Non-Invasive Assessment of the Intraventricular Pressure Using Novel Color M-Mode Echocardiography in Animal Studies: Current Status and Future Perspectives in Veterinary Medicine
Abstract
:Simple Summary
Abstract
1. Introduction
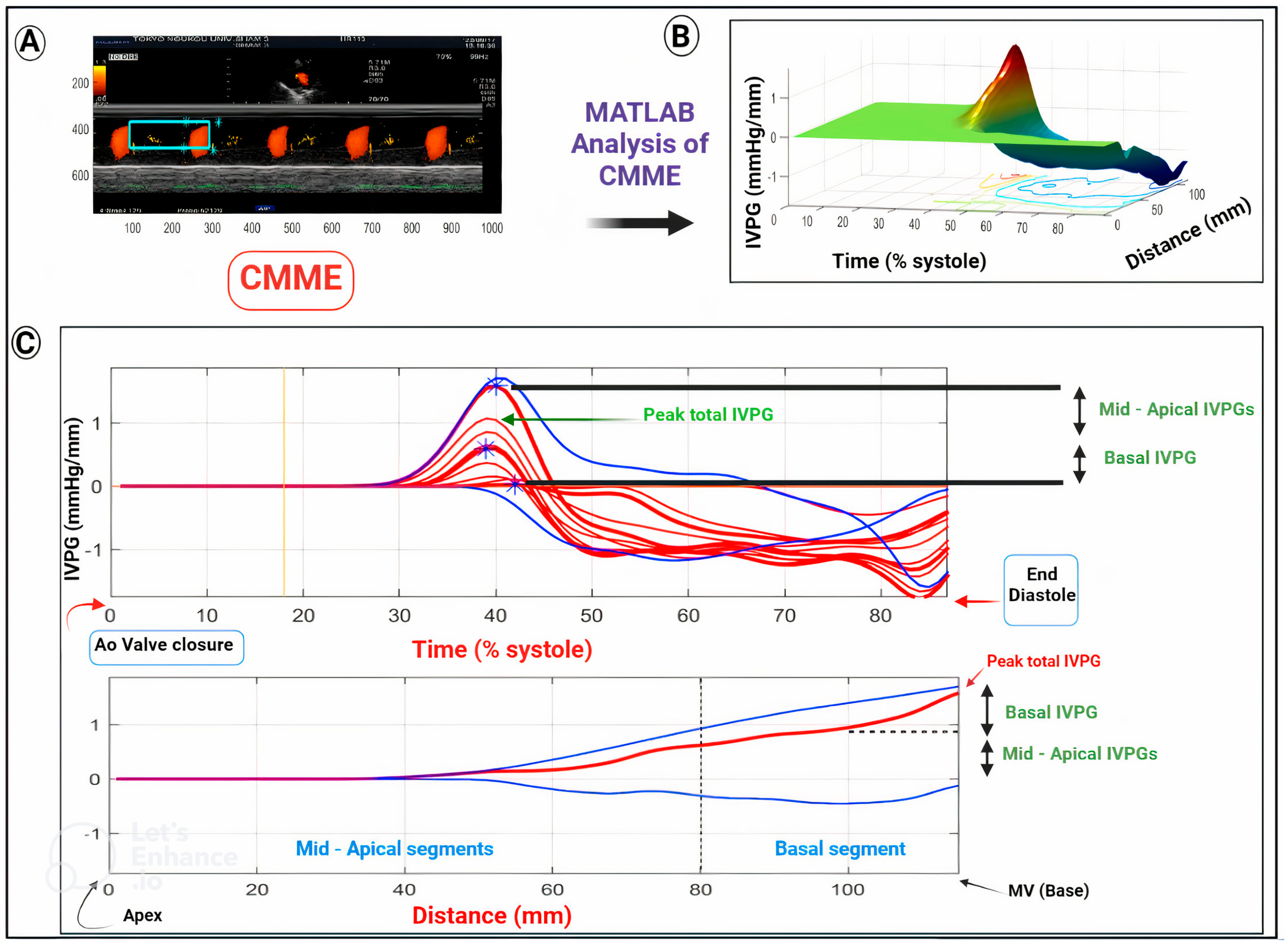
2. CMME-Derived IVPG/IVPD for Assessment of Cardiac Function
3. CMME Scanning and the IVPD and IVPG Calculation
4. Usability of IVPD and IVPG in Heart Failure
- A.
