Sperm Head Morphology Alterations Associated with Chromatin Instability and Lack of Protamine Abundance in Frozen-Thawed Sperm of Indonesian Local Bulls
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Design
2.2. Frozen-Thawed Sperm Analysis
2.2.1. DNA Damage Assessment by Acridine Orange Staining
2.2.2. DNA Damage Assessment with Sperm-Bos-Halomax
2.3. Measurement of Bovine Sperm Protamine 1 Protein and Gene Abundance
2.4. Protamine Deficiency Assessment with CMA3 Staining
2.5. Statistical Analysis
3. Results
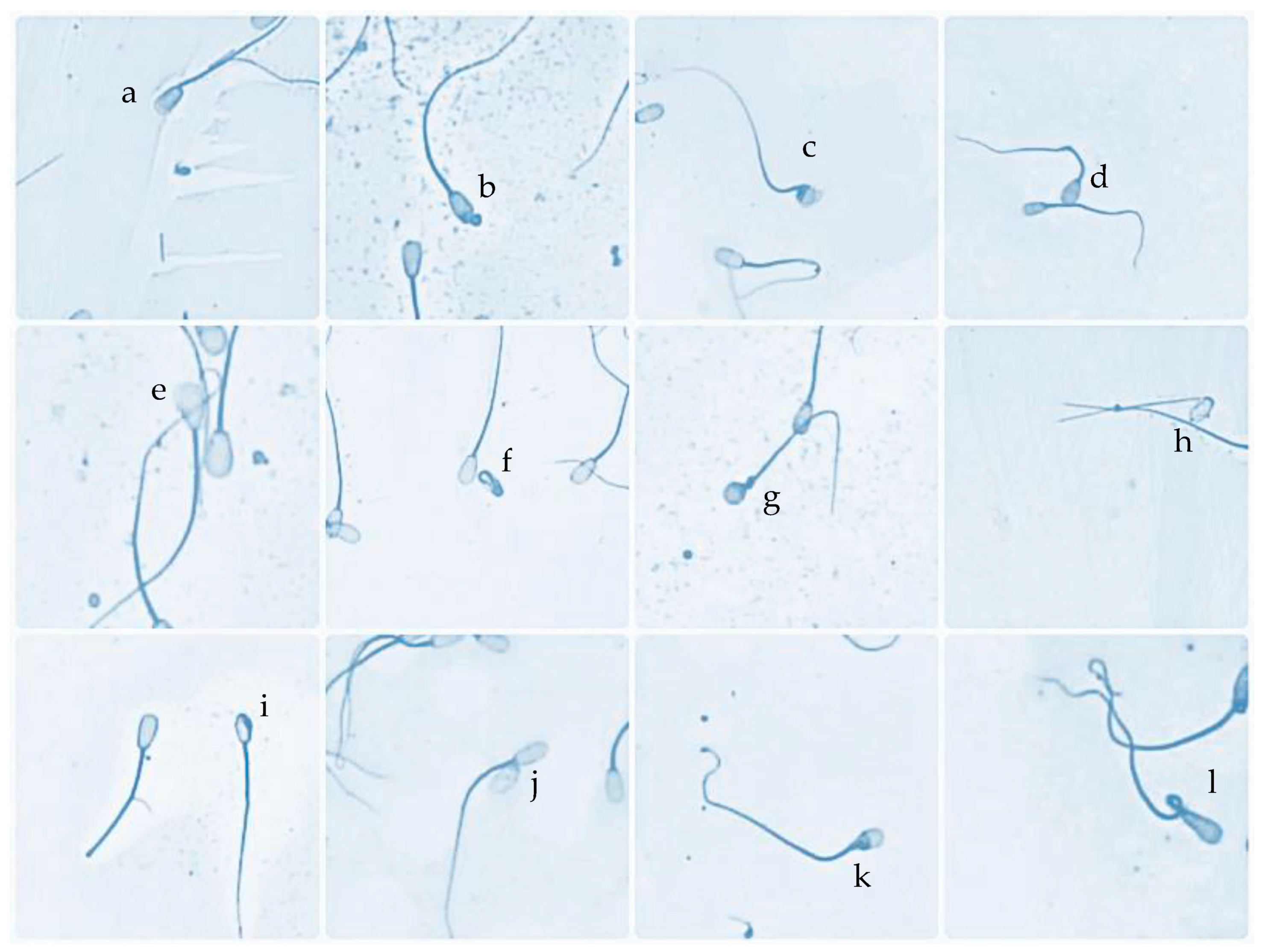
3.1. Characteristic Sperm Head Morphological Defects in HD and LD Bulls
3.2. Sperm DNA, PRM Deficiency, and PRM1 Abundance in HD and LD Bulls
3.3. Correlation of PRM Deficiency, Sperm Head Defects, DNA Damage, and PRM1 Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sitanggang, G. Environmental effects and repeatability estimates on semen quality in Ongole grade bulls. J. Ilmu Pertan. Indones. (JIPI) 2018, 23, 88–92. [Google Scholar] [CrossRef]
- Zulyazaini, D.; Wahyuni, S.; Akmal, M.; Abdullah, M.A.N. The characteristics of semen and chemical composition of the seminal plasma of Aceh cattle maintained in BIBD Saree Aceh Besar. J. Agripet 2016, 16, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Pardede, B.P.; Supriatna, I.; Yudi, Y.; Agil, M. Relationship of frozen-thawed semen quality with the fertility rate after being distributed in the Brahman Cross Breeding Program. Vet. World 2020, 13, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.L.-l.; Zhang, H.G.; Wang, R.X.; Jiang, Y.T.; Liu, R.Z. Re-evaluation of the value of sperm morphology in classical in vitro fertilization in a Northeastern Chinese population. J. Int. Med. Res. 2019, 47, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Ball, P.J.; Peters, A.R. Reproduction in Cattle, 3rd ed.; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Simon, L.; Castillo, J.; Oliva, R.; Lewis, S.E.M. Relationships between human sperm protamines, DNA damage, and assisted reproduction outcomes. Reprod. Biomed. Online 2019, 23, 724–734. [Google Scholar] [CrossRef] [Green Version]
- Boe-Hansen, G.B.; Christensen, P.; Vibjerg, D.; Nielsen, M.B.F.; Hedeboe, A.M. Sperm chromatin structure integrity in liquid stored boar semen and its relationships with field fertility. Theriogenology 2008, 69, 728–736. [Google Scholar] [CrossRef]
- Pardede, B.P.; Supriatna, I.; Agil, M. Protamine and other proteins in sperm and seminal plasma as molecular markers of bull fertility. Vet. World 2020, 13, 556–562. [Google Scholar] [CrossRef]
