complete in the non-parous specimen but incomplete in parous ones (
Figure 5). The caudal end of the lateral
vaginae opens into a medial and impar duct: the urogenital sinus that serves as a common passage for the reproductive and urinary systems (
Figure 6). The median vagina, very short in the sugar glider, extends from the caudal wall of the vaginal sinus to the cranial end of the urogenital sinus in recently parous females but does not exist in non-pregnant animals (
Figure 3c and
Figure 7).
The urinary bladder is ventral to the median vagina and urogenital sinus (
Figure 2a). A large external urethral orifice is located on the ventral wall of the urogenital sinus, the ending point of a short urethra. The ureters, originating in the ipsilateral kidney, run dorsolaterally to the uterus. Each ureter passes dorsally to the cranial branch of the ipsilateral lateral vagina and is incorporated into the peritoneum fold between the two branches. Their course is parallel to the caudal branch until they end in the vicinity of the neck of the urinary bladder, in a dorsal position. The two ureters terminate very close to each other by means of two elongated orifices located near to the internal urethral orifice, where they empty their content (
Figure 5 and
Figure 6).
The female sugar glider has neither a vulva nor an anus. Instead, there is a single opening, ventral to the beginning of the tail that communicates with a small internal cavity, the cloaca, a common organ of the digestive system and urogenital apparatus.
The urogenital sinus is a long tube that begins next to the caudal wall of the vaginal sinus and runs ventrally to the final part of the digestive system (large intestine) (
intestinum crassum) to the vicinity of the cloacal orifice (
Figure 7). It opens into the floor, close to the rectum that empties into the cranial part of the cloacal cavity.
The genital and urinary ducts empty into the cranial part of the urogenital sinus. The caudal loop of the lateral
vaginae and the external urethral orifice (
ostium urethrae externum) flow at the same level, but the lateral
vaginae open into the dorsolateral wall, whereas the external urethral orifice is on the ventral one (
Figure 6).
In the caudal part of the urogenital sinus, next to its cloacal opening, there is a ventral fossa (
fossa clitoridis) where the clitoris is located. This pit is covered by two folds of the lateral mucosa, one right and one left, which practically hide the fossa. The clitoris is forked. Each of its halves is elongated, with a constant thickness except at its terminal end, near the cloaca, where it tapers to a conical point. Given its great length, at rest, its caudal part must be folded inside the fossa (
Figure 8).
4. Discussion
The name ‘marsupials’ designates a group of mammals whose females have a pouch, or marsupium, where the young (joeys) finish their development after their birth. The marsupium is usually attached to the ventral wall of the abdomen, but there are considerable variations between species. Sometimes it opens cranially, sometimes caudally or centrally [
9]. There are even marsupials that lack a pouch [
9,
10]. Russell (1982) [
11] described six types of marsupium. In type 1, the mammary area is not covered by the skin although lateral ridges of skin develop during the breeding season; in type 2, the mammary area is partially covered; in types 3, 4, 5 and 6, the mammary area is covered by a fold of skin forming a real pouch; they differ in the position of their openings: central (type 3), cranial or anterior (types 4 and 5) and caudal (type 6). In addition, in type 4, the teats are located in two pockets, right and left, projecting forward from the cranial margin of the skin fold. Since the mammary area is completely covered by a skin fold and the opening of the bursa is located in its cranial part, the sugar glider marsupium is included in type 5, according to Russell’s (1982) [
11] classification.
The morphology of the pouch in the sugar glider matches the description made by Smith in 1973 [
7]. It opens near its cranial end and has two pockets extending onto the flanks of the female, where the
mamae are. According to Tyndale-Biscoe and Renfree (1987) [
12], Johnson-Delaney (2021) [
13] and our own data, the female sugar glider has four
mamae (nipples) inside the marsupium, two on the right and two on the left. Nevertheless, other authors point out that they are only two teats in the pouch [
7,
8,
14,
15]. Variations between individuals could be possible, as indicated by Smith (1973) [
7] and Girling (2013) [
16].