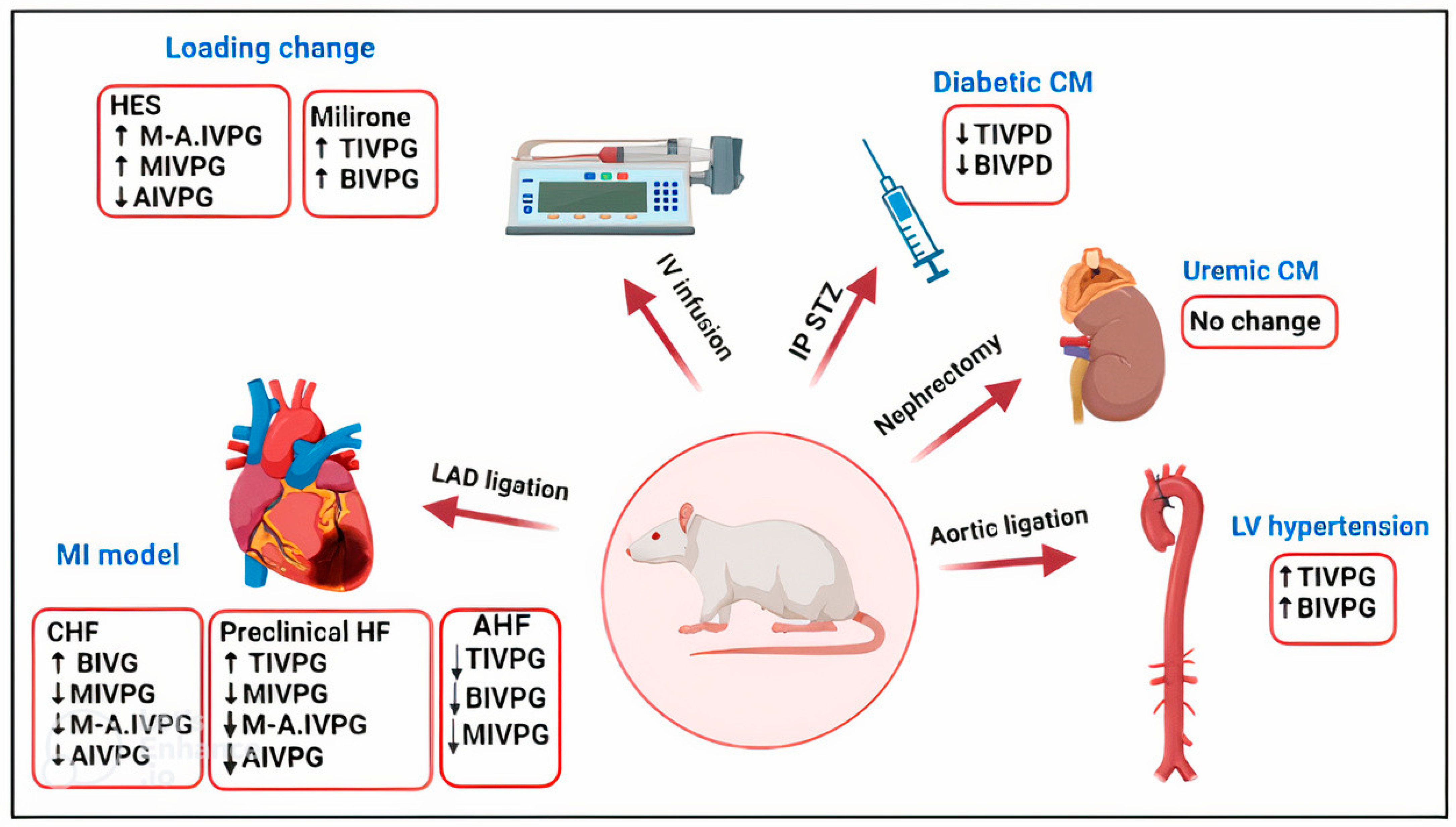
- Rat models of cardiovascular disorders
- (a)
- Changes in IVPD and IVPG in loading conditions
- (b)
- Hypertrophic cardiomyopathy (HCM)
- (c)
- Diabetic cardiomyopathy
- (d)
- Myocardial infarction model
- (e)
- Uremic cardiomyopathy
| Model | Left Ventricular Hypertrophy | Uremic CM | Diabetic CM | Loading Change | Infarction for 6 Months | Infarction for 1 Month | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | LVH | Carvedilol | Sal-B | UCM | Sal-B | DM | MIL | HES | +ve CHF | −ve CHF | MI | MI + Tre® |
| Induction | Aortic coarctation | kidney dissection | Streptozotocin | IV infusion | LAD ligation | |||||||
| Total IVPG | ↑ | ↓ | ↓ | NS | NS | ND | NS | ↑ | NS | NS | ↓ | NS |
| Basal IVPG | ↑ | ↓ | ↓ | NS | NS | ND | NS | ↑ | ↑ | ↑ | ↓ | NS |
| Mid-to-apical IVPG | NS | NS | NS | NS | NS | ND | ↑ | NS | ↓ | ↓ | NS | NS |
| Mid IVPG | ND | ND | ND | ND | ND | ND | ↑ | NS | ↓ | ↓ | ↓ | ↓ |
| AIVPG | ND | ND | ND | ND | ND | ND | ↑ | NS | ↓ | ↓ | NS | NS |
| Total IVPD | ND | ND | ND | ND | ND | ↓ | NS | ↑ | ND | ND | ND | ND |
| Basal IVPD | ND | ND | ND | ND | ND | ↓ | ↓ | ↑ | ND | ND | ND | ND |
| Mid IVPD | ND | ND | ND | ND | ND | NS | ↑ | NS | ND | ND | ND | ND |
| Mid-to-apical IVPD | ND | ND | ND | ND | ND | ND | ↑ | NS | ND | ND | ND | ND |
| Apical IVPD | ND | ND | ND | ND | ND | NS | ↑ | ↑ | ND | ND | ND | ND |
| Reference | [24] | [55] | [23] | [22] | [48,49] | [50] | ||||||
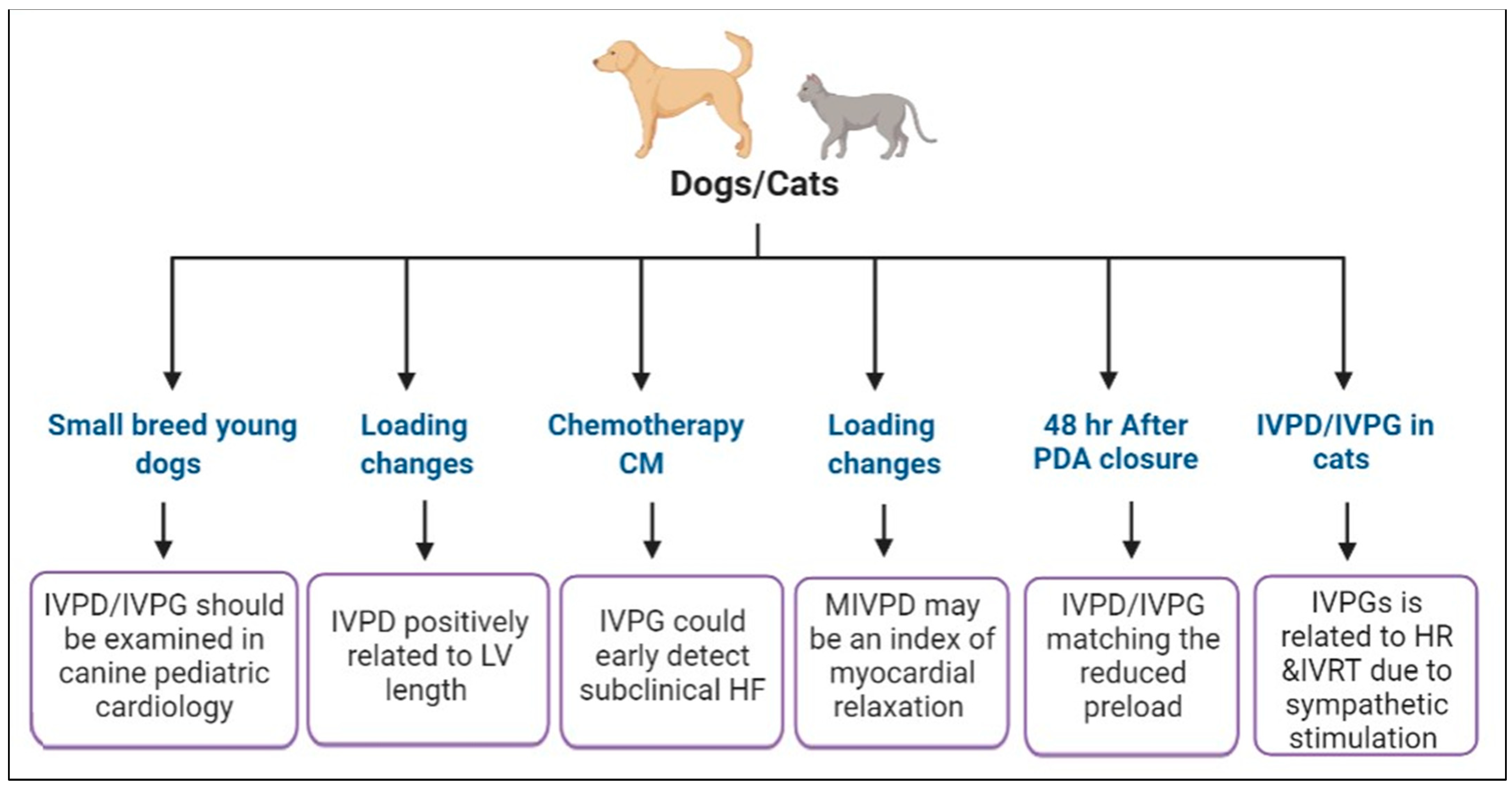
- B.
- IVPD and IVPG in experimental studies in dogs
- (a)
- Changes in IVPD and IVPG in loading conditions
- (b)
- Chemotherapy-induced heart failure
- C.
- IVPD and IVPG measurements in the clinical setting in companion animal medicine
- D.
- IVPD and IVPG in pigs
- E.
- CMME-derived IVPD and IVPG in sheep and goats
5. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roger, V.L. Epidemiology of Heart Failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sakata, Y.; Ohtani, T.; Takeda, Y.; Mano, T. Heart failure with preserved ejection fraction. Circ. J. 2009, 73, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Mandour, A.S.; Matsuura, K.; Shimada, K.; El-Husseiny, H.M.; Hamabe, L.; Yilmaz, Z.; Uemura, A.; Tanaka, R. Changes in the Pulmonary Artery Wave Reflection in Dogs with Experimentally-Induced Acute Pulmonary Embolism and the Effect of Vasodilator. Animals 2021, 11, 1977. [Google Scholar] [CrossRef] [PubMed]
- Boon, J.A. Veterinary Echocardiography; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Otto, C.M. Textbook of Clinical Echocardiography; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagueh, S.F. Non-invasive assessment of left ventricular filling pressure. Eur. J. Heart Fail. 2018, 20, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faggiano, P.; Vizzardi, E.; Pulcini, E.; Maffeo, D.; Fracassi, F.; Nodari, S.; Dei Cas, L. The study of left ventricular diastolic function by Doppler echocardiography: The essential for the clinician. Heart Int. 2007, 3, 42. [Google Scholar] [CrossRef]
- Nagueh, S.F. Left Ventricular Diastolic Function: Understanding Pathophysiology, Diagnosis, and Prognosis with Echocardiography. JACC Cardiovasc. Imaging 2020, 13, 228–244. [Google Scholar] [CrossRef]
- Vizzardi, E.; Faggiano, P.; Chiari, E.; Maffeo, D.; Frattini, S.; Bellandi, F.; Nodari, S.; Dei Cas, L. The study of left ventricular diastolic function by Doppler echocardiography: The essential for the clinician. Monaldi Arch. Chest Dis. 2009, 72, 23–28. [Google Scholar] [CrossRef]
- Hidekatsu, F.; Nobuyuki, O.; Kazuaki, W.; Toshihiko, G.; Tomomitsu, T.; Genjiro, K. Prognostic Value of Left Ventricular Diastolic Dysfunction in Patients Undergoing Cardiac Catheterization for Coronary Artery Disease. Cardiol. Res. Pract. 2012, 2012, 243735. [Google Scholar]
