Biological Matrices from Cairina moschata as Non-Destructive Biomonitoring Tools to Study Environmental Quality of Urban and Extra-Urban Areas: A Case Study of Palermo (Sicily, Italy)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
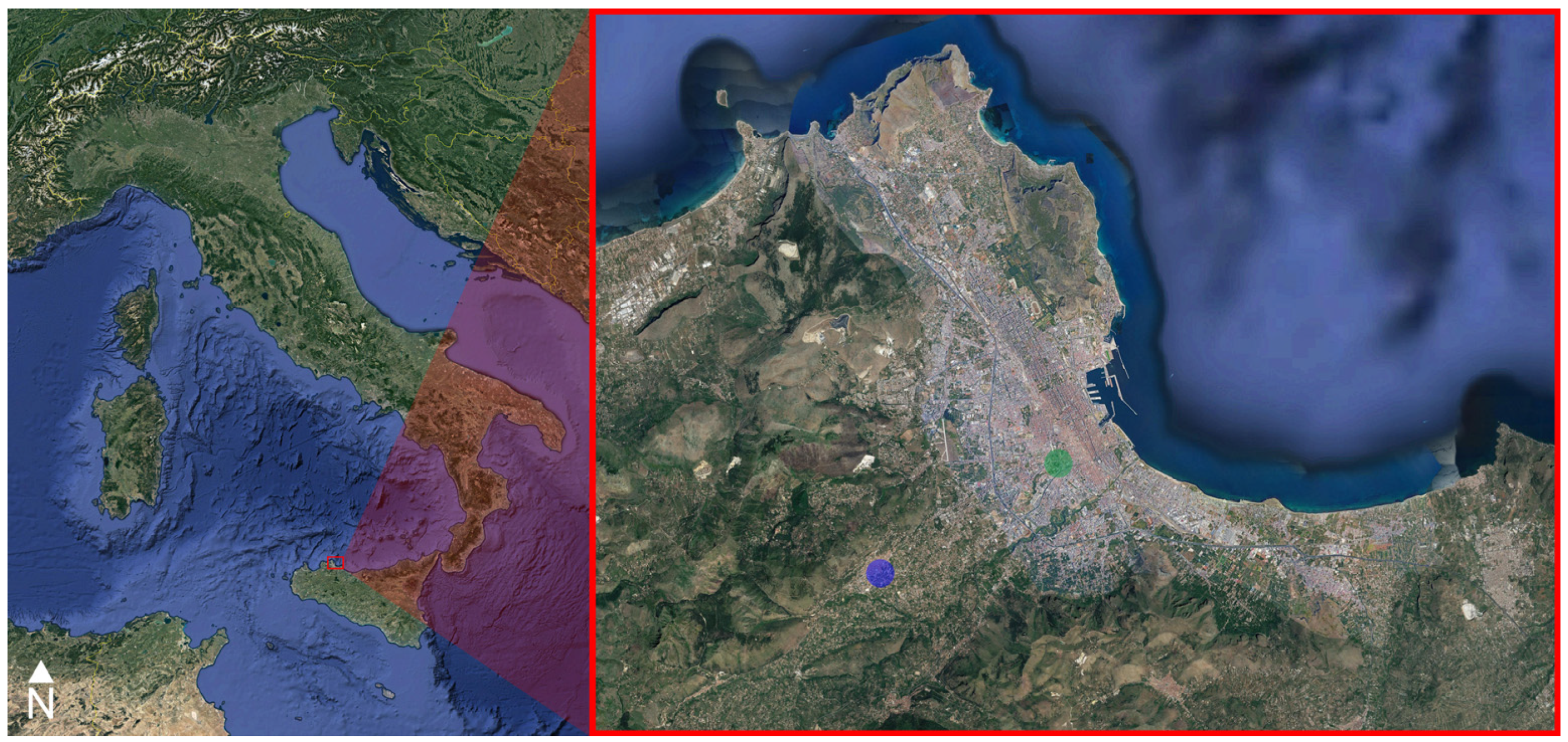
2.1. Sampling
- -
- 10 blood samples drawn from the brachial vein in Parco D’Orleans;
- -
- 10 blood samples drawn from the brachial vein in Monreale;
- -
- 10 feather samples in Parco D’Orleans;
- -
- 10 feather samples in Monreale.
2.2. Sample Preparation
2.3. Analytical Methods
2.4. Data Analyses
3. Results
3.1. Comparison between Sampling and Control Area
3.2. Comparison between Different Matrices from the Same Individuals
3.3. Comparison between Washed and Unwashed Feathers
4. Discussion
4.1. Comparison between Sampling and Control Area
4.2. Comparison between Different Matrices from the Same Individuals
4.3. Comparison between Washed and Unwashed Feathers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budnik, L.T.; Baur, X. The Assessment of Environmental and Occupational Exposure to Hazardous Substances by Biomonitoring. Dtsch. Ärztebl. Int. 2009, 106, 91. [Google Scholar] [CrossRef] [PubMed]
- Amadi, C.N.; Frazzoli, C.; Orisakwe, O.E. Sentinel Species for Biomonitoring and Biosurveillance of Environmental Heavy Metals in Nigeria. J. Environ. Sci. Health Part C 2020, 38, 21–60. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P. Biomonitoring and Environmental Management. Environ. Monit. Assess. 1990, 14, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU Of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance. OJ L 276, 20.10.2010, p. 33–79. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 20 March 2023).