We observed macroscopic secretory glands in the mucosa lining the inner surface of the pouch. The presence of apocrine glands in the pouch skin has previously been described in the sugar glider by Smith (1973) [
7] and in other marsupials such as the tammar wallaby [
17] and red kangaroo [
18]. Various functions have been suggested for the secretion of these glands. The role of the chemical protection of the young by the secretions are not well known, but they could have antimicrobial activity as found in the tammar wallaby, whose pouch skin expresses genes for the antimicrobial peptide cathelicidin [
19]. The maternal glandular secretions could also provide moisture and their smell could play an important role in guiding the joey on its journey from the cloaca to the pouch after birth [
18]. When the young marsupial is born, many physiological processes are still immature for a time after parturition, among them pulmonary respiration. The lungs are immature, so gas exchange takes place through the skin for some days. Inside the pouch, the humid environment may aid this integumental gas exchange [
20].
Interestingly, although marsupials have a dual hormonal control of genital organ development, pouch development is not under this control [
21]. The initial development of the pouch, as well as of the mammary glands and the scrotum in the male, is evident before the differentiation of the gonads and depends on the expression of a gene or genes on the X-chromosome [
22]. In fact, it is the number of X-chromosomes that determines whether a marsupium or a scrotum will form: animals with one X-chromosome will develop a scrotum while those with two X-chromosomes will form a pouch [
22,
23].
The female genital apparatus of eutherian mammals basically consists of paired organs responsible for producing gametes (ovary) and a system of ducts that communicate with the outside. Each part of the genital tract is specialized to perform one or more functions. Thus, the ovary produces gametes and reproductive hormones, fertilization takes place in the oviduct (pair), and the uterus (unpaired, except in lagomorphs and most rodents which have duplex uteri) provides the environment and adequate nourishment for the embryo development until its birth. In eutherian mammals, the vagina (unpaired) serves both as copulatory organ and as birth canal. The vestibule continues the vagina to the vulva, which opens externally. The genital anatomy of the female sugar glider is similar to that of other marsupials and quite different from that of eutherian mammals. They have double reproductive organs: two ovaries, two oviducts, two uteri and two lateral
vaginae. The origin of the double reproductive tract is the result of a difference between eutherian and marsupial mammals in the embryonic development of the genital and urinary ducts. In eutherians, when the ureters migrate from the roof of the celomic cavity ventrally to connect with the bladder, they are placed laterally to the paramesonephric (Müllerian) ducts. Therefore, these can fuse with each other to form the body of the uterus and the cranial portion of the vagina. Conversely, in marsupials the ureters migrate medially to the genital ducts, preventing their fusion [
10].
As the ovaries were not enclosed in any bursa in the female sugar gliders that we studied, we suggest that there is no true ovarian bursa in this species unlike those described in some South American marsupials such as
Didelphis sp. [
24] and in
Dendrolagus [
25]. Johnson-Delany (2002) [
14] described the ovaries as lying against the medioventral side of the uterus, near the junction of the uterus and the oviduct. However, in all the specimens we observed, the gonads were located dorsolateral to the uterus and could not be seen in a ventral view of the genitalia.
Uterine tubes vary in length and their degree of convolution depends on the marsupial species [
26]. There are three parts of the mammalian uterine tubes:
infundibulum,
ampulla and
isthmus. However, unlike in eutherian mammals, in the sugar glider, the widest tubular part is the
isthmus, as seen in the anatomy of other marsupials referred to by Taggart (1994) [
26] and Hughes (2000) [
27].