- Flachskampf, F.A.; Biering-Sørensen, T.; Solomon, S.D.; Duvernoy, O.; Bjerner, T.; Smiseth, O.A. Cardiac Imaging to Evaluate Left Ventricular Diastolic Function. JACC Cardiovasc. Imaging 2015, 8, 1071–1093. [Google Scholar] [CrossRef] [Green Version]
- Little, W.C. Diastolic dysfunction beyond distensibility: Adverse effects of ventricular dilatation. Circulation 2005, 112, 2888–2890. [Google Scholar] [CrossRef] [Green Version]
- Courtois, M.; Kovács, S.J., Jr.; Ludbrook, P.A. Transmitral pressure-flow velocity relation. Importance of regional pressure gradients in the left ventricle during diastole. Circulation 1988, 78, 661–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, D.; Rankin, J.S.; Edwards, C.H., 2nd; McHale, P.A.; Anderson, R.W. Regional diastolic mechanics of the left ventricle in the conscious dog. Am. J. Physiol. 1979, 236, H323–H330. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, H.L.; Verani, M.S.; Chen, C.J.; Cramer, J.A. Regional pressure differences in the left ventricle. Catheter. Cardiovasc. Diagn. 1980, 6, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.L.; Vandervoort, P.M.; Firstenberg, M.S.; Garcia, M.J.; Thomas, J.D. Estimation of diastolic intraventricular pressure gradients by Doppler M-mode echocardiography. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2507–H2515. [Google Scholar] [CrossRef]
- Takahashi, K.; Nii, M.; Takigiku, K.; Toyono, M.; Iwashima, S.; Inoue, N.; Tanaka, N.; Matsui, K.; Shigemitsu, S.; Yamada, M.; et al. Development of suction force during early diastole from the left atrium to the left ventricle in infants, children, and adolescents. Heart Vessel. 2019, 34, 296–306. [Google Scholar] [CrossRef]
- Iwano, H.; Kamimura, D.; Fox, E.; Hall, M.; Vlachos, P.; Little, W.C. Altered spatial distribution of the diastolic left ventricular pressure difference in heart failure. J. Am. Soc. Echocardiogr. 2015, 28, 597–605.e591. [Google Scholar] [CrossRef] [Green Version]
- Ohara, T.; Niebel, C.L.; Stewart, K.C.; Charonko, J.J.; Pu, M.; Vlachos, P.P.; Little, W.C. Loss of adrenergic augmentation of diastolic intra-LV pressure difference in patients with diastolic dysfunction: Evaluation by color M-mode echocardiography. JACC Cardiovasc. Imaging 2012, 5, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, K.; Sato, K.; Shimada, K.; Goya, S.; Uemura, A.; Iso, T.; Yazaki, K.; Yilmaz, Z.; Takahashi, K.; Tanaka, R. Changes in left ventricular blood flow during diastole due to differences in chamber size in healthy dogs. Sci. Rep. 2020, 10, 1106. [Google Scholar] [CrossRef] [Green Version]
- Yairo, A.; Mandour, A.S.; Matsuura, K.; Yoshida, T.; Ma, D.; Kitpipatkun, P.; Kato, K.; Cheng, C.J.; El-Husseiny, H.M.; Tanaka, T.; et al. Effect of Loading Changes on the Intraventricular Pressure Measured by Color M-Mode Echocardiography in Rats. Diagnostics 2021, 11, 1403. [Google Scholar] [CrossRef]
- Kitpipatkun, P.; Matsuura, K.; Shimada, K.; Uemura, A.; Goya, S.; Yoshida, T.; Ma, D.; Takahashi, K.; Tanaka, R. Key factors of diastolic dysfunction and abnormal left ventricular relaxation in diabetic rats. J. Med. Ultrason. 2020, 47, 347–356. [Google Scholar] [CrossRef]
- Ma, D.; Mandour, A.S.; Yoshida, T.; Matsuura, K.; Shimada, K.; Kitpipatkun, P.; Uemura, A.; Ifuku, M.; Takahashi, K.; Tanaka, R. Intraventricular pressure gradients change during the development of left ventricular hypertrophy: Effect of salvianolic acid B and beta-blocker. Ultrasound 2021, 29, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Bach, M.B.T.; Takahashi, K.; Willesen, J.L.; Koch, J.; Tanaka, R. Non-invasive assessment of left ventricular relaxation property using color M-mode-derived intraventricular pressure gradients in cats. J. Vet. Cardiol. 