- Fossi, M.C. Nondestructive Biomarkers in Ecotoxicology. Environ. Health Perspect. 1994, 102, 49–54. [Google Scholar] [CrossRef]
- Justino, C.; Duarte, A.; Rocha-Santos, T. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An Appealing Tool for Assessment of Metal Pollution in the Aquatic Ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef]
- Thakur, S.; Dhyani, S.; Bramhanwade, K.; Pandey, K.K.; Bokade, N.; Janipella, R.; Pujari, P. Non-Invasive Biomonitoring of Mercury in Birds near Thermal Power Plants: Lessons from Maharashtra, India. Environ. Monit. Assess. 2020, 192, 260. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A Review of Toxicity and Mechanisms of Individual and Mixtures of Heavy Metals in the Environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Hanfi, M.Y.; Mostafa, M.Y.A.; Zhukovsky, M.V. Heavy Metal Contamination in Urban Surface Sediments: Sources, Distribution, Contamination Control, and Remediation. Environ. Monit. Assess. 2020, 192, 32. [Google Scholar] [CrossRef]
- Kim, R.-Y.; Yoon, J.-K.; Kim, T.-S.; Yang, J.E.; Owens, G.; Kim, K.-R. Bioavailability of Heavy Metals in Soils: Definitions and Practical Implementation—A Critical Review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Fasola, M.; Muhammad, A.; Malik, S.A.; Bostan, N.; Bokhari, H.; Kamran, M.A.; Shafqat, M.N.; Alamdar, A.; Khan, M.; et al. Avian Feathers as a Non-Destructive Bio-Monitoring Tool of Trace Metals Signatures: A Case Study from Severely Contaminated Areas. Chemosphere 2015, 119, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Lemly, A.D. A Protocol for Aquatic Hazard Assessment of Selenium. Ecotoxicol. Environ. Saf. 1995, 32, 280–288. [Google Scholar] [CrossRef]
- Solgi, E.; Mirzaei-Rajeouni, E.; Zamani, A. Feathers of Three Waterfowl Bird Species from Northern Iran for Heavy Metals Biomonitoring. Bull. Environ. Contam. Toxicol. 2020, 104, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Furness, R.W. Birds as Monitors of Pollutants. In Birds as Monitors of Environmental Change; Furness, R.W., Greenwood, J.J.D., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 86–143. ISBN 978-94-015-1324-1. [Google Scholar]
- Malik, R.N.; Zeb, N. Assessment of Environmental Contamination Using Feathers of Bubulcus Ibis L., as a Biomonitor of Heavy Metal Pollution, Pakistan. Ecotoxicology 2009, 18, 522–536. [Google Scholar] [CrossRef]
- Scheuhammer, A.M. The Chronic Toxicity of Aluminium, Cadmium, Mercury, and Lead in Birds: A Review. Environ. Pollut. 1987, 46, 263–295. [Google Scholar] [CrossRef]
- Binkowski, Ł.J.; Sawicka-Kapusta, K.; Szarek, J.; Strzyżewska, E.; Felsmann, M. Histopathology of Liver and Kidneys of Wild Living Mallards Anas Platyrhynchos and Coots Fulica Atra with Considerable Concentrations of Lead and Cadmium. Sci. Total Environ. 2013, 450–451, 326–333. [Google Scholar] [CrossRef]
- Braune, B.M.; Scheuhammer, A.M. TRACE ELEMENT AND METALLOTHIONEIN CONCENTRATIONS IN SEABIRDS FROM THE CANADIAN ARCTIC. Environ. Toxicol. Chem. 2008, 27, 645. [Google Scholar] [CrossRef]
- Di Giulio, R.T.; Scanlon, P.F. Heavy Metals in Tissues of Waterfowl from the Chesapeake Bay, USA. Environ. Pollut. Ser. Ecol. Biol. 1984, 35, 29–48. [Google Scholar] [CrossRef]
- Garcá-Fernández, A.J.; Sanchez-Garcia, J.A.; Gomez-Zapata, M.; Luna, A. Distribution of Cadmium in Blood and Tissues of Wild Birds. Arch. Environ. Contam. Toxicol. 1996, 30, 252–258. [Google Scholar] [CrossRef]
- Ansara-Ross, T.M.; Ross, M.J.; Wepener, V. The Use of Feathers in Monitoring Bioaccumulation of Metals and Metalloids in the South African Endangered African Grass-Owl (Tyto Capensis). Ecotoxicology 2013, 22, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.-H.S.; Hassanpour, M.; Pourkhabbaz, A.-R.; Błaszczyk, M.; Paluch, J.; Binkowski, Ł.J. Trace Element Concentrations in Feathers of Five Anseriformes in the South of the Caspian Sea, Iran. Environ. Monit. Assess. 2016, 188, 22. [Google Scholar] [CrossRef] [PubMed]
- Kertész, V.; Bakonyi, G.; Farkas, B. Water Pollution by Cu and Pb Can Adversely Affect Mallard Embryonic Development. Ecotoxicol. Environ. Saf. 2006, 65, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, F.; Migani, F.; Andreotti, A.; Baccetti, N.; Bianchi, N.; Birke, M.; Dinelli, E. Metals and Trace Elements in Feathers: A Geochemical Approach to Avoid Misinterpretation of Analytical Responses. Sci. Total Environ. 2016, 544, 476–494. [Google Scholar] [CrossRef]
- Goutner, V.; Furness, R.W.; Papakostas, G. Mercury in Feathers of Squacco Heron (Ardeola Ralloides) Chicks in Relation to Age, Hatching Order, Growth, and Sampling Dates. Environ. Pollut. 2001, 111, 107–115. [Google Scholar] [CrossRef]
- Frazzoli, C.; Bocca, B.; Mantovani, A. The One Health Perspective in Trace Elements Biomonitoring. J. Toxicol. Environ. Health Part B 2015, 18, 344–370. [Google Scholar] [CrossRef]
- Available online: https://store.uni.com/p/CEN11013888/en-138052002-197145/CEN11013888_OEN (accessed on 20 March 2023).
- Craighead, D.; Bedrosian, B. Blood Lead Levels of Common Ravens With Access to Big-Game Offal. J. Wildl. Manag. 2008, 72, 240–245. [Google Scholar] [CrossRef]
- Commission Decision Of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (2002/657/EC) (Notified under Document Number C(2002) 3044). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32002D0657 (accessed on 20 March 2023).
- Commission Regulation (EU) No 836/2011 of 19 August 2011 Amending Regulation (EC) No 333/2007 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Lead, Cadmium, Mercury, Inorganic Tin, 3-MCPD and Benzo(a)Pyrene in Foodstuffs Text with EEA Relevance. OJ L 215, 20.8.2011, p. 9–16. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:215:0009:0016:EN:PDF (accessed on 20 March 2023).
- Commission Regulation (EC) No 333/2007 of 28 March 2007 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Lead, Cadmium, Mercury, Inorganic Tin, 3-MCPD and Benzo(a)Pyrene in Foodstuffs (Text with EEA Relevance). OJ L 88, 29.3.2007, p. 29–38. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32007R0333 (accessed on 20 March 2023).
- Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32014R0488&from=DE (accessed on 20 March 2023).
- Available online: https://eur-lex.europa.eu/legal-content/it/ALL/?uri=CELEX%3A32002D0657 (accessed on 20 March 2023).
- Available online: https://store.uni.com/uni-cei-en-iso-iec-17025-2005 (accessed on 20 March 2023).
- Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32007R0333&from=LV (accessed on 20 March 2023).
- Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:215:0009:0016:IT:PDF (accessed on 20 March 2023).
- Available online: https://store.uni.com/uni-en-15763-2010 (accessed on 20 March 2023).
