Airway Hyperresponsiveness, but Not Bronchoalveolar Inflammatory Cytokines Profiles, Is Modified at the Subclinical Onset of Severe Equine Asthma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
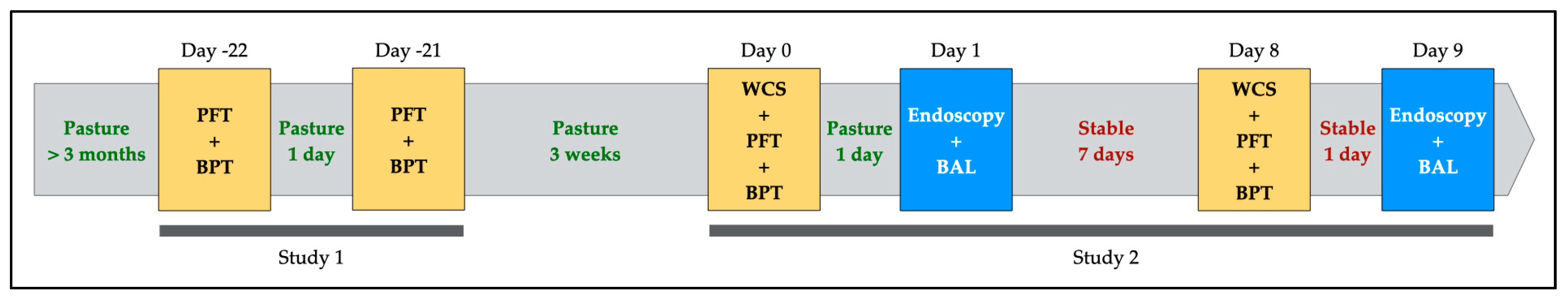
2.2. Experimental Design
2.2.1. Study 1: Within-Subject Repeatability of Respiratory Resistance and Bronchoprovocation Test
2.2.2. Study 2: Effect of Mild Environmental Challenge on Clinical Score, Respiratory Parameters, Airway Hyperresponsiveness and Bronchoalveolar Inflammation
2.3. Clinical Procedures
2.3.1. Weighted Clinical Score (WCS)
2.3.2. Airway Endoscopy and Bronchoalveolar Lavage Fluid (BALf) Collection
2.3.3. Pulmonary Function Test (PFT)
2.3.4. Methacholine Bronchoprovocation Test (BPT)
2.4. Laboratory Procedures
2.4.1. Bronchoalveolar Inflammatory Cytokines Quantification via Enzyme-Linked Immunosorbent Assay (ELISA)
2.4.2. BALf Cytology
2.4.3. Measurement of Gene Expression of Leukocytes via Real-Time Quantitative Polymerase Chain Reactions (RT-qPCR)
2.5. Statistical Analysis
2.5.1. Study 1: Within-Subject Repeatability of Respiratory Resistance and BPT
2.5.2. Study 2: Effect of Mild Environmental Challenge on Clinical Score, Respiratory Parameters, Airway Hyperresponsiveness and Alveolar Inflammation
3. Results
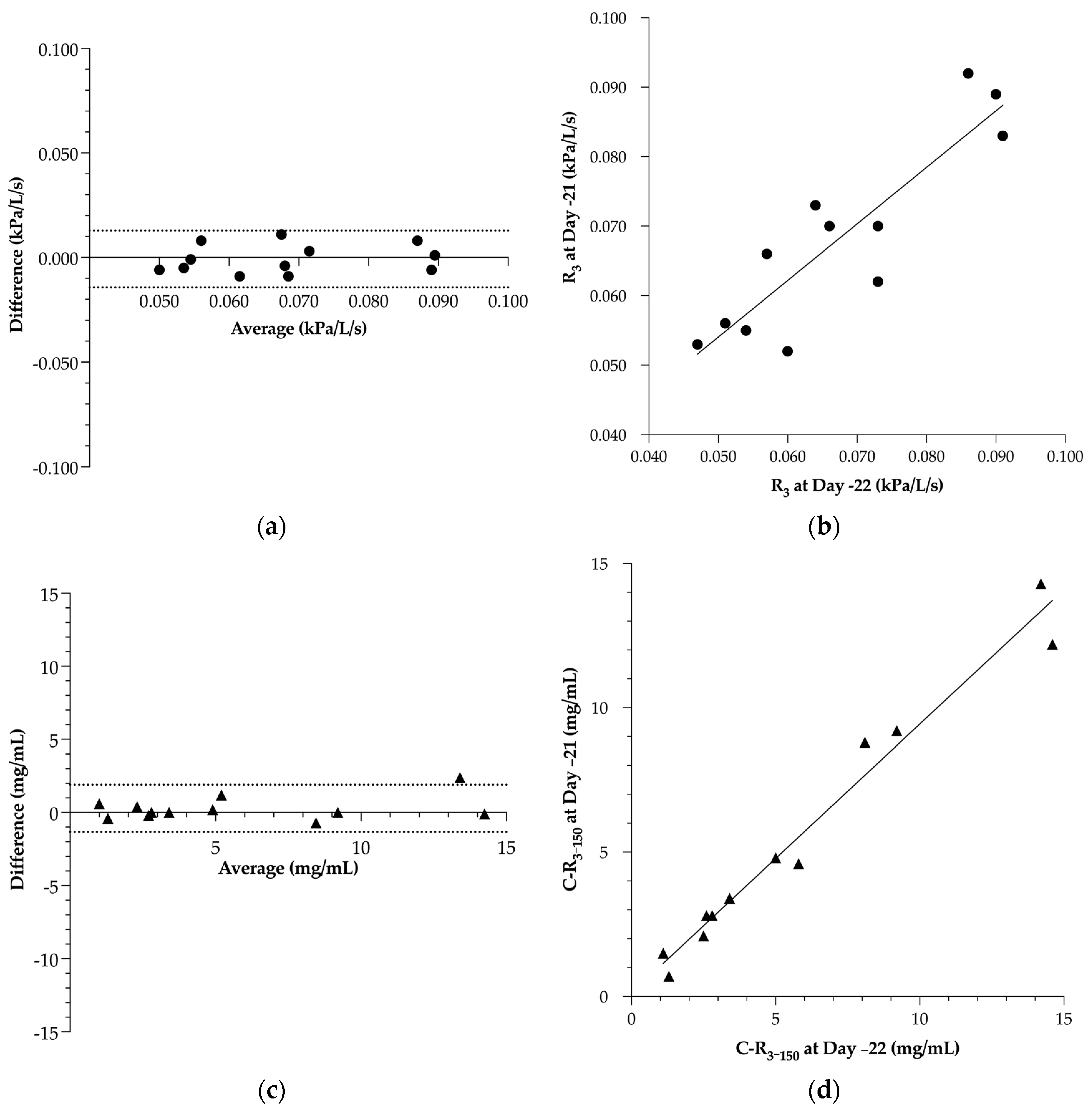
3.1. Within-Subject Repeatability of Respiratory Resistance and BPT
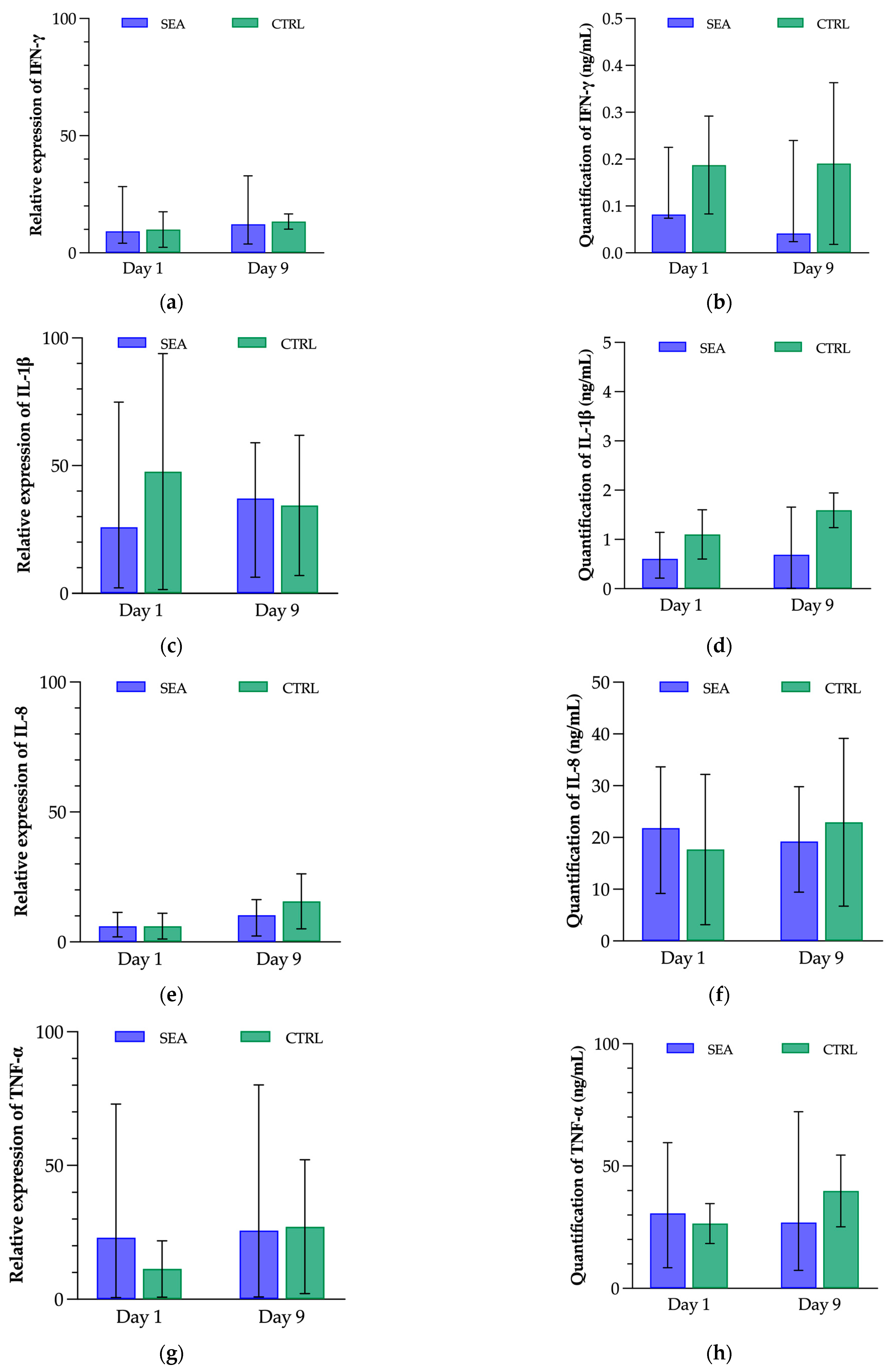
3.2. Effect of Mild Environmental Challenge on Clinical Score, Respiratory Parameters, Airway Hyperresponsiveness and Bronchoalveolar Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couëtil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.P.; Léguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses-Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Chupp, G. Phenotypes and Endotypes of Adult Asthma: Moving toward Precision Medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef]
- Lowell, F.C. Observations on Heaves. An Asthma-like Syndrome in the Horse. Allergy Proc. 1990, 11, 149–150. [Google Scholar] [CrossRef]