2022, 41, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Shiraishi, K.; Mandour, A.S.; Sato, K.; Shimada, K.; Goya, S.; Yoshida, T.; Kitpipatkun, P.; Hamabe, L.; Uemura, A.; et al. The Utility of Intraventricular Pressure Gradient for Early Detection of Chemotherapy-Induced Subclinical Cardiac Dysfunction in Dogs. Animals 2021, 11, 1122. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takahashi, K.; Yamada, M.; Yazaki, K.; Matsui, K.; Tanaka, N.; Shigemitsu, S.; Akimoto, K.; Kishiro, M.; Nakanishi, K.; et al. Assessment of early diastolic intraventricular pressure gradient in the left ventricle among patients with repaired tetralogy of Fallot. Heart Vessel. 2017, 32, 1364–1374. [Google Scholar] [CrossRef]
- Yotti, R.; Bermejo, J.; Antoranz, J.C.; Desco, M.M.; Cortina, C.; Rojo-Alvarez, J.L.; Allué, C.; Martín, L.; Moreno, M.; Serrano, J.A.; et al. A noninvasive method for assessing impaired diastolic suction in patients with dilated cardiomyopathy. Circulation 2005, 112, 2921–2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popović, Z.B.; Richards, K.E.; Greenberg, N.L.; Rovner, A.; Drinko, J.; Cheng, Y.; Penn, M.S.; Fukamachi, K.; Mal, N.; Levine, B.D.; et al. Scaling of diastolic intraventricular pressure gradients is related to filling time duration. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H762–H769. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.S.; Rowin, E.J.; Manning, W.J.; Maron, M.S.; Nezafat, R. Machine Learning for Predicting Heart Failure Progression in Hypertrophic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 647857. [Google Scholar] [CrossRef] [PubMed]
- Ironside, V.A.; Tricklebank, P.R.; Boswood, A. Risk indictors in cats with preclinical hypertrophic cardiomyopathy: A prospective cohort study. J. Feline Med. Surg. 2020, 23, 149–159. [Google Scholar] [CrossRef]
- Elliott, P.; McKenna, W.J. Hypertrophic cardiomyopathy. Lancet 2004, 363, 1881–1891. [Google Scholar] [CrossRef]
- Kittleson, M.D.; Côté, E. The Feline Cardiomyopathies: 2. Hypertrophic cardiomyopathy. J. Feline Med. Surg. 2021, 23, 1028–1051. [Google Scholar] [CrossRef] [PubMed]
- Häggström, J.; Luis Fuentes, V.; Wess, G. Screening for hypertrophic cardiomyopathy in cats. J. Vet. Cardiol. 2015, 17, S134–S149. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.R.; Borgeat, K.; Connolly, D.J.; Boswood, A.; Dennis, S.; Wagner, T.; Menaut, P.; Maerz, I.; Evans, D.; Simons, V.E.; et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 2013, 27, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.R.; Borgeat, K.; Brodbelt, D.C.; Connolly, D.J.; Luis Fuentes, V. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J. Vet. Cardiol. 2015, 17 (Suppl. 1), S318–S328. [Google Scholar] [CrossRef] [Green Version]
- Rohrbaugh, M.N.; Schober, K.E.; Rhinehart, J.D.; Bonagura, J.D.; Habing, A.; Yildiz, V. Detection of congestive heart failure by Doppler echocardiography in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 2020, 34, 1091–1101. [Google Scholar] [CrossRef]
- Williams, L.J.; Nye, B.G.; Wende, A.R. Diabetes-Related Cardiac Dysfunction. Endocrinol. Metab. 2017, 32, 171–179. [Google Scholar] [CrossRef]
- Domanski, M.; Krause-Steinrauf, H.; Deedwania, P.; Follmann, D.; Ghali, J.K.; Gilbert, E.; Haffner, S.; Katz, R.; Lindenfeld, J.; Lowes, B.D.; et al. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J. Am. Coll. Cardiol. 2003, 42, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Leese, G.P.; Savage, M.W.; Chattington, P.D.; Vora, J.P. The diabetic patient with hypertension. Postgrad. Med. J. 1996, 72, 263. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, Z.-G.; Gao, Y.; Xie, L.-J.; Jiang, L.; Hu, B.-Y.; Diao, K.-Y.; Shi, K.; Xu, H.-Y.; Shen, M.-T.; et al. Left ventricular subclinical myocardial dysfunction in uncomplicated type 2 diabetes mellitus is associated with impaired myocardial perfusion: A contrast-enhanced cardiovascular magnetic resonance study. Cardiovasc. Diabetol. 2018, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Voulgari, C.; Papadogiannis, D.; Tentolouris, N. Diabetic cardiomyopathy: From the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc. Health Risk Manag. 2010, 6, 883–903. [Google Scholar] [CrossRef] [Green Version]