Although the uterine function is similar in marsupial and eutherian mammals, there are important differences in uterine morphology. In marsupials, there are no uterine horns, only a body and neck (
cervix) are present. The uterine neck length varies between species [
26], as does the uterine body shape: usually, it is fusiform, as we have observed in the sugar glider, in accordance to that previously described by Johnson-Delany and Lennox (2017) [
28], but was abnormally enlarged in the extinct Tasmanian tiger (
Thylacinus cynocephalus) [
27] and is flattened dorsoventrally in the Tasmanian devil (
Sarcophilus satanicus) [
29]. However, in most species, the caudal ends of both uteri (
cervices) are surrounded by a common sheath of connective tissue [
14,
28], as we found in
Petaurus breviceps.The uterine papilla formed by the opening of each cervix into the vaginal sinus in the
Petaurus breviceps was previously described by Johnson-Delany (2002) [
14]. This author also reported an
os uteri ventrolaterally on the papilla that, in contrast, we could not identify in our macroscopic study. This papilla seems to be quite common in marsupials since it has also been observed in
Sarcophilus satanicus [
29], in
Thylacinus cynecephalus [
27] and in
Dendrolagus [
25].
The function of the lateral
vaginae is to facilitate the arrival of sperm to the
uteri [
30]. The male marsupial deposits semen in the upper part of the urogenital sinus, where the caudal ends of the lateral
vaginae empty. From here, spermatozoa progress up through these ducts and penetrate the vaginal sinus, near the
cervices [
31,
32]. Since there is no direct communication between the urogenital sinus and the
uteri, this is the only possible pathway in all non-parous and most parous female marsupials. However, in macropods, the birth canal (median vagina) is permanent, so it allows direct access from the urogenital sinus to the cervices in parous females [
33].
The shape of the lateral
vaginae varies among marsupials. In
Petaurus breviceps, they are long and U-shaped in accordance with our study, but they can become long and convoluted as in
Caenolestes obscurus or short and straight such as in
Trichosurus vulpecula and
Tarsupes rostratus. Additionally, in some species, the cranial part of the lateral vagina expands to form a chamber that serves as a seminal receptacle [
12].
In pregnant females, a median vagina is formed in the midline, lying between the lateral
vaginae [
7,
16,
22]. In most marsupials, this forms a transient birth canal in the medial connective tissue between the vaginal and urogenital sinuses. It opens before each birth and closes soon after the passage of the foetus, and reforms in each subsequent parturition [
9]. However, in most
Macropodidae (kangaroos), the median vagina becomes a permanent structure after the first birth [
12]. Judah and Nutall (2008) [
8] point out that it is a temporary structure in
Petaurus breviceps, which coincides with our own macroscopical observations. However, the birth canal could persist even if it is blocked by tissue separating the vaginal and urogenital sinuses. Histological study would be required to confirm that it disappears completely after parturition, as seems to be the case in the Tasmanian tiger [
27].
According to our studies, in the sugar glider, the medial vagina is very short whereas the lateral ones are long and thin. Thus, our results are in accordance with those previously described by Johnson-Delaney and Lennox (2017) [
28]. However, it is not always so in marsupials. For instance, in opossums and macropods, the medial and lateral
vaginae are nearly equal in length [
28]. In
Petaurus breviceps, both structures, the vaginal and the urogenital sinuses, are very close, but it could be the same as in other marsupials such as
phalangeridae,
dasyuridae or
vombatidae [
30]: they are contiguous, but there is no communication between them except in pregnant or recently calved females.
We observed an incomplete septum in the midline of the vaginal sinus, separating the beginning of the two cranial loops of the lateral
vaginae, in parous females (six of seven specimens). This was previously described by Johnson-Delany (2002) [
14] and Johnson-Delany and Lennox (2017) [
28]. According to Rodger (2020) [
32], in non-parous marsupial females this median vaginal septum separates the left and right parts of the genital tract, but after the first parturition, in most marsupials this septum is perforated and the right and left sides of the vaginal sinus communicate. Our observations support this statement in the sugar glider. In some species such as
Dendrolagus matschiei, the division of the sinus vaginalis is only partial and due to a longitudinal fold, whose height decreases as it advances in the caudal direction until it disappears. It is only attached to the ventral wall but not to the dorsal one, only dividing the upper half of the sinus [
25]. Differences in the septum between species (complete, incomplete, or absent) have previously been described by Tyndale-Biscoe and Renfree (1987) [
12].