- Adout, A.; Hawlena, D.; Maman, R.; Paz-Tal, O.; Karpas, Z. Determination of Trace Elements in Pigeon and Raven Feathers by ICPMS. Int. J. Mass Spectrom. 2007, 267, 109–116. [Google Scholar] [CrossRef]
- Berglund, Å.M.M. Evaluating Blood and Excrement as Bioindicators for Metal Accumulation in Birds. Environ. Pollut. 2018, 233, 1198–1206. [Google Scholar] [CrossRef]
- Carneiro, M.; Colaço, B.; Brandão, R.; Ferreira, C.; Santos, N.; Soeiro, V.; Colaço, A.; Pires, M.J.; Oliveira, P.A.; Lavín, S. Biomonitoring of Heavy Metals (Cd, Hg, and Pb) and Metalloid (As) with the Portuguese Common Buzzard (Buteo Buteo). Environ. Monit. Assess. 2014, 186, 7011–7021. [Google Scholar] [CrossRef] [PubMed]
- Ek, K.H.; Morrison, G.M.; Lindberg, P.; Rauch, S. Comparative Tissue Distribution of Metals in Birds in Sweden Using ICP-MS and Laser Ablation ICP-MS. Arch. Environ. Contam. Toxicol. 2004, 47, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Grúz, A.; Mackle, O.; Bartha, A.; Szabó, R.; Déri, J.; Budai, P.; Lehel, J. Biomonitoring of Toxic Metals in Feathers of Predatory Birds from Eastern Regions of Hungary. Environ. Sci. Pollut. Res. 2019, 26, 26324–26331. [Google Scholar] [CrossRef]
- Hanć, A.; Zduniak, P.; Erciyas-Yavuz, K.; Sajnóg, A.; Barałkiewicz, D. Laser Ablation-ICP-MS in Search of Element Pattern in Feathers. Microchem. J. 2017, 134, 1–8. [Google Scholar] [CrossRef]
- Mikoni, N.A.; Poppenga, R.; Ackerman, J.T.; Foley, J.; Hazlehurst, J.; Purdin, G.; Aston, L.; Hargrave, S.; Jelks, K.; Tell, L.A. Trace Element Contamination in Feather and Tissue Samples from Anna’s Hummingbirds. Ecol. Indic. 2017, 80, 96–105. [Google Scholar] [CrossRef]
- Willie, S.; Brophy, C.; Clancy, V.; Lam, J.; Sturgeon, R.; Yang, L. DORM-4: Matériau de Référence Certifié de Protéines de Poissons Pour l’analyse Des Métaux à l’état de Traces. In DORM-4: Fish Protein Certified Reference Material for Trace Metals; National Research Council of Canada: Ottawa, ON, Canada, 2012. [Google Scholar] [CrossRef]