- Wang, T.; Gao, H.; Li, W.; Liu, C. Essential role of histone replacement and modifications in male fertility. Front. Genet. 2019, 10, 962. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, U.; Ganguly, I.; Deb, R.; Singh, R.; Mann, S.; Senger, G.; Mandal, D.K.; Kumar, M.; Sharma, A. Protamine 3 expressions in crossbred bull spermatozoa may not be a prognostic marker for differentiating good and poor-quality semen. African J. Biotech. 2014, 13, 1999–2003. [Google Scholar]
- Carreira, J.T.; Trevizan, J.T.; Carvalho, I.R.; Kipper, B.; Rodrigues, L.H.; Silva, C.; Perri, S.H.V.; Drevet, J.R.; Koivisto, M.B. Does sperm quality and DNA integrity differ in cryopreserved semen samples from young, adult, and aged Nellore bulls? Basic. Clin. Androl. 2017, 27, 12. [Google Scholar] [CrossRef] [Green Version]
- Pardede, B.P.; Maulana, T.; Kaiin, E.M.; Agil, M.; Karja, N.W.K.; Sumantri, C.; Supriatna, I. The potential of sperm bovine protamine as a protein marker of semen production and quality at the National Artificial Insemination Center of Indonesia. Vet. World 2021, 14, 2473–2481. [Google Scholar] [CrossRef]
- Pardede, B.P.; Agil, M.; Karja, N.W.K.; Sumantri, C.; Supriatna, I.; Purwantara, B. PRM1 Gene Expression and Its Protein Abundance in Frozen-Thawed Spermatozoa as Potential Fertility Markers in Breeding Bulls. Vet. Sci. 2022, 9, 111. [Google Scholar] [CrossRef]
- Bao, J.; Bedford, M.T. Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction 2016, 151, R55–R70. [Google Scholar] [CrossRef] [Green Version]
- Ni, K.; Spiess, A.-N.; Schuppe, H.-C.; Steger, K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility: A systematic review and meta-analysis. Andrology 2016, 4, 7890–7899. [Google Scholar]
- Chenoweth, P.J. Genetic sperm defects. Theriogenology 2005, 64, 457–468. [Google Scholar] [CrossRef]
- Jakubik-Uljasz, J.; Gill, K.; Rosiak-Gill, A.; Piasecka, M. Relationship between sperm morphology and sperm DNA dispersion. Transl. Androl. Urol. 2020, 9, 405–415. [Google Scholar] [CrossRef]
- Watanabe, S. Chromosome analysis of human spermatozoa with morphologically abnormal heads by injection into mouse oocytes. Reprod. Med. Biol. 2004, 3, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.S.; Gao, H.; Zhao, Y.; Ma, S. Aneuploidy and DNA fragmentation in morphologically abnormal sperm. Int. J. Androl. 2010, 33, e163–e179. [Google Scholar] [CrossRef] [Green Version]
- Enciso, M.; Cisale, H.; Johnston, S.D.; Sarasa, J.; Fernandez, J.L.; Gosalvez, J. Major morphological sperm abnormalities in the bull are related to sperm DNA damage. Theriogenology 2011, 76, 21–32. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in sperm during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef] [Green Version]