- Iso, T.; Takahashi, K.; Yazaki, K.; Ifuku, M.; Nii, M.; Fukae, T.; Yazawa, R.; Ishikawa, A.; Haruna, H.; Takubo, N.; et al. In-Depth Insight Into the Mechanisms of Cardiac Dysfunction in Patients With Type 1 Diabetes Mellitus Using Layer-Specific Strain Analysis. Circ. J. 2019, 83, 1330–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ifuku, M.; Takahashi, K.; Hosono, Y.; Iso, T.; Ishikawa, A.; Haruna, H.; Takubo, N.; Komiya, K.; Kurita, M.; Ikeda, F.; et al. Left atrial dysfunction and stiffness in pediatric and adult patients with Type 1 diabetes mellitus assessed with speckle tracking echocardiography. Pediatr. Diabetes 2021, 22, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Mandour, A.S.; Hamabe, L.; Yoshida, T.; Shimada, K.; Tanaka, R. Novel protocol to establish the myocardial infarction model in rats using a combination of medetomidine-midazolam-butorphanol (MMB) and atipamezole. Front. Vet. Sci. 2022, 9, 1064836. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Mandour, A.S.; Hendawy, H.; Elhaieg, A.; Elfadadny, A.; Tanaka, R. A Review on Experimental Surgical Models and Anesthetic Protocols of Heart Failure in Rats. Front. Vet. Sci. 2023, 10, 386. [Google Scholar] [CrossRef]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 2019, 114, 19. [Google Scholar] [CrossRef]
- El-Husseiny, H.; Mady, E.; Shimada, K.; Hamabe, L.; Yoshida, T.; Ma, D.; Mandour, A.; Hendawy, H.; Sasaki, K.; Fukuzumi, S.; et al. Intraventricular pressure gradient: A promising tool to predict the post-infarction chronic congestive heart failure in rats. Eur. Heart J.—Cardiovasc. Imaging 2022, 23, jeab289.390. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Ma, D.; Hamabe, L.; Takahashi, K.; Tanaka, R. Intraventricular pressure gradient: A novel tool to assess the post-infarction chronic congestive heart failure. Front. Cardiovasc. Med. 2022, 9, 944171. [Google Scholar] [CrossRef]
- Farag, A.; Mandour, A.S.; Kaneda, M.; Elfadadny, A.; Elhaieg, A.; Shimada, K.; Tanaka, R. Effect of trehalose on heart functions in rats model after myocardial infarction: Assessment of novel intraventricular pressure and heart rate variability. Front. Cardiovasc. Med. 2023, 10, 1182628. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [Green Version]
- de Albuquerque Suassuna, P.G.; Sanders-Pinheiro, H.; de Paula, R.B. Uremic Cardiomyopathy: A New Piece in the Chronic Kidney Disease-Mineral and Bone Disorder Puzzle. Front. Med. 2018, 5, 206. [Google Scholar] [CrossRef] [Green Version]
- Alhaj, E.; Alhaj, N.; Rahman, I.; Niazi, T.O.; Berkowitz, R.; Klapholz, M. Uremic cardiomyopathy: An underdiagnosed disease. Congest. Heart Fail. 2013, 19, E40–E45. [Google Scholar] [CrossRef]
- Ganau, A.; Devereux, R.B.; Roman, M.J.; de Simone, G.; Pickering, T.G.; Saba, P.S.; Vargiu, P.; Simongini, I.; Laragh, J.H. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J. Am. Coll. Cardiol. 1992, 19, 1550–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Mandour, A.S.; Elfadadny, A.; Hendawy, H.; Yoshida, T.; El-Husseiny, H.M.; Nishifuji, K.; Takahashi, K.; Zhou, Z.; Zhao, Y.; et al. Changes in Cardiac Function During the Development of Uremic Cardiomyopathy and the Effect of Salvianolic Acid B Administration in a Rat Model. Front. Vet. Sci. 2022, 9, 905759. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Shiraishi, K.; Sato, K.; Shimada, K.; Goya, S.; Uemura, A.; Ifuku, M.; Iso, T.; Takahashi, K.; Tanaka, R. Left ventricular vortex and intraventricular pressure difference in dogs under various loading conditions. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H882–H888. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M. Leite-Moreira A, F. Relevance of intraventricular pressure gradients in left ventricular diastolic and systolic function: Clinical implications. Rev. Port. Cir. Cardiotorac. Vasc. 2018, 25, 19–26. [Google Scholar]