- Boncompagni, E.; Muhammad, A.; Jabeen, R.; Orvini, E.; Gandini, C.; Sanpera, C.; Ruiz, X.; Fasola, M. Egrets as Monitors of Trace-Metal Contamination in Wetlands of Pakistan. Arch. Environ. Contam. Toxicol. 2003, 45, 399–406. [Google Scholar] [CrossRef]
- Burger, J. Metals in Feathers of Brown Noddy (Anous Stolidus): Evidence for Bioaccumulation or Exposure Levels? Environ. Monit. Assess. 1993, 24, 181–187. [Google Scholar] [CrossRef]
- Ritchie, B.W.; Harrison, G.J.; Harrison, L.R. Avian Medicine: Principles and Application; HBD International Inc.: Delray Beach, FL, USA, 1994; pp. 1034–1038. [Google Scholar]
- Church, H.J.; Day, J.P.; Braithwaite, R.A.; Brown, S.S. Binding of Lead to a Metallothionein-like Protein in Human Erythrocytes. J. Inorg. Biochem. 1993, 49, 55–68. [Google Scholar] [CrossRef]
- Edwards, W.R.; Smith, K.E. Exploratory Experiments on the Stability of Mineral Profiles of Feathers. J. Wildl. Manag. 1984, 48, 853. [Google Scholar] [CrossRef]
- Buggiani, S.S.; Rindi, S. Lead Toxicosis and Salt Glands in Domestic Ducks. Bull. Environ. Contam. Toxicol. 1980, 24, 152–155. [Google Scholar] [CrossRef]
| Solution | Concentration | Volume and Solution for Extraction | Final Volume (Flask) | Solvent | |
|---|---|---|---|---|---|
| MRC | 1000 mg/L | H2O/HNO3 2% | |||
| MR1 | 10 mg/L | 100 μL | MRC | 10 mL | H2O/HNO3 2% |
| MR2 | 1 mg/L | 1000 μL | MR1 | 10 mL | H2O/HNO3 2% |
| MR3 | 500 mg/L | 500 μL | MR1 | 10 mL | H2O/HNO3 2% |
| MR4 | 100 µg/L | 1000 μL | MR2 | 10 mL | H2O/HNO3 2% |
| MR5 | 50 µg/L | 1000 μL | MR3 | 10 mL | H2O/HNO3 2% |
| MR6 | 10 µg/L | 1000 μL | MR4 | 10 mL | H2O/HNO3 2% |
| MR7 | 5 µg/L | 1000 μL | MR5 | 10 mL | H2O/HNO3 2% |
| MR8 | 1 µg/L | 1000 μL | MR6 | 10 mL | H2O/HNO3 2% |
| MR9 | 0.5 µg/L | 1000 μL | MR7 | 10 mL | H2O/HNO3 2% |
| MR10 | 0.1 µg/L | 1000 μL | MR8 | 10 mL | H2O/HNO3 2% |
| MR11 | 0.05 µg/L | 1000 μL | MR9 | 10 mL | H2O/HNO3 2% |
| Element | Measured Value (mg/kg) | Assigned Value (mg/kg) | Acceptability Interval (mg/kg) |
