Enhanced Autophagy in Damaged Laminar Tissue of Acute Laminitis Induced by Oligofructose Overloading in Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design and Treatment
2.3. Clinical Examination of Dairy Cows
2.4. Laminar Tissue Sampling
2.5. RNA Isolation and cDNA Synthesis
2.6. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.7. Western Blot
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. Clinical Manifestation of Dairy Cow Laminitis
3.2. Autophagy-Associated Genes Expression in Laminar Tissue of Laminitis Dairy Cows
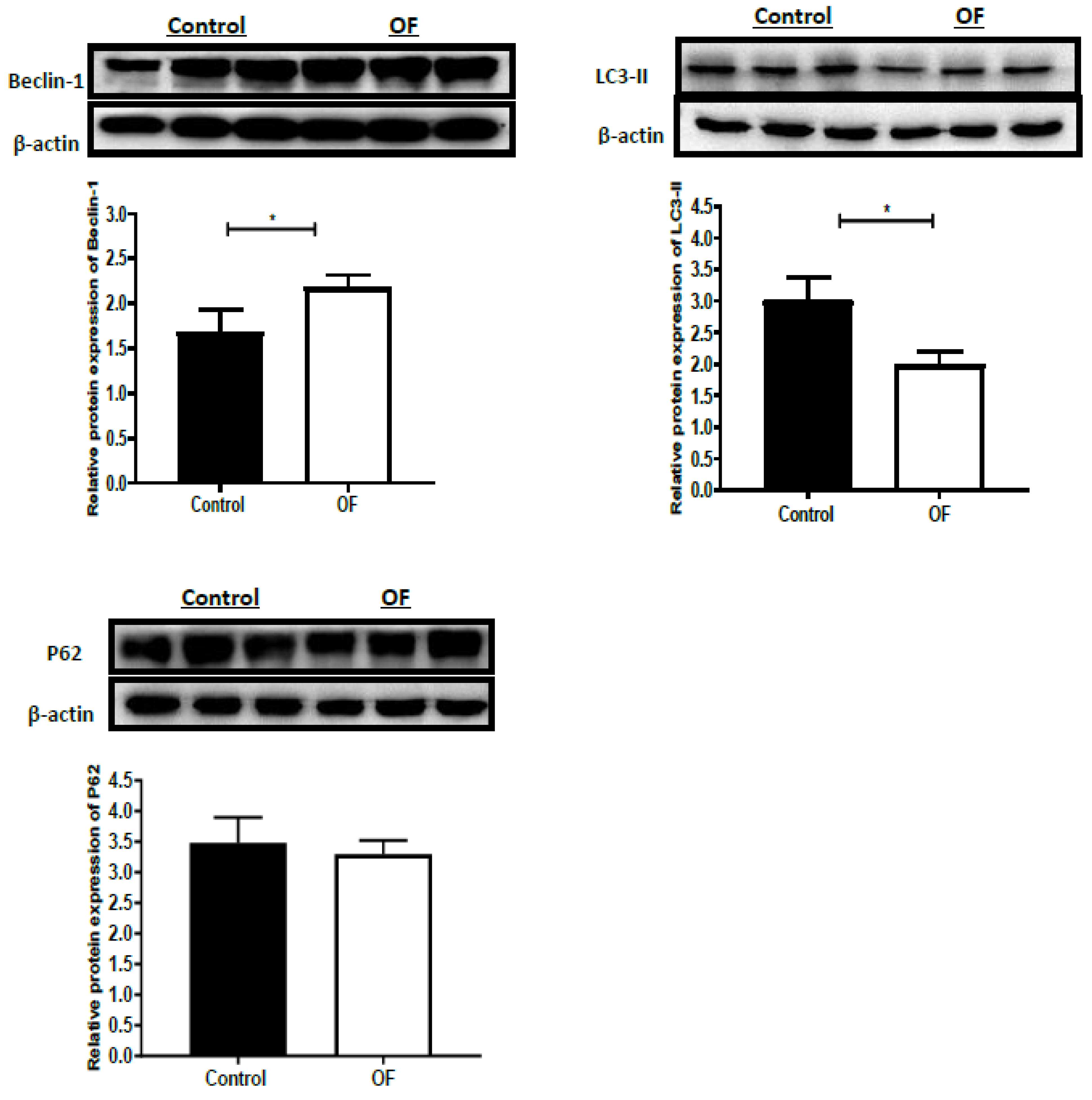
3.3. Autophagy-Associated Protein Expression in Laminar Tissue of Laminitis Dairy Cows
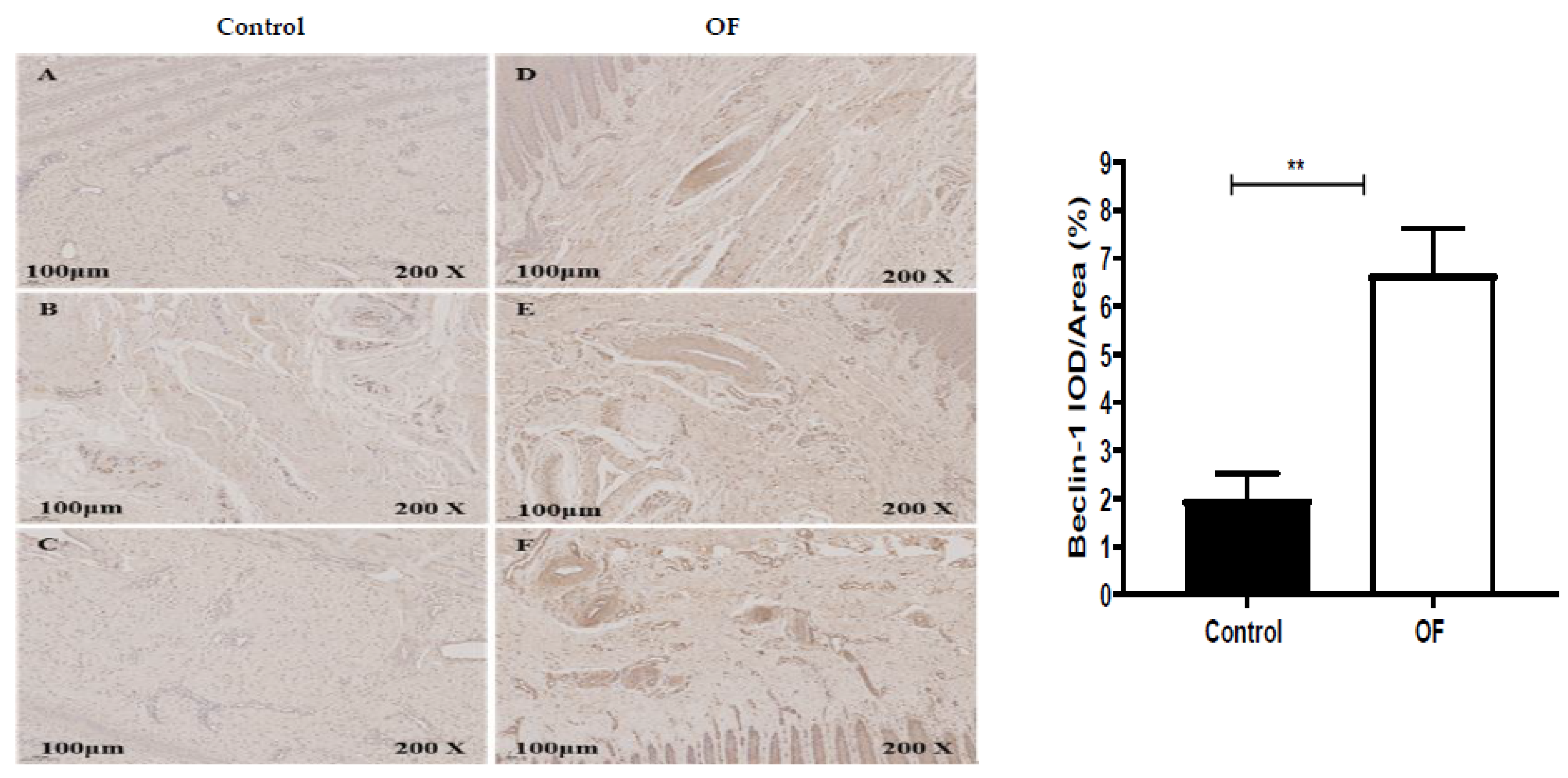
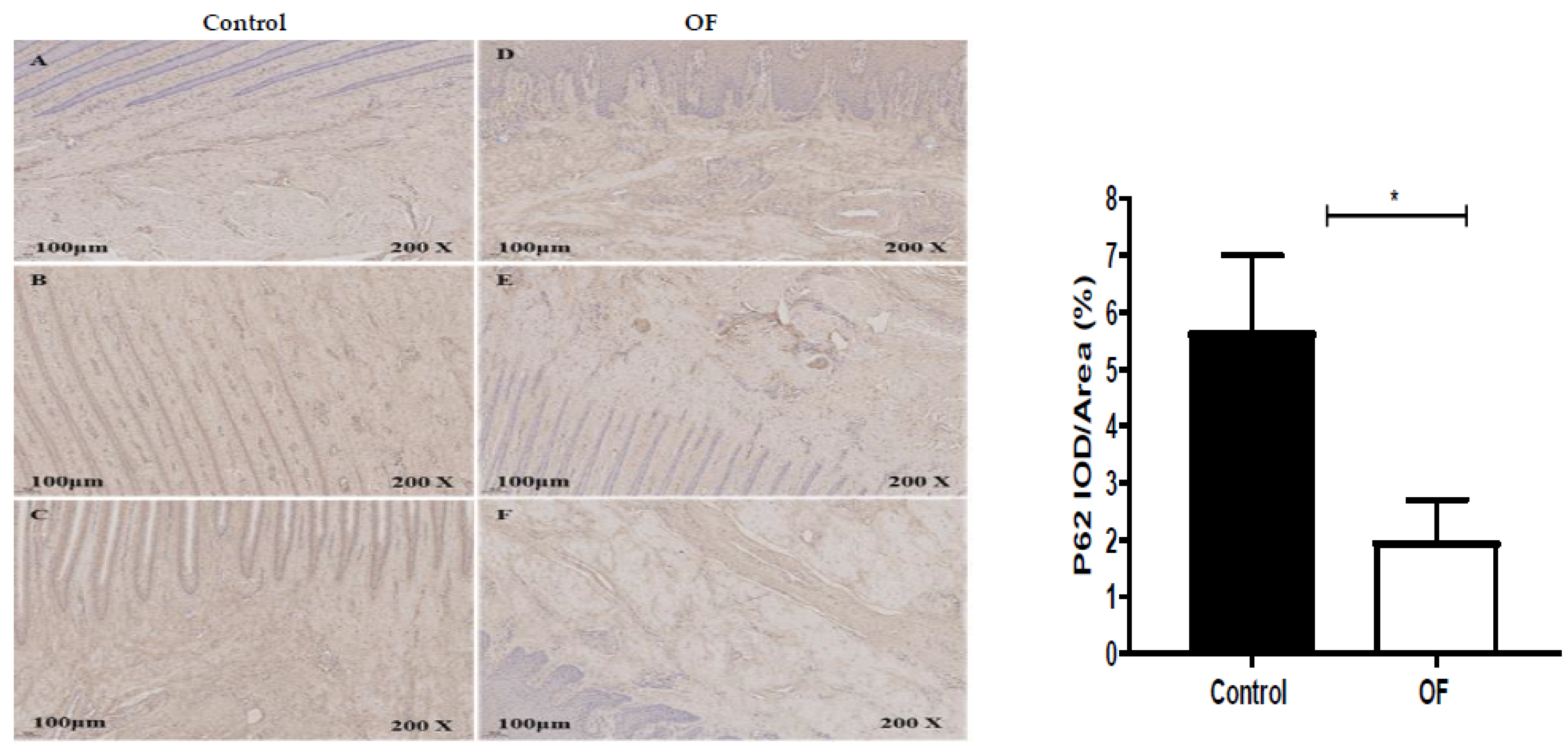
3.4. Immuno-Expression of Beclin1 and P62 Proteins in Laminar Tissue of Laminitis Dairy Cows
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hendry, K.A.; Knight, C.H.; Galbraith, H.; Wilde, C.J. Basement membrane integrity and keratinization in healthy and ulcerated bovine hoof tissue. J. Dairy Res. 2003, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Boosman, R.; Nemeth, F.; Gruys, E. Bovine laminitis: Clinical aspects, pathology and pathogenesis with reference to acute equine laminitis. Vet. Q. 1991, 13, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.V.; Green, M.J.; Huxley, J.N. Use of statistical modelling to investigate the pathogenesis of claw horn disruption lesions in dairy cattle. Vet. J. 2018, 238, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, J. “Subclinical” laminitis in dairy cattle. N. Z. Vet. J. 1992, 40, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Alvergnas, M.; Strabel, T.; Rzewuska, K.; Sell-Kubiak, E. Claw disorders in dairy cattle: Effects on production, welfare and farm economics with possible prevention methods. Livest. Sci. 2019, 222, 54–64. [Google Scholar] [CrossRef]