- Kaup, F.J.; Drommer, W.; Damsch, S.; Deegen, E. Ultrastructural Findings in Horses with Chronic Obstructive Pulmonary Disease (COPD) II: Pathomorphological Changes of the Terminal Airways and the Alveolar Region. Equine Vet. J. 1990, 22, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Herszberg, B.; Ramos-Barbón, D.; Tamaoka, M.; Martin, J.G.; Lavoie, J.P. Heaves, an Asthma-like Equine Disease, Involves Airway Smooth Muscle Remodeling. J. Allergy Clin. Immunol. 2006, 118, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.M.; Clark, E.G.; Clayton, H.M. Chronic Obstructive Pulmonary Disease: Usefulness of Clinical Signs, Bronchoalveolar Lavage, and Lung Biopsy as Diagnostic and Prognostic Aids. Can. Vet. J. 1992, 33, 591–598. [Google Scholar]
- Fairbairn, S.M.; Page, C.P.; Lees, P.; Cunningham, F.M. Early Neutrophil but Not Eosinophil or Platelet Recruitment to the Lungs of Allergic Horses Following Antigen Exposure. Clin. Exp. Allergy 1993, 23, 821–828. [Google Scholar] [CrossRef] [PubMed]
- McGorum, B.C.; Dixon, P.M.; Halliwell, R.E. Responses of Horses Affected with Chronic Obstructive Pulmonary Disease to Inhalation Challenges with Mould Antigens. Equine Vet. J. 1993, 25, 261–267. [Google Scholar] [CrossRef]
- Joubert, P.; Cordeau, M.E.; Boyer, A.; Silversides, D.W.; Lavoie, J.P. Cytokine Expression by Peripheral Blood Neutrophils from Heaves-Affected Horses before and after Allergen Challenge. Vet. J. 2008, 178, 227–232. [Google Scholar] [CrossRef]
- Tesarowski, D.B.; Viel, L.; McDonell, W.N. Pulmonary Function Measurements during Repeated Environmental Challenge of Horses with Recurrent Airway Obstruction (Heaves). Am. J. Vet. Res. 1996, 57, 1214–1219. [Google Scholar]
- Leclere, M.; Lavoie-Lamoureux, A.; Lavoie, J.-P. Heaves, an Asthma-like Disease of Horses. Respirology 2011, 16, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; de Solis, C.N.; Coleman, M.C. Case-Control Study of Risk Factors for Equine Asthma in Texas. J. Equine Vet. Sci. 2021, 103, 103644. [Google Scholar] [CrossRef]