|---|---|---|---|
| Cadmium | 0.291 ± 0.030 | 0.299 | 0.281–0.317 |
| Lead | 0.392 ± 0.040 | 0.404 | 0.342–0.466 |
| Arsenic | 6.980 ± 0.004 | 6.87 | 6.43–7.31 |
| Solution | Concentration | Volume and Solution for Extraction | Final Volume (Flask) | Solvent | |
|---|---|---|---|---|---|
| MRC | 1000 mg/L | HCl 5% | |||
| MR1 | 10 mg/L | 100 μL | MRC | 10 mL | HCl 5% |
| MR2 | 1 mg/L | 1000 μL | MR1 | 10 mL | HCl 5% |
| MR3 | 100μg/L | 1000 μL | MR2 | 10 mL | HCl 5% |
| QC2 | 20 μg/L | 200μl | MR2 | 10 mL | HCl 5% |
| QC3 | 500 μg/L | 500 μl | MR1 | 10 mL | HCl 5% |
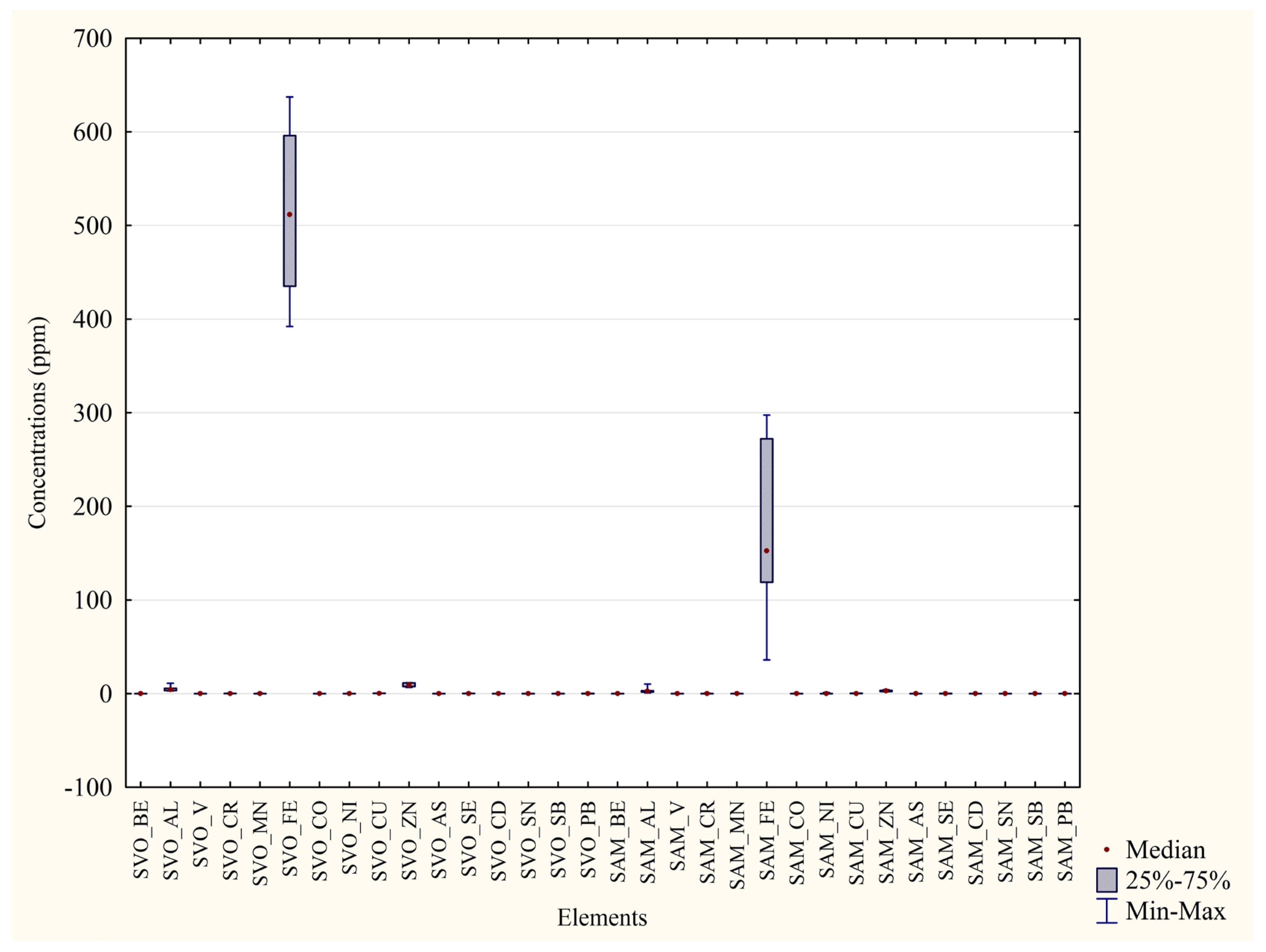
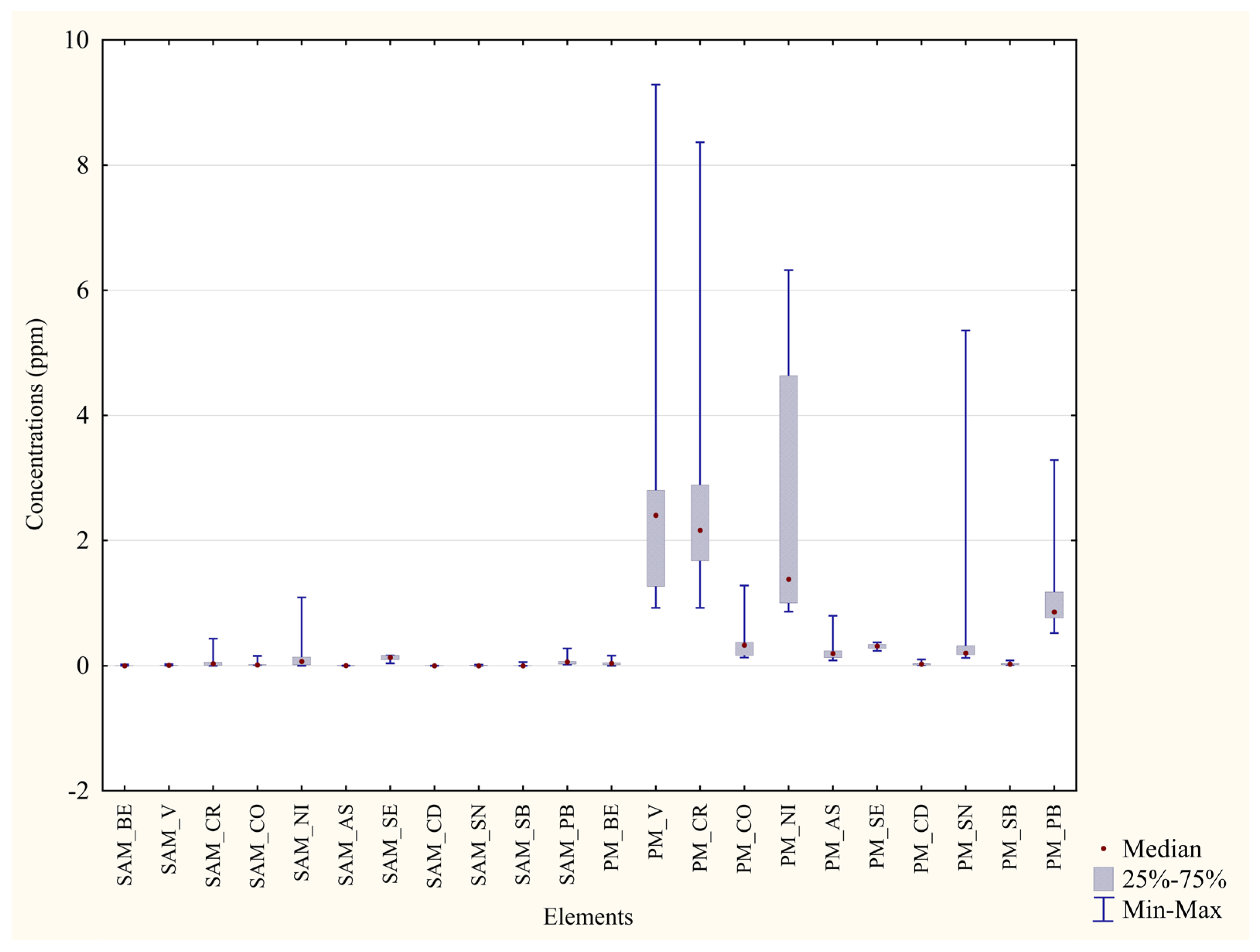
| Metal | Blood P. D’Orleans ±Median min–max (ppm) | Blood Monreale ±Median min–max (ppm) | Significance (p Value) | Percentage Difference between the Two Areas |
|---|---|---|---|---|
| Al | ±4.27 2.92–11.15 | ±2.62 0.98–10.24 | p = 0.0082 ** | 38% |
| Cr | ±0.19 0.13–0.29 | ±0.031 0.00–0.43 | p = 0.02 * | 83% |
| Mn | ±0.12 0.069–0.46 | ±0.061 0.016–0.28 | p = 0.0156 * | 48% |
| Fe | ±511.81 392.08–637.37 | ±152.61 36.25–297.52 | p = 0.0002 *** | 70% |
| Co | ±0.0035 0.0016–0.014 | ±0.012 0.0079–0.16 | p = 0.0012 ** | 71% |
| Ni | ±0.00 0.00–0.0069 | ±0.068 0.00–1.09 | p = 0.0007 *** | 100% |
| Cu | ±0.33 0.28–0.51 | ±0.27 0.19–0.39 | p = 0.004 * | 19% |
| Zn | ±9.27 6.78–11.66 | ±3.06 2.10–4.18 | p = 0.0002 *** | 67% |
| As | ±0.0049 0.0038–0.014 | ± 0.0018 0.00–0.0044 | p = 0.0012 ** | 62% |
| Se | ±0.20 0.15–0.34 | ±0.13 0.038–0.17 | p = 0.0041 ** | 33% |
| Sn | ±0.048 0.0048–0.13 | ±0.00 0.00–0.018 | p = 0.003 *** | 100% |
| Pb | ±0.10 0.055–0.17 | ±0.059 0.018–0.28 | p = 0.0413 * | 41% |
| Metal | Feathers P. D’Orleans ±Median min–max (ppm) | Feathers Monreale ±Median min–max (ppm) | Significance (p Value) | Percentage Difference between the Two Areas |
|---|---|---|---|---|
| Al | ±402.24 79.67–1472.25 | ±1260.76 550.71–5668.88 | p = 0.0041 ** | 68% |
| Cr | ±0.93 0.40–2.18 | ±2.16 0.93–8.37 | p = 0.0052 ** | 57% |