- Potterton, S.L.; Bell, N.J.; Whay, H.R.; Berry, E.A.; Atkinson, O.C.; Dean, R.S.; Main, D.C.; Huxley, J.N. A descriptive review of the peer and non-peer reviewed literature on the treatment and prevention of foot lameness in cattle published between 2000 and 2011. Vet. J. 2012, 193, 612–616. [Google Scholar] [CrossRef]
- Greenough, P.R. Bovine Laminitis and Lameness: A Hands on Approach; Elsevier Health Sciences: London, UK, 2007. [Google Scholar]
- Tian, M.Y.; Fan, J.H.; Zhuang, Z.W.; Dai, F.; Wang, C.Y.; Hou, H.T.; Ma, Y.Z. Effects of silymarin on p65 NF-κB, p38 MAPK and CYP450 in LPS-induced hoof dermal inflammatory cells of dairy cows. BMC Vet. Res. 2019, 15, 127. [Google Scholar] [CrossRef]
- Thoefner, M.B.; Pollitt, C.C.; Van-Eps, A.W.; Milinovich, G.J.; Trott, D.J.; Wattle, O.; Andersen, P.H. Acute bovine laminitis: A new induction model using alimentary oligofructose overload. J. Dairy Sci. 2004, 87, 2932–2940. [Google Scholar] [CrossRef]
- Danscher, A.M.; Enemark, J.M.D.; Telezhenko, E.; Capion, N.; Ekstrom, C.T.; Thoefner, M.B. Oligofructose overload induces lameness in cattle. J. Dairy Sci. 2009, 92, 607–616. [Google Scholar] [CrossRef]
- Thoefner, M.B.; Wattle, O.; Pollitt, C.C.; French, K.R.; Nielsen, S.S. Histopathology of oligofructose-induced acute laminitis in heifers. J. Dairy Sci. 2005, 88, 2774–2782. [Google Scholar] [CrossRef]
- Mendes, H.M.; Casagrande, F.P.; Lima, I.R.; Souza, C.H.; Gontijo, L.D.; Alves, G.E.; Vasconcelos, A.C.; Faleiros, R.R. Histopathology of dairy cows’ hooves with signs of naturally acquired laminitis. Pesqui. Vet. Brasil. 2013, 33, 613–619. [Google Scholar] [CrossRef]
- Sousa, R.D.S.; Oliveira, F.L.C.; Dias, M.R.B.; Minami, N.S.; Amaral, L.; Santos, J.A.A.D.; Barreto, J.R.A.; Minervino, A.H.H.; Ortolani, E.L. Characterization of oligofructose-induced acute rumen lactic acidosis and the appearance of laminitis in zebu cattle. Animals 2020, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Leise, B.S.; Faleiros, R.R.; Watts, M.; Johnson, P.J.; Black, S.J.; Belknap, J.K. Laminar inflammatory gene expression in the carbohydrate overload model of equine laminitis. Equine Vet. J. 2011, 43, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Dern, K.; van-Eps, A.; Wittum, T.; Watts, M.; Pollitt, C.; Belknap, J. Effect of continuous digital hypothermia on lamellar inflammatory signaling when applied at a clinically-relevant timepoint in the oligofructose laminitis model. J. Vet. Int. Med. 2018, 32, 450–458. [Google Scholar] [CrossRef]
- Singh, R.; Cuervo, A.M. Autophagy in the cellular energetic balance. Cell Metab. 2011, 13, 495–504. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Shen, T.; Xu, F.; Fang, Z.; Loor, J.J.; Ouyang, H.; Chen, M.; Jin, B.; Wang, X.; Shi, Z.; Zhu, Y.; et al. Hepatic autophagy and mitophagy status in dairy cows with subclinical and clinical ketosis. J. Dairy Sci. 2021, 104, 4847–4857. [Google Scholar] [CrossRef]
- Chen, M.; Loor, J.J.; Zhai, Q.; Liang, Y.; Yu, H.; Du, X.; Shen, T.; Fang, Z.; Shi, Z.; Wang, X.; et al. Enhanced autophagy activity in liver tissue of dairy cows with mild fatty liver. J. Dairy Sci. 2020, 103, 3628–3635. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Mizushima, N.L.B.C.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Xie, W.; Wang, Y.; Ma, N.; Chang, G.; Shen, X. Subacute ruminal acidosis downregulates FOXA2, changes oxidative status, and induces autophagy in the livers of dairy cows fed a high-concentrate diet. J. Dairy Sci. 2023, 106, 2007–2018. [Google Scholar] [CrossRef]
- Sprecher, D.E.; Hostetler, D.E.; Kaneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Ding, J.; Li, S.; Jiang, L.; Li, Y.; Zhang, X.; Song, Q.; Hayat, M.A.; Zhang, J.T.; Wang, H. Laminar inflammation responses in the oligofructose overload induced model of bovine laminitis. Front. Vet. Sci. 2020, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Shi, M.; Wang, L.; Qi, D.; Tao, Z.; Hayat, M.A.; Liu, T.; Zhang, J.T.; Wang, H. Gene Expression of Metalloproteinases and Endogenous Inhibitors in the Lamellae of Dairy Heifers with Oligofructose-Induced Laminitis. Front. Vet. Sci. 2020, 7, 597827. [Google Scholar] [CrossRef]