- Elfadadny, A.; El-Husseiny, H.M.; Abugomaa, A.; Ragab, R.F.; Mady, E.A.; Aboubakr, M.; Samir, H.; Mandour, A.S.; El-Mleeh, A.; El-Far, A.H.; et al. Role of multidrug resistance-associated proteins in cancer therapeutics: Past, present, and future perspectives. Environ. Sci. Pollut. Res. 2021, 28, 49447–49466. [Google Scholar] [CrossRef]
- Bhagat, A.; Kleinerman, E.S. Anthracycline-Induced Cardiotoxicity: Causes, Mechanisms, and Prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192. [Google Scholar]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef]
- Shigemitsu, S.; Takahashi, K.; Yazaki, K.; Kobayashi, M.; Yamada, M.; Akimoto, K.; Tamaichi, H.; Fujimura, J.; Saito, M.; Nii, M.; et al. New insight into the intraventricular pressure gradient as a sensitive indicator of diastolic cardiac dysfunction in patients with childhood cancer after anthracycline therapy. Heart Vessel. 2019, 34, 992–1001. [Google Scholar] [CrossRef]
- Hirose, M.; Mandour, A.S.; Goya, S.; Hamabe, L.; Matsuura, K.; Yoshida, T.; Watanabe, M.; Shimada, K.; Uemura, A.; Takahashi, K.; et al. Color M-Mode Echocardiography for Non-Invasive Assessment of the Intraventricular Pressure in Dogs Before and After Ductus Arteriosus Occlusion: A Retrospective Study. Front. Vet. Sci. 2022, 9, 908829. [Google Scholar] [CrossRef] [PubMed]
- Mandour, A.; Hirose, M.; Hamabe, L.; Momoko, W.; Uemuhara, K.; Shimada, K.; Tanaka, R. Color M-mode echocardiography-derived intraventricular pressure measurements in clinically healthy small breed dogs. In Proceedings of the 115th Japanese Society of Veterinary Cardiology, Tokyo, Japan, 18–19 December 2021. [Google Scholar]
- Saunders, A.B.; Gordon, S.G.; Boggess, M.M.; Miller, M.W. Long-term outcome in dogs with patent ductus arteriosus: 520 cases (1994–2009). J. Vet. Intern. Med. 2014, 28, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Edwards, J.; Ferguson-Mignan, T.F.; Cobb, M.; Mongan, N.P.; Rutland, C.S. Genetics of Human and Canine Dilated Cardiomyopathy. Int. J. Genom. 2015, 2015, 204823. [Google Scholar] [CrossRef] [Green Version]
- Cortina, C.; Bermejo, J.; Yotti, R.; Desco, M.M.; Rodríguez-Pérez, D.; Antoranz, J.C.; Rojo-Alvarez, J.L.; Garcia, D.; García-Fernández, M.A.; Fernández-Avilés, F. Noninvasive assessment of the right ventricular filling pressure gradient. Circulation 2007, 116, 1015–1023. [Google Scholar] [CrossRef] [Green Version]
- McCauley, S.R.; Clark, S.D.; Quest, B.W.; Streeter, R.M.; Oxford, E.M. Review of canine dilated cardiomyopathy in the wake of diet-associated concerns. J. Anim. Sci. 2020, 98, skaa155. [Google Scholar] [CrossRef]
- Hamabe, L.; Mandour, A.S.; Shimada, K.; Uemura, A.; Yilmaz, Z.; Nagaoka, K.; Tanaka, R. Role of Two-Dimensional Speckle-Tracking Echocardiography in Early Detection of Left Ventricular Dysfunction in Dogs. Animals 2021, 11, 2361. [Google Scholar] [CrossRef]
- Hamabe, L.; Shimada, K.; Mandour, A.S.; Yoshida, T.; Hirose, M.; Hendawy, H.; El-Husseiny, H.M.; Tanaka, R. Evaluation of Left Ventricular Function in Healthy Retrievers Using Standard and 2D Speckle-Tracking Echocardiography. Vet. Sci. 2022, 9, 529. [Google Scholar] [CrossRef]
- O’Sullivan, M.L.; O’Grady, M.R.; Minors, S.L. Assessment of diastolic function by Doppler echocardiography in normal Doberman Pinschers and Doberman Pinschers with dilated cardiomyopathy. J. Vet. Intern. Med. 2007, 21, 81–91. [Google Scholar] [CrossRef]
- Freid, K.J.; Freeman, L.M.; Rush, J.E.; Cunningham, S.M.; Davis, M.S.; Karlin, E.T.; Yang, V.K. Retrospective study of dilated cardiomyopathy in dogs. J. Vet. Intern. Med. 2021, 35, 58–67. [Google Scholar] [CrossRef]