- Zukerman, Z.; Sagiv, M.; Ravid, A.; Ben-Bassat, M.; Malik, Z.; Shohat, B.; Tadir, Y.; Ovadia, Y.; Singer, R. A high proportion of double-headed and double-tailed sperm in semen of a human male. A case report. Andrologia 1986, 18, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Donald, H.P.; Hancock, J.L. Evidence of a gene-controlled sterility in bulls. J. Agric. Sci. 1953, 43, 178–181. [Google Scholar] [CrossRef]
- Durairajanayagam, D. Lifestyle causes of male infertility. Arab. J. Urol. 2018, 16, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccetti, B.; Burini, A.G.; Coilodel, G.; Magnano, A.R.; Piomboni, P.; Renieri, T.; Sensini, C. Morphogenesis of the decapitated and decaudated sperm defect in two brothers. Gamete Res. 1989, 23, 181–188. [Google Scholar] [CrossRef]
- Patel, G.K.; Haque, N.; Madhavatar, M.; Chaudhari, A.K.; Patel, D.K.; Bhalakiya, N.; Jamnesha, N.; Patel, P.; Kumar, R. Artificial insemination: A tool to improve livestock productivity. J. Pharm. Phytochem. 2017, 6, 307–313. [Google Scholar]
- Petac, D.; Kosec, M. Some morphological characters of bull spermatozoa and their effect on fertility. Zb. Bioteh. Fak. Univerze Edvarda Kardelja V Ljubljani Vet. 1989, 26, 65–72. [Google Scholar]
- Nöthling, J.O.; Arndt, E.P. Fertility of two bulls with poor sperm morphology. J. S. Afr. Vet. Assoc. 1995, 2, 74–76. [Google Scholar]
- Andersson, M.; Vierula, M.; Alanko, M. Acrosomal morphology and fertility in AI bulls. In Proceedings of the 11th International Congress on Animal Reproduction and Artificial Insemination, Dublin, Ireland, 26–30 June 1988; p. 222. [Google Scholar]
- Evenson, D.P. The Sperm Chromatin Structure Assay (SCSA) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef] [Green Version]
- Pardede, B.P.; Supriatna, I.; Yudi, Y.; Agil, M. Decreased bull fertility: Age-related changes in sperm motility and DNA fragmentation. E3S Web Conf. 2020, 151, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Fathi, Z.; Tavalaee, M.; Kiani, A.; Deemeh, M.R.; Modaresi, M.; Nasr-Esfahani, M.H. Flow cytometry: A novel approach for indirect assessment of protamine deficiency by CMA3 staining, taking into account the presence of M540 or apoptotic bodies. Int. J. Fertil. Steril. 2011, 5, 128–133. [Google Scholar]
- Nasr-Esfahani, M.H.; Aboutorabi, R.; Razavi, S. Credibility of Chromomycin A3 Staining in Prediction of Fertility. Int. J. Fertil. Steril. 2009, 3, 5–10. [Google Scholar]
- Indriastuti, R.; Pardede, B.P.; Gunawan, A.; Ulum, M.F.; Arifiantini, R.I.; Purwantara, B. Sperm Transcriptome Analysis Accurately Reveals Male Fertility Potential in Livestock. Animals 2022, 12, 2955. [Google Scholar] [CrossRef]