- Hayat, M.A.; Ding, J.; Li, Y.U.; Zhang, X.; Zhang, J.I.; Li, S.; Wang, H.B. Determination of the activity of selected antioxidant enzymes during bovine laminitis, induced by oligofructose overload. Med. Weter. 2020, 76, 289–295. [Google Scholar] [CrossRef]
- Concha, C.; Carretta, M.D.; Alarcón, P.; Conejeros, I.; Gallardo, D.; Hidalgo, A.I.; Tadich, N.; Cáceres, D.D.; Hidalgo, M.A.; Burgos, R.A. Oxidative response of neutrophils to platelet-activating factor is altered during acute ruminal acidosis induced by oligofructose in heifers. J. Vet. Sci. 2014, 15, 217–224. [Google Scholar] [CrossRef]
- Alarcon, P.; Hidalgo, A.I.; Manosalva, C.; Cristi, R.; Teuber, S.; Hidalgo, M.A.; Burgos, R.A. Metabolic disturbances in synovial fluid are involved in the onset of synovitis in heifers with acute ruminal acidosis. Sci. Rep. 2019, 9, 5452. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.; Credille, B. Diagnosis and treatment of clinical rumen acidosis. Vet. Clin. Food Anim. Pract. 2017, 33, 451–461. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.; Plaizier, J. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Abaker, J.A.; Xu, T.L.; Jin, D. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J. Dairy Sci. 2017, 100, 666–678. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Li, X.; Guan, Y.; Wang, Y.; Yuan, X.; Sun, G.; Wang, Z.; Li, X. Inflammatory mechanism of Rumenitis in dairy cows with subacute ruminal acidosis. BMC Vet. Res. 2018, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, G.; Du, X.; Sun, X.; Peng, Z.; Zhao, C.; Xu, Q.; Abdelatty, A.M.; Mohamed, F.F.; Wang, Z.; et al. Increased autophagy mediates the adaptive mechanism of the mammary gland in dairy cows with hyperketonemia. J. Dairy Sci. 2020, 103, 2545–2555. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Meijer, A.J.; Codogno, P. Regulation and role of autophagy in mammalian cells. Int. J. Biochem. Cell Biol. 2004, 36, 2445–2462. [Google Scholar] [CrossRef]
- Kaur, S.; Changotra, H. The beclin 1 interactome: Modification and roles in the pathology of autophagy-related disorders. Biochimie 2020, 175, 34–49. [Google Scholar] [CrossRef]