| V | ±0.78 0.19–2.51 | ±2.40 0.92–9.29 | p = 0.0032 ** | 67% |
| Fe | ±330.29 90.84–1237.44 | ±863.91 344.35–4217.01 | p = 0.01 ** | 62% |
| Co | ±0.12 0.048–0.34 | ±0.33 0.13–1.28 | p = 0.0082 ** | 62% |
| Ni | ±0.44 0.18–1.14 | ±1.38 0.87–6.32 | p = 0.0004 *** | 67% |
| Cu | ±9.42 7.63–10.86 | ±7.44 5.75–12.77 | p = 0.049 * | 21% |
| Se | ±0.46 0.27–0.66 | ±0.31 0.24–0.37 | p = 0.0015 ** | 31% |
| Sn | ±1.28 0.30–2.68 | ±0.20 0.13–5.36 | p = 0.0052 ** | 84% |
| Pb | ±2.43 1.02–7.07 | ±0.86 0.52–3.29 | p = 0.0032 ** | 64% |
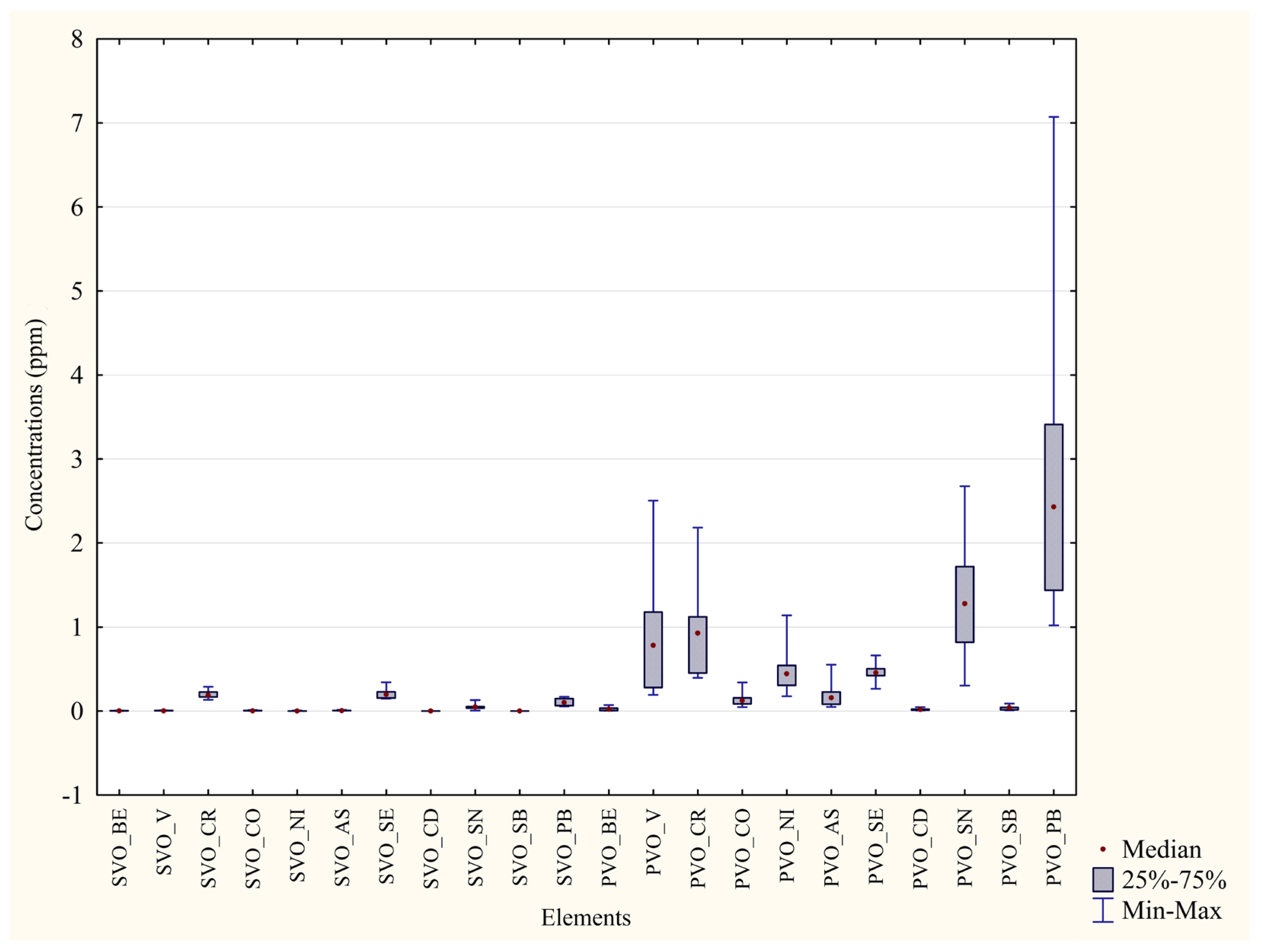
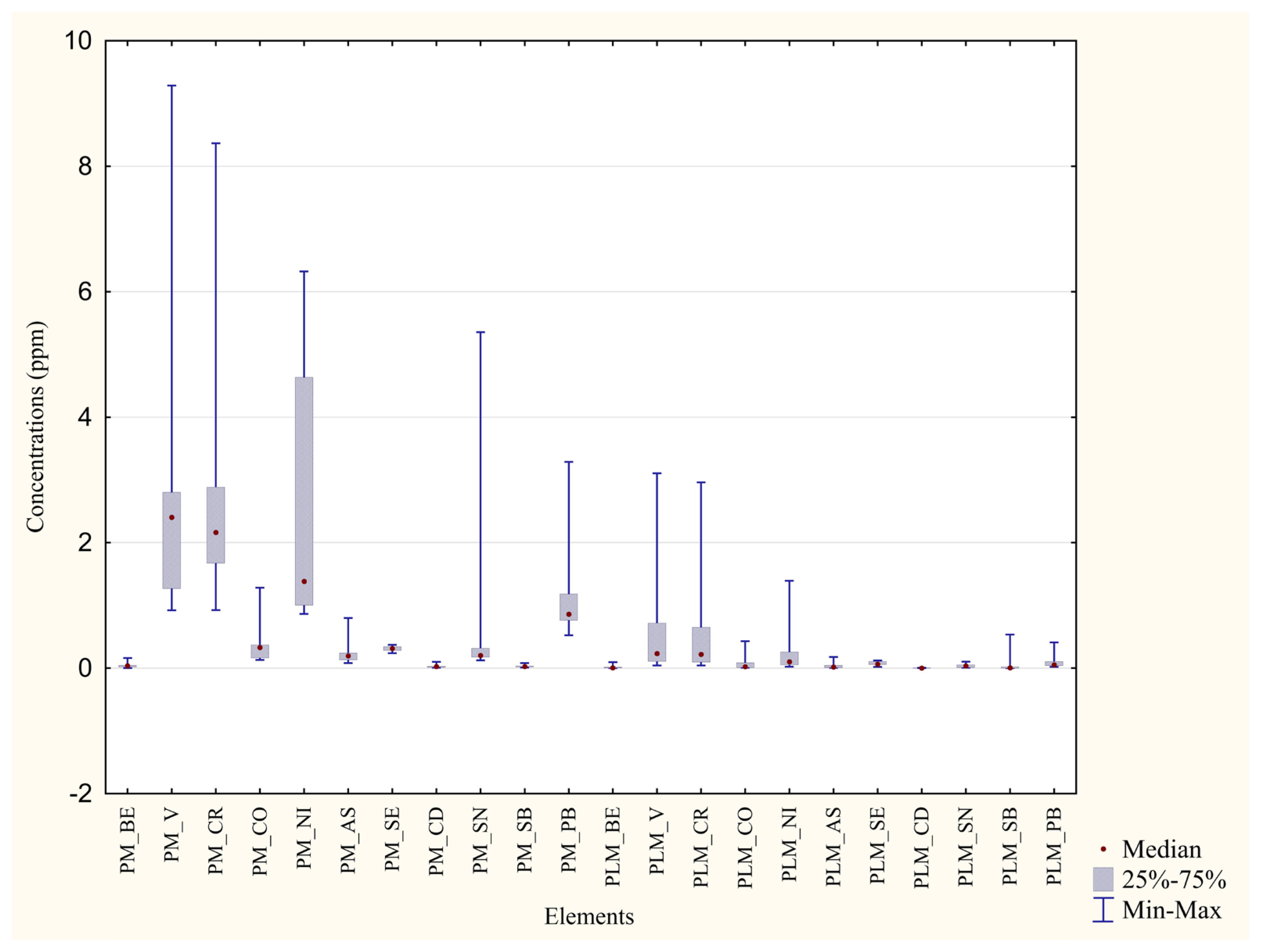
| Metal | Blood P. D’Orleans ±Median min–max (ppm) | Feathers P. D’Orleans ±Median min–max (ppm) | Significance (p Value) | Percentage Difference between the Two Areas |
|---|---|---|---|---|
| V | ±0.0042 0.0028–0.0081 | ±0.78 0.19–2.51 | p = 0.0002 *** | 99% |
| Cr | ±0.19 0.13–0.29 | ±0.93 0.40–2.18 | p = 0.0002 *** | 79% |
| Co | ±0.0035 0.0016–0.014 | ±0.12 0.048–0.34 | p = 0.0002 *** | 97% |
| Ni | ±0.00 0.00–0.0069 | ±0.44 0.18–1.14 | p = 0.0001 *** | 100% |
| As | ±0.0049 0.0038–0.014 | ±0.16 0.048–0.55 | p = 0.0002 *** | 96% |
| Se | ±0.20 0.15–0.34 | ±0.46 0.27–0.66 | p = 0.0002 *** | 56% |
| Cd | ±0.00043 0.00033–0.00076 | ±0.017 0.0081–0.047 | p = 0.0002 *** | 97% |
| Sn | ±0.048 0.0048–0.13 | ±1.28 0.30–2.68 | p = 0.0002 *** | 96% |
| Pb | ±0.10 0.055–0.17 | ±2.43 1.02–7.07 | p = 0.0002 *** | 95% |
| Metal | Blood Monreale ±Median min–max (ppm) | Feathers Monreale ±Median min-max (ppm) | Significance (p Value) | Percentage Difference between the Two Areas |
|---|---|---|---|---|
| V | ±0.0062 0.0017–0.024 | ±2.40 0.92–9.29 | p = 0.0002 *** | 99% |
| Cr | ±0.031 0.00–0.43 | ±2.16 0.93–8.37 | p = 0.0002 *** | 98% |
| Co | ±0.012 0.0079–0.16 | ±0.33 0.13–1.28 | p = 0.0003 *** | 96% |
| Ni | ±0.068 0.00–1.09 | ±1.38 0.87–6.32 | p = 0.0004 *** | 95% |
| As | ±0.0018 0.00–0.0044 | ±0.19 0.082–0.80 | p = 0.0002 *** | 99% |