Because of the close relationship of the ureters and vagina in marsupials, unlike eutherian mammals, neutering of female sugar gliders is not a routine procedure [
34]. Given the technical complexity of the surgery, the animal’s small size [
34], and the fact that it has not been confirmed to have a favourable impact on their health [
28], to avoid unwanted gestations in this species, only males are usually neutered [
34]. However, due to the presence of the cloaca, which connects the urogenital and digestive tracts, this species is relatively prone to ascending infections from the cloaca, which can cause vaginitis and metritis [
35]. Sometimes, these pathologies can be resolved conservatively [
35]. However, when removal of the female genitalia (ovary-vagina-hysterectomy) is necessary for medical reasons, careful consideration should be given to the course of the ureters, isolating their last section from the ipsilateral lateral vagina. In obese animals it may not be possible to separate these ducts, in which case, only the ovaries and uteri could be removed (ovariohysterectomy) [
14].
As stated by Pearson (1945) [
30], the long urogenital sinus of the sugar glider seems to be characteristic of marsupials. He also described a clitoris in
Petaurus breviceps, located on the ventral wall of the urogenital sinus, near its posterior extremity. According to Pavlicev et al. (2022) [
36], there seems to be a relationship between the anatomy of the penis and that of the clitoris in marsupials: when the
glans penis is bifurcated, the clitoris is also forked. Thus, a bifurcated clitoris was found in female short-tailed opossums [
14], the Tasmanian devil (
Sarcophilus satanicus) [
29], the Tasmanian tiger (
Thylacinus cynecephalus) [
27] and the sugar glider (
Petarurus breviceps). In contrast, it is unforked in macropodids [
37]. Matthews (1947) [
25] described the clitoris in
Dendrolagus ursinus as a conical structure, flattened on its dorsal side, convex on its ventral side and lying in a pocket, so it did not project into the urogenital sinus lumen. In addition, Gonçalves et al. (2009) [
24] reported the existence of erectile tissue in the ventral wall of the urogenital sinus, next to its end, forming a genital tubercle that resembles a clitoris.
The short urethra of the sugar glider follows the general rule of marsupials indicated by Pearson (1945) [
30]. According to his studies, the only exceptions were the genders/genere
Parameles and
Bettongia, due to the cranial displacement of the urinary bladder. The urethral orifice (
ostium urethrae externum), placed on the ventral floor of the urogenital sinus, was previously identified by Girling (2013) [
16] at the same level of the lateral
vaginae ends and had also been described in other species (Hughes, 2000) [
27], although it does not seem to be a constant rule in marsupials. Thus, in some South American species, such as
Didelphis albiventris [
38], the external urethral orifice is located cranially at the end of the lateral
vaginae, but caudally in
Dendrolagus ursinus [
25].
Although we did not observe it in the sugar glider, a papilla has been described at the end of the urethra (external urethral orifice) in some marsupial species [
25].
The existence of a reduced cloaca, such as the sugar glider one, seems to be a common feature of marsupials [
39], although in some South American species, such as
Didelphis albiventris [
38],
Chinorectes minimus [
40] and
Monodelphis [
36], the urogenital sinus and the anus open separately on the surface of the body.
 ). A joey can be seen deep inside the pouch.
). A joey can be seen deep inside the pouch.
 ). A joey can be seen deep inside the pouch.
). A joey can be seen deep inside the pouch.