- Wang, S.; Wuniqiemu, T.; Tang, W.; Teng, F.; Bian, Q.; Yi, L.; Qin, J.; Zhu, X.; Wei, Y.; Dong, J. Luteolin inhibits autophagy in allergic asthma by activating PI3K/Akt/mTOR signaling and inhibiting Beclin-1-PI3KC3 complex. Int. Immunopharmacol. 2021, 94, 107460. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.C.; Fang, C.; Russell, R.C.; Kim, J.H.; Fan, W.; Liu, R.; Zhong, Q.; Guan, K.L. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 2013, 152, 290–303. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.-Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.-L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Noda, N.N.; Satomi, Y.; Ichimura, Y.; Fujioka, Y.; Takao, T.; Inagaki, F.; Ohsumi, Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007, 282, 37298–37302. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef]
- Hurley, J.H.; Young, L.N. Mechanisms of autophagy initiation. Annu. Rev. Biochem. 2017, 86, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, A.; Mayoral, R.; Agra, N.; Valdecantos, M.P.; Pardo, V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lo-Iacono, O.; Corazzari, M.; Fimia, G.M.; et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death. Dis. 2014, 5, e1179. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Walker, S.J.; Brugge, J.S. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J. Cell Biol. 2003, 163, 315–326. [Google Scholar] [CrossRef] [PubMed]

| Genes | RefSeq Accession No. | Primer Sequences (5′-3′) |
|---|---|---|
| ATG-5 | NM_001034579.2 | Forward: ACACCTTTGCAGTGGCTGAGTG Reverse: TCTGTTGGTTGCGGGATGATGC |
| ATG-12 | NM_001076982.1 | Forward: GAGCGAACCCGAACCATCCAAG Reverse: AGGGTCCCAACTTCCTGGTCTG |
| Beclin-1 | NM_001033627.2 | Forward: GCAGGTGAGCTTCGTGTGTCAG Reverse: GCTGGGCTGTGGCAAGTAATGG |
| mTOR | XM_002694043.6 | Forward: GCACGTCAGCACCATCAACCTC Reverse: CAGCCGCCGCAGCCATTC |
| P-62 | NM_176641.1 | Forward: CCAGGAGGTGCCCAGAAACATG Reverse: AGGCGGAGCATAGGTCGTAGTC |