- Kim, D.H.; Morris, B.; Guerrero, J.L.; Sullivan, S.M.; Hung, J.; Levine, R.A. Ovine Model of Ischemic Mitral Regurgitation. Methods Mol. Biol. 2018, 1816, 295–308. [Google Scholar]
- Leroux, A.A.; Moonen, M.L.; Farnir, F.; Sandersen, C.F.; Deleuze, S.; Salciccia, A.; Amory, H. Two-dimensional and M-mode echocardiographic reference values in healthy adult Saanen goats. Vet. Rec. 2012, 170, 154. [Google Scholar] [CrossRef] [PubMed]
- Mandour, A.S.; Samir, H.; El-Beltagy, M.A.; Hamabe, L.; Abdelmageed, H.A.; Watanabe, I.; Elfadadny, A.; Shimada, K.; El-Masry, G.; Al-Rejaie, S.; et al. Monthly Dynamics of Plasma Elements, Hematology, Oxidative Stress Markers, and Hormonal Concentrations in Growing Male Shiba Goats (Capra hircus) Reared in Tokyo-Japan. Animals 2022, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Mandour, A.S.; Samir, H.; Yoshida, T.; Matsuura, K.; Abdelmageed, H.A.; Elbadawy, M.; Al-Rejaie, S.; El-Husseiny, H.M.; Elfadadny, A.; Ma, D.; et al. Assessment of the Cardiac Functions Using Full Conventional Echocardiography with Tissue Doppler Imaging before and after Xylazine Sedation in Male Shiba Goats. Animals 2020, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Asada-Kamiguchi, J.; Jones, M.; Greenberg, N.L.; Popovic, Z.B.; Tsujino, H.; Zetts, A.D.; Qin, J.X.; Garcia, M.J.; Thomas, J.D.; Shiota, T. Intraventricular pressure gradients in left ventricular aneurysms determined by color M-mode Doppler method: An animal study. J. Am. Soc. Echocardiogr. 2006, 19, 1112–1118. [Google Scholar] [CrossRef]
- Mandour, A.S.; Samir, H.; Yoshida, T.; Matsuura, K.; Hamabe, L.; Shimada, K.; Abdelmageed, H.A.; Elbadawy, M.; Uemura, A.; Takahashi, K.; et al. Novel color M-mode echocardiography for non-invasive assessment of the intraventricular pressure in goats: Feasibility, repeatability, and the effect of sedation. Front. Vet. Sci. 2022, 9, 935437. [Google Scholar] [CrossRef]
- Samir, H.; Mandour, A.S.; Radwan, F.; Ahmed, A.E.; Momenah, M.A.; Aldawood, N.A.; Yoshida, T.; Watanabe, G.; El-Sherbiny, H.R. Effect of Acute Melatonin Injection on Metabolomic and Testicular Artery Hemodynamic Changes and Circulating Hormones in Shiba Goats under Sub-Tropical Environmental Conditions. Animals 2023, 13, 1794. [Google Scholar] [CrossRef]
- Ahmed, M.; Samir, H.; Yoshida, T.; Matsuura, K.; Elhussieny, H.; Ma, D.; Abdelmageed, H.A.; Hamble, L.; Watanabe, G.; Tanaka, R. Effect of acute melatonin administration on heart function in goats: Full conventional and speckle tracking echocardiography and intraventricular pressure gradients change. In Proceedings of the 114th Annual Meeting of Japanese Society of Veterinary Cardiology, Tokyo, Japan, 3–4 July 2021. [Google Scholar]
- Panesar, D.K.; Burch, M. Assessment of Diastolic Function in Congenital Heart Disease. Front. Cardiovasc. Med. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Berli, A.S.; Jud Schefer, R.; Steininger, K.; Schwarzwald, C.C. The use of strain, strain rate, and displacement by 2D speckle tracking for assessment of systolic left ventricular function in goats: Applicability and influence of general anesthesia. Cardiovasc. Ultrasound 2015, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Ma, D.; Mandour, A.S.; Ozai, Y.; Yoshida, T.; Matsuura, K.; Takeuchi, A.; Cheng, C.-J.; El-Husseiny, H.M.; Hendawy, H.; et al. Evaluation of Changes in the Cardiac Function before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography. Animals 2022, 12, 56. [Google Scholar] [CrossRef]
- Samir, H.; Nyametease, P.; Elbadawy, M.; Fathi, M.; Mandour, A.S.; Radwan, F.; Nagaoka, K.; Sasaki, K.; Watanabe, G. Assessment of correlations and concentrations of salivary and plasma steroids, testicular morphometry, and semen quality in different climatic conditions in goats. Theriogenology 2020, 157, 238–244. [Google Scholar] [CrossRef]