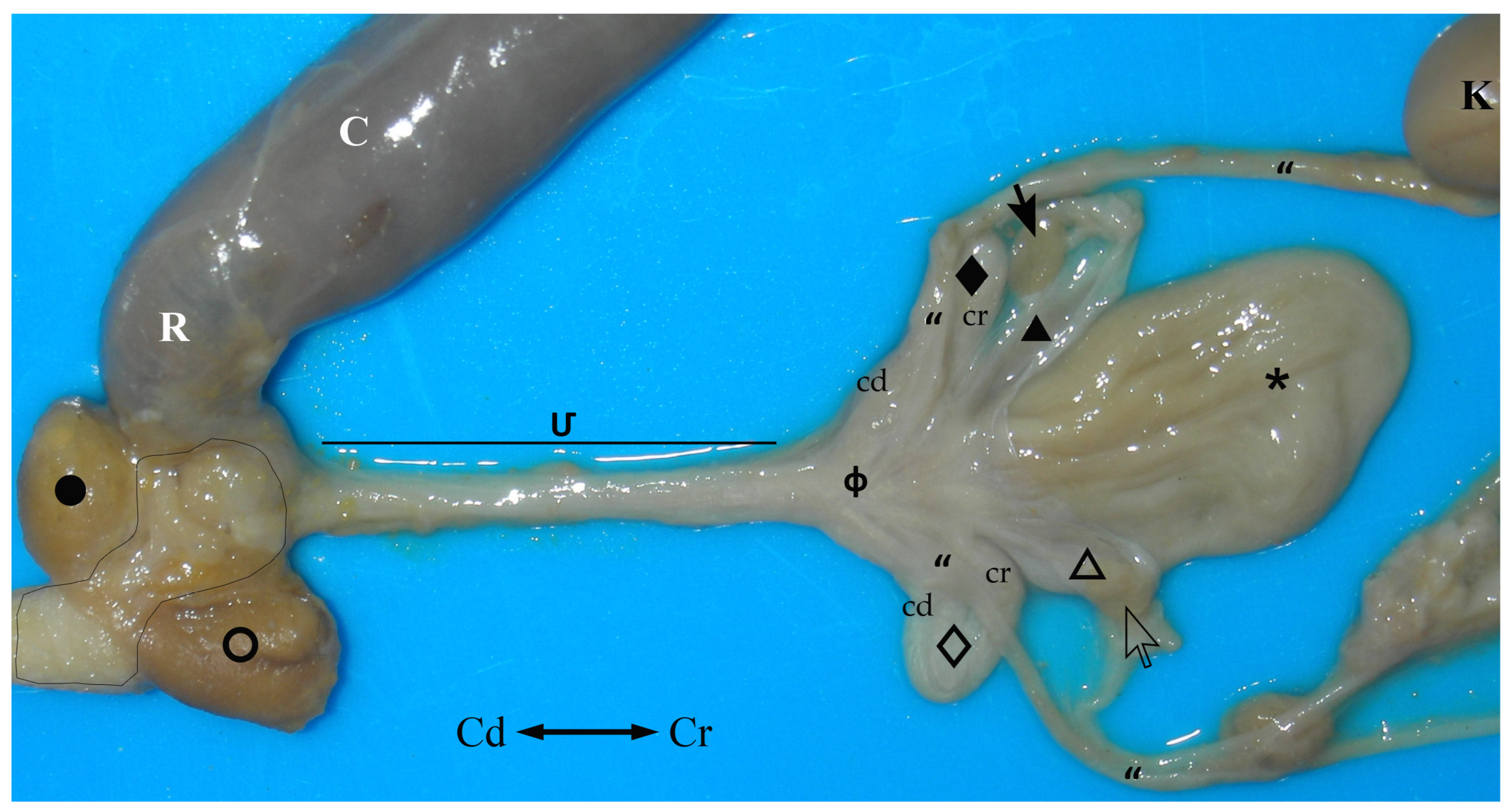
 ) cloacal orifice; (➝) clitoris; (>) fossa clitoridis; (○) right paracloacal gland.
) cloacal orifice; (➝) clitoris; (>) fossa clitoridis; (○) right paracloacal gland.
 ) cloacal orifice; (➝) clitoris; (>) fossa clitoridis; (○) right paracloacal gland.
) cloacal orifice; (➝) clitoris; (>) fossa clitoridis; (○) right paracloacal gland.