| GAPDH | DQ402990 | Forward: GGGTCATAAGTCCCTCCACGA Reverse: GGTCATAAGTCCCTCCACGA |
| Time | Control Cows (n = 6) | OF-Treated Cows (n = 6) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| −72 h | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 0 h | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6 h | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| 12 h | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 |
| 18 h | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 3 | 2 | 2 |
| 24 h | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 |
| 36 h | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 2 | 3 | 3 | 3 |
| 48 h | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 2 | 3 | 3 | 3 |
| 60 h | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 4 | 3 | 5 | 4 | 5 |
| 72 h | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 4 | 3 | 5 | 4 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, M.A.; Ding, J.; Zhang, X.; Liu, T.; Zhang, J.; Bokhari, S.G.; Akbar, H.; Wang, H. Enhanced Autophagy in Damaged Laminar Tissue of Acute Laminitis Induced by Oligofructose Overloading in Dairy Cows. Animals 2023, 13, 2478. https://doi.org/10.3390/ani13152478
Hayat MA, Ding J, Zhang X, Liu T, Zhang J, Bokhari SG, Akbar H, Wang H. Enhanced Autophagy in Damaged Laminar Tissue of Acute Laminitis Induced by Oligofructose Overloading in Dairy Cows. Animals. 2023; 13(15):2478. https://doi.org/10.3390/ani13152478
Chicago/Turabian StyleHayat, Muhammad Abid, Jiafeng Ding, Xianhao Zhang, Tao Liu, Jiantao Zhang, Shehla Gul Bokhari, Hamid Akbar, and Hongbin Wang. 2023. "Enhanced Autophagy in Damaged Laminar Tissue of Acute Laminitis Induced by Oligofructose Overloading in Dairy Cows" Animals 13, no. 15: 2478. https://doi.org/10.3390/ani13152478
APA StyleHayat, M. A., Ding, J., Zhang, X., Liu, T., Zhang, J., Bokhari, S. G., Akbar, H., & Wang, H. (2023). Enhanced Autophagy in Damaged Laminar Tissue of Acute Laminitis Induced by Oligofructose Overloading in Dairy Cows. Animals, 13(15), 2478. https://doi.org/10.3390/ani13152478