- Couëtil, L.L.; Ward, M.P. Analysis of Risk Factors for Recurrent Airway Obstruction in North American Horses: 1,444 Cases (1990–1999). J. Am. Vet. Med. Assoc. 2003, 223, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, S.; Duvivier, D.H.; Votion, D.; Art, T.; Lekeux, P. Environmental Control to Maintain Stabled COPD Horses in Clinical Remission: Effects on Pulmonary Function. Equine Vet. J. 1998, 30, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Derksen, F.J.; Robinson, N.E.; Armstrong, P.J.; Stick, J.A.; Slocombe, R.F. Airway Reactivity in Ponies with Recurrent Airway Obstruction (Heaves). J. Appl. Physiol. 1985, 58, 598–604. [Google Scholar] [CrossRef]
- Armstrong, P.J.; Derksen, F.J.; Slocombe, R.F.; Robinson, N.E. Airway Responses to Aerosolized Methacholine and Citric Acid in Ponies with Recurrent Airway Obstruction (Heaves). Am. Rev. Respir. Dis. 1986, 133, 357–361. [Google Scholar]
- Hargreave, F.E.; Ryan, G.; Thomson, N.C.; O’Byrne, P.M.; Latimer, K.; Juniper, E.F.; Dolovich, J. Bronchial Responsiveness to Histamine or Methacholine in Asthma: Measurement and Clinical Significance. J. Allergy Clin. Immunol. 1981, 68, 347–355. [Google Scholar] [CrossRef]
- Beckett, W.S.; Marenberg, M.E.; Pace, P.E. Repeated Methacholine Challenge Produces Tolerance in Normal but Not in Asthmatic Subjects. Chest 1992, 102, 775–779. [Google Scholar] [CrossRef]
- Singh, S. Bronchial Challenge Test in Patients with a History Suggestive of Bronchial Asthma with Normal Spirometric Studies. Med. J. Armed Forces India 2021, 77, 82–85. [Google Scholar] [CrossRef]
- Klein, H.J.; Deegen, E. Histamine Inhalation Provocation Test: Method to Identify Nonspecific Airway Reactivity in Equids. Am. J. Vet. Res. 1986, 47, 1796–1800. [Google Scholar]
- Doucet, M.Y.; Vrins, A.A.; Ford-Hutchinson, A.W. Histamine Inhalation Challenge in Normal Horses and in Horses with Small Airway Disease. Can. J. Vet. Res. 1991, 55, 285–293. [Google Scholar]
- Vandenput, S.; Votion, D.; Duvivier, D.H.; Van Erck, E.; Anciaux, N.; Art, T.; Lekeux, P. Effect of a Set Stabled Environmental Control on Pulmonary Function and Airway Reactivity of COPD Affected Horses. Vet. J. 1998, 155, 189–195. [Google Scholar] [CrossRef]