- Mandour, A.S.; Elsayed, R.F.; Ali, A.O.; Mahmoud, A.E.; Samir, H.; Dessouki, A.A.; Matsuura, K.; Watanabe, I.; Sasaki, K.; Al-Rejaie, S.; et al. The utility of electrocardiography and echocardiography in copper deficiency-induced cardiac damage in goats. Environ. Sci. Pollut. Res. Int. 2021, 28, 7815–7827. [Google Scholar] [CrossRef]
- Mandour, A.S.; Mahmoud, A.E.; Ali, A.O.; Matsuura, K.; Samir, H.; Abdelmageed, H.A.; Ma, D.; Yoshida, T.; Hamabe, L.; Uemura, A.; et al. Expression of cardiac copper chaperone encoding genes and their correlation with cardiac function parameters in goats. Vet. Res. Commun. 2021, 45, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Mandour, A.S.; Samir, H.; El-Beltagy, M.A.; Abdel-Daim, M.M.; Izumi, W.; Ma, D.; Matsuura, K.; Tanaka, R.; Watanabe, G. Effect of supra-nutritional selenium-enriched probiotics on hematobiochemical, hormonal, and Doppler hemodynamic changes in male goats. Environ. Sci. Pollut. Res. Int. 2020, 27, 19447–19460. [Google Scholar] [CrossRef] [PubMed]
- Samir, H.; Mandour, A.S.; Radwan, F.; Swelum, A.A.; Yoshida, T.; Tanaka, R.; Nagaoka, K.; Watanabe, G. Diurnal rhythms in testicular blood flow, testicular morphometry and reproductive hormones in Shiba goats. Reprod. Fertil. Dev. 2022, 34, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Samir, H.; Mandour, A.S.; Radwan, F.; Swelum, A.A.; Nagaoka, K.; Sasaki, K.; Watanabe, G. Effect of xylazine sedation on testicular blood flow, testicular echotexture, and circulating hormones in Shiba goats. Vet. Res. Commun. 2022, 47, 849–859. [Google Scholar] [CrossRef] [PubMed]
| Experimental Model | Chemotherapy CM | Overloading | |||
|---|---|---|---|---|---|
| Time | After 4.5 Months | After 18 Months | Pressure Load | Volume Load | Milrinone |
| Total IVPD | ND | ND | ↓ | ↑ | NS |
| Basal IVPD | ND | ND | NS | NS | NS |
| Mid-to-apical IVPD | ND | ND | NS | ND | ND |
| Mid IVPD | ND | ND | ↓ | NS | ↑ |
| Apical IVPD | ND | ND | NS | ↑ | NS |
| Total IVPG | NS | ↓ | ND | ND | ND |
| Basal IVPG | NS | NS | ND | ND | ND |
| Mid-to-apical IVPG | NS | ↓ | ND | ND | ND |
| Mid IVPG | ↓ | ↓ | ND | ND | ND |
| Apical IVPG | NS | NS | ND | ND | ND |
| References | [26] | [56] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandour, A.S.; Farag, A.; Helal, M.A.Y.; El-Masry, G.; Al-Rejaie, S.; Takahashi, K.; Yoshida, T.; Hamabe, L.; Tanaka, R. Non-Invasive Assessment of the Intraventricular Pressure Using Novel Color M-Mode Echocardiography in Animal Studies: Current Status and Future Perspectives in Veterinary Medicine. Animals 2023, 13, 2452. https://doi.org/10.3390/ani13152452
Mandour AS, Farag A, Helal MAY, El-Masry G, Al-Rejaie S, Takahashi K, Yoshida T, Hamabe L, Tanaka R. Non-Invasive Assessment of the Intraventricular Pressure Using Novel Color M-Mode Echocardiography in Animal Studies: Current Status and Future Perspectives in Veterinary Medicine. Animals. 2023; 13(15):2452. https://doi.org/10.3390/ani13152452
Chicago/Turabian StyleMandour, Ahmed S., Ahmed Farag, Mahmoud A. Y. Helal, Gamal El-Masry, Salim Al-Rejaie, Ken Takahashi, Tomohiko Yoshida, Lina Hamabe, and Ryou Tanaka. 2023. "Non-Invasive Assessment of the Intraventricular Pressure Using Novel Color M-Mode Echocardiography in Animal Studies: Current Status and Future Perspectives in Veterinary Medicine" Animals 13, no. 15: 2452. https://doi.org/10.3390/ani13152452
APA StyleMandour, A. S., Farag, A., Helal, M. A. Y., El-Masry, G., Al-Rejaie, S., Takahashi, K., Yoshida, T., Hamabe, L., & Tanaka, R. (2023). Non-Invasive Assessment of the Intraventricular Pressure Using Novel Color M-Mode Echocardiography in Animal Studies: Current Status and Future Perspectives in Veterinary Medicine. Animals, 13(15), 2452. https://doi.org/10.3390/ani13152452