| Se | ±0.13 0.038–0.17 | ±0.31 0.24–0.37 | p = 0.0002 *** | 58% |
| Cd | ±0.00042 0.00–0.0013 | ±0.026 0.0094–0.10 | p = 0.0001 *** | 98% |
| Sn | ±0.00 0.00–0.018 | ±0.20 0.13–5.36 | p = 0.0012 ** | 100% |
| Pb | ±0.059 0.018–0.28 | ±0.86 0.52–3.29 | p = 0.0002 *** | 93% |
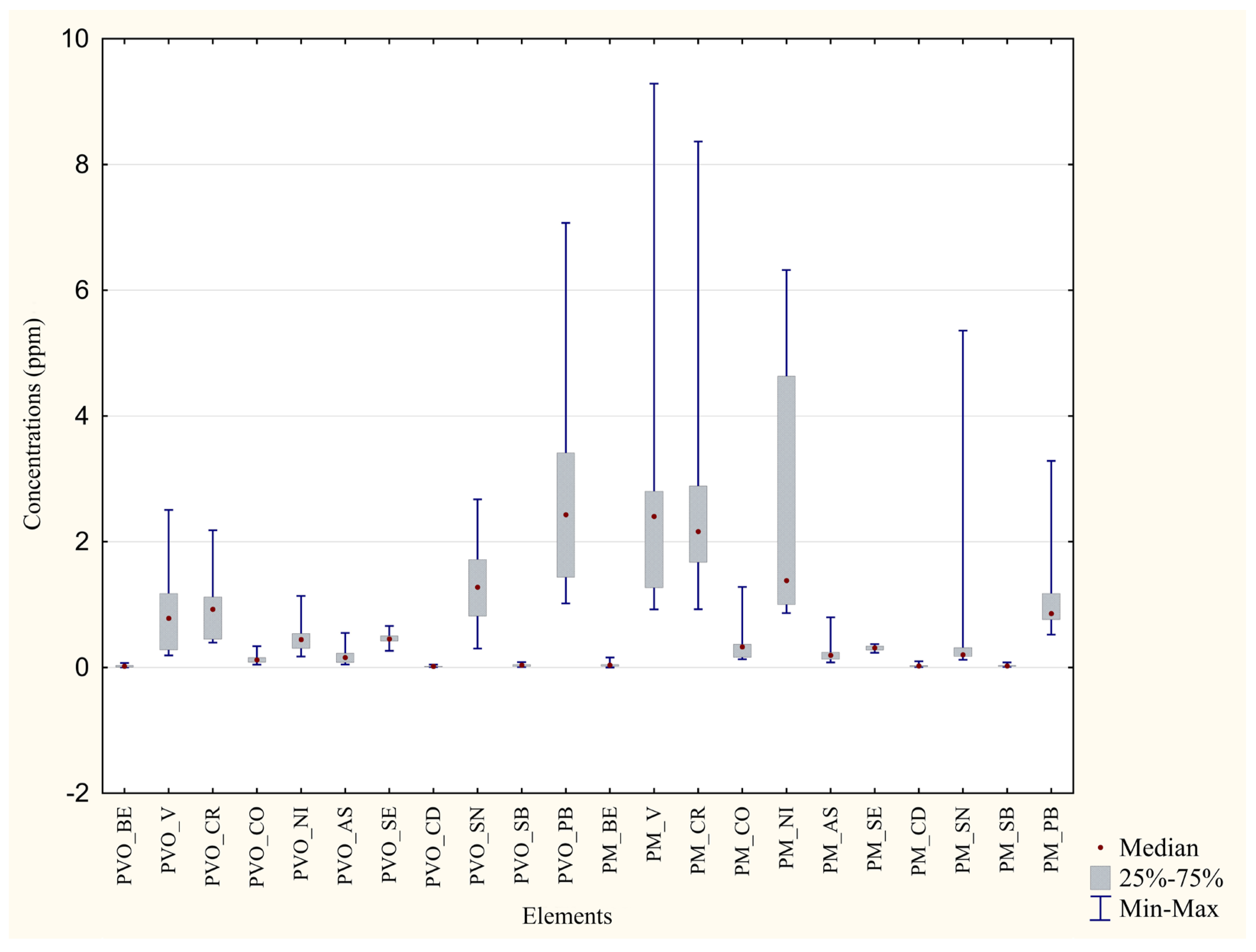
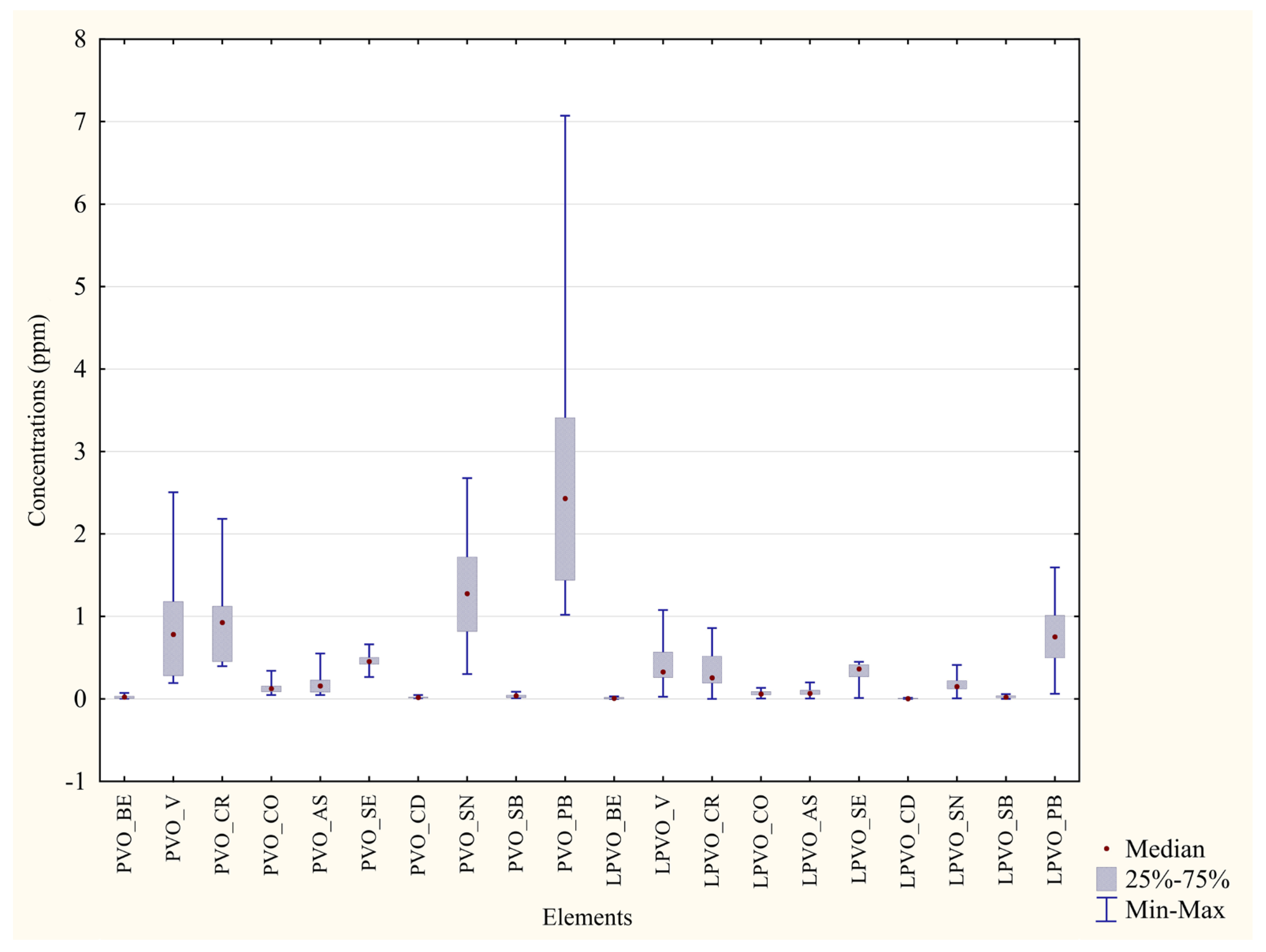
| Metal | Unwashed Feathers P.D’Orleans ±Median min–max (ppm) | Washed Feathers P. D’Orleans ±Median min–max (ppm) | Significance (p Value) | Percentage Difference between the Two Areas |
|---|---|---|---|---|
| Cr | ±0.93 0.40–2.18 | ±0.26 0.00–0.86 | p = 0.006 ** | 72% |
| Mn | ±11.50 7.48–37.70 | ±3.46 0.23–6.82 | p = 0.0002 *** | 72% |
| Co | ±0.12 0.048–0.34 | ±0.060 0.0050–0.14 | p = 0.015 * | 51% |
| Cu | ±9.42 7.63–10.86 | ±5.42 0.23–6.27 | p =0.0002 *** | 42% |
| Zn | ±109.65 68.79–142.87 | ±39.39 1.64–58.56 | p = 0.0002 *** | 64% |
| As | ±0.16 0.048–0.55 | ±0.067 0.0060–0.20 | p = 0.041 * | 58% |
| Se | ±0.46 0.27–0.66 | ±0.36 0.011–0.45 | p = 0.019 * | 20% |
| Cd | ±0.017 0.0081–0.047 | ±0.0045 0.00–0.013 | p = 0.0009 *** | 73% |
| Sn | ±1.28 0.30–2.68 | ±0.15 0.0070–0.41 | p = 0.0003 *** | 88% |
| Pb | ±2.43 1.02–7.07 | ±0.75 0.061–1.59 | p = 0.0009 *** | 68% |
| Metal | Unwashed Feathers Monreale ±Median min–max (ppm) | Washed Feathers Monreale ±Median min–max (ppm) | Significance (p Value) | Percentage Difference between the Two Areas |
|---|---|---|---|---|
| Al | ±1260.76 550.71–5668.88 | ±142.75 26.20–1799.51 | p =0.0025 ** | 89% |
| V | ±2.40 0.92–9.29 | ±0.23 0.043–3.11 | p = 0.0025 ** | 90% |
| Cr | ±2.16 0.93–8.37 | ±0.22 0.043–2.96 | p = 0.0025 ** | 89% |
| Mn | ±15.43 6.33–60.63 | ±0.23 0.094–2.89 | p = 0.0002 *** | 98% |
| Fe | ±863.91 344.35–4217.01 | ±98.97 27.15–1476.18 | p = 0.0041 ** | 88% |
| Co | ±0.33 0.13–1.28 | ±0.022 0.0075–0.43 | p = 0.0025 ** | 93% |
| Ni | ±1.38 0.87–6.32 | ±0.10 0.023–1.39 | p = 0.0007 *** | 92% |
| Cu | ±7.44 5.75–12.77 | ±0.68 0.18–1.70 | p = 0.0002 *** | 90% |