- Miller, R.L.; Grayson, M.H.; Strothman, K. Advances in Asthma: New Understandings of Asthma’s Natural History, Risk Factors, Underlying Mechanisms, and Clinical Management. J. Allergy Clin. Immunol. 2021, 148, 1430–1441. [Google Scholar] [CrossRef]
- Coeshott, C.; Ohnemus, C.; Pilyavskaya, A.; Ross, S.; Wieczorek, M.; Kroona, H.; Leimer, A.H.; Cheronis, J. Converting Enzyme-Independent Release of Tumor Necrosis Factor α and IL-1β from a Stimulated Human Monocytic Cell Line in the Presence of Activated Neutrophils or Purified Proteinase 3. Proc. Natl. Acad. Sci. USA 1999, 96, 6261–6266. [Google Scholar] [CrossRef]
- Osei, E.T.; Brandsma, C.-A.; Timens, W.; Heijink, I.H.; Hackett, T.-L. Current Perspectives on the Role of Interleukin-1 Signalling in the Pathogenesis of Asthma and COPD. Eur. Respir. J. 2020, 55, 1900563. [Google Scholar] [CrossRef]
- Niessen, N.M.; Gibson, P.G.; Simpson, J.L.; Scott, H.A.; Baines, K.J.; Fricker, M. Airway Monocyte Modulation Relates to Tumour Necrosis Factor Dysregulation in Neutrophilic Asthma. ERJ Open Res. 2021, 7, 00131–02021. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.J.; Yucesoy, B.; Luster, M.I. Prevention of IL-1 Signaling Attenuates Airway Hyperresponsiveness and Inflammation in a Murine Model of Toluene Diisocyanate-Induced Asthma. J. Allergy Clin. Immunol. 2005, 116, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Provost, K.; Niu, N.; Homer, R.; Cohn, L. IFN-γ Acts on the Airway Epithelium to Inhibit Local and Systemic Pathology in Allergic Airway Disease. J. Immunol. 2011, 187, 3815–3820. [Google Scholar] [CrossRef]
- Figueiredo, C.A.; Rodrigues, L.C.; Alcantara-Neves, N.M.; Cooper, P.J.; Amorim, L.D.; Silva, N.B.; Cruz, A.A.; Barreto, M.L. Does IFN-γ Play a Role on the Pathogenesis of Non-Atopic Asthma in Latin America Children? Allergy Asthma Clin. Immunol. 2012, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, C.L.; Shaughnessy, T.E.; Matthay, M.A.; Fahy, J.V. Increased Neutrophil Numbers and IL-8 Levels in Airway Secretions in Acute Severe Asthma: Clinical and Biologic Significance. Am. J. Respir. Crit. Care Med. 2000, 161, 1185–1190. [Google Scholar] [CrossRef]