- Rosyada, Z.N.A.; Pardede, B.P.; Kaiin, E.M.; Tumbelaka, L.I.T.A.; Solihin, D.D.; Purwantara, B.; Ulum, M.F. Identification of HSP70-2 and PRM-1 mRNA, proteins, and analyses of their association with fertility using frozen-thawed sperm in Madura bulls. Anim. Biosci. 2023, 26. [Google Scholar] [CrossRef]



| Bull No. | Sperm Head Morphological Defects Status | Average Sperm Head Morphological Defects (%) | Difference from Population Average (%) |
|---|---|---|---|
| 1 | High sperm head morphological defects (HD) | 4.04 | 1.076 |
| 2 | 4.02 | 1.056 | |
| 3 | 4.10 | 1.136 | |
| 4 | 4.06 | 1.096 | |
| 5 | 3.96 | 0.996 | |
| 6 | 4.00 | 1.036 | |
| 7 | 4.00 | 1.036 | |
| 8 | 4.06 | 1.096 | |
| 9 | 4.02 | 1.056 | |
| 10 | 4.08 | 1.116 | |
| 11 | Low sperm head morphological defects (LD) | 1.90 | −1.064 |
| 12 | 1.92 | −1.044 | |
| 13 | 1.88 | −1.084 | |
| 14 | 1.92 | −1.044 | |
| 15 | 1.92 | −1.044 | |
| 16 | 1.92 | −1.044 | |
| 17 | 1.92 | −1.004 | |
| 18 | 1.96 | −1.004 | |
| 19 | 1.92 | −1.044 | |
| 20 | 1.88 | −1.084 | |
 | |||
| Sperm Parameters | PRM Def | Head Defects | AO | Bos-Halomax | PRM1-p | PRM1-g |
|---|---|---|---|---|---|---|
| PRM def | 1 | 0.964 ** | 0.982 ** | 0.995 ** | −0.936 ** | −0.906 ** |
| Head defects | 1 | 0.954 ** | 0.953 ** | −0.952 ** | −0.912 ** | |
| AO | 1 | 0.991 ** | −0.926 ** | −0.917 ** | ||
| Bos-Halomax | 1 | −0.916 ** | −0.921 ** | |||
| PRM1-p | 1 | 0.932 ** | ||||
| PRM1-g | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusumawati, A.; Satrio, F.A.; Indriastuti, R.; Rosyada, Z.N.A.; Pardede, B.P.; Agil, M.; Purwantara, B. Sperm Head Morphology Alterations Associated with Chromatin Instability and Lack of Protamine Abundance in Frozen-Thawed Sperm of Indonesian Local Bulls. Animals 2023, 13, 2433. https://doi.org/10.3390/ani13152433
Kusumawati A, Satrio FA, Indriastuti R, Rosyada ZNA, Pardede BP, Agil M, Purwantara B. Sperm Head Morphology Alterations Associated with Chromatin Instability and Lack of Protamine Abundance in Frozen-Thawed Sperm of Indonesian Local Bulls. Animals. 2023; 13(15):2433. https://doi.org/10.3390/ani13152433
Chicago/Turabian StyleKusumawati, Asmarani, Faisal Amri Satrio, Rhesti Indriastuti, Zulfi Nur Amrina Rosyada, Berlin Pandapotan Pardede, Muhammad Agil, and Bambang Purwantara. 2023. "Sperm Head Morphology Alterations Associated with Chromatin Instability and Lack of Protamine Abundance in Frozen-Thawed Sperm of Indonesian Local Bulls" Animals 13, no. 15: 2433. https://doi.org/10.3390/ani13152433
APA StyleKusumawati, A., Satrio, F. A., Indriastuti, R., Rosyada, Z. N. A., Pardede, B. P., Agil, M., & Purwantara, B. (2023). Sperm Head Morphology Alterations Associated with Chromatin Instability and Lack of Protamine Abundance in Frozen-Thawed Sperm of Indonesian Local Bulls. Animals, 13(15), 2433. https://doi.org/10.3390/ani13152433