| Zn | ±97.12 71.91–173.87 | ±4.51 0.96–9.58 | p = 0.0002 *** | 95% |
| As | ±0.19 0.082–0.80 | ±0.016 0.0070–0.18 | p = 0.0005 *** | 91% |
| Se | ±0.31 0.24–0.37 | ±0.062 0.020–0.12 | p = 0.0002 *** | 80% |
| Cd | ±0.026 0.0094–0.10 | ±0.00043 0.00022–0.0033 | p = 0.0002 *** | 98% |
| Sn | ±0.20 0.13–5.36 | ±0.036 0.0086–0.10 | p = 0.0002 *** | 82% |
| Sb | ±0.026 0.011–0.082 | ±0.0041 0.00–0.54 | p = 0.0018 ** | 84% |
| Pb | ±0.86 0.52–3.29 | ±0.053 0.021–0.41 | p = 0.0002 *** | 93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nicola, M.R.; Novello, C.; Lo Valvo, M.; Lo Dico, G.M.; Bianchi, V.G.; Mercuri, S.R.; Giornetti, M. Biological Matrices from Cairina moschata as Non-Destructive Biomonitoring Tools to Study Environmental Quality of Urban and Extra-Urban Areas: A Case Study of Palermo (Sicily, Italy). Animals 2023, 13, 2474. https://doi.org/10.3390/ani13152474
Di Nicola MR, Novello C, Lo Valvo M, Lo Dico GM, Bianchi VG, Mercuri SR, Giornetti M. Biological Matrices from Cairina moschata as Non-Destructive Biomonitoring Tools to Study Environmental Quality of Urban and Extra-Urban Areas: A Case Study of Palermo (Sicily, Italy). Animals. 2023; 13(15):2474. https://doi.org/10.3390/ani13152474
Chicago/Turabian StyleDi Nicola, Matteo Riccardo, Christian Novello, Mario Lo Valvo, Gianluigi Maria Lo Dico, Vittoria Giulia Bianchi, Santo Raffaele Mercuri, and Marcella Giornetti. 2023. "Biological Matrices from Cairina moschata as Non-Destructive Biomonitoring Tools to Study Environmental Quality of Urban and Extra-Urban Areas: A Case Study of Palermo (Sicily, Italy)" Animals 13, no. 15: 2474. https://doi.org/10.3390/ani13152474
APA StyleDi Nicola, M. R., Novello, C., Lo Valvo, M., Lo Dico, G. M., Bianchi, V. G., Mercuri, S. R., & Giornetti, M. (2023). Biological Matrices from Cairina moschata as Non-Destructive Biomonitoring Tools to Study Environmental Quality of Urban and Extra-Urban Areas: A Case Study of Palermo (Sicily, Italy). Animals, 13(15), 2474. https://doi.org/10.3390/ani13152474