- Franchini, M.; Gill, U.; Von Fellenberg, R.; Bracher, V.D. Interleukin-8 Concentration and Neutrophil Chemotactic Activity in Bronchoalveolar Lavage Fluid of Horses with Chronic Obstructive Pulmonary Disease Following Exposure to Hay. Am. J. Vet. Res. 2000, 61, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, D.M.; Grünig, G.; Matychak, M.B.; Young, J.; Wagner, B.; Erb, H.N.; Antczak, D.F. Recurrent Airway Obstruction (RAO) in Horses Is Characterized by IFN-γ and IL-8 Production in Bronchoalveolar Lavage Cells. Vet. Immunol. Immunopathol. 2003, 96, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.B.; Husulak, M.L.; Kosolofski, H.; Dos Santos, S.; Burgess, H.; Meachem, M.D. Tumor Necrosis Factor-Alpha Protein Concentrations in Bronchoalveolar Lavage Fluid from Healthy Horses and Horses with Severe Equine Asthma. Vet. Immunol. Immunopathol. 2018, 202, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Giguère, S.; Viel, L.; Lee, E.; MacKay, R.J.; Hernandez, J.; Franchini, M. Cytokine Induction in Pulmonary Airways of Horses with Heaves and Effect of Therapy with Inhaled Fluticasone Propionate. Vet. Immunol. Immunopathol. 2002, 85, 147–158. [Google Scholar] [CrossRef]
- Perkins, G.A.; Viel, L.; Wagner, B.; Hoffman, A.; Erb, H.N.; Ainsworth, D.M. Histamine Bronchoprovocation Does Not Affect Bronchoalveolar Lavage Fluid Cytology, Gene Expression and Protein Concentrations of IL-4, IL-8 and IFN-γ. Vet. Immunol. Immunopathol. 2008, 126, 230–235. [Google Scholar] [CrossRef]
- Pirie, R.S.; Mueller, H.; Engel, O.; Albrecht, B.; Salis-Soglio, M. Inhaled Ciclesonide Is Efficacious and Well Tolerated in the Treatment of Severe Equine Asthma in a Large Prospective European Clinical Trial. Equine Vet. J. 2021, 53, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Beeler-Marfisi, J.; Clark, M.E.; Wen, X.; Sears, W.; Huber, L.; Ackerley, C.; Viel, L.; Bienzle, D. Experimental Induction of Recurrent Airway Obstruction with Inhaled Fungal Spores, Lipopolysaccharide, and Silica Microspheres in Horses. Am. J. Vet. Res. 2010, 71, 682–689. [Google Scholar] [CrossRef]
- Lavoie, J.P.; Bullone, M.; Rodrigues, N.; Germim, P.; Albrecht, B.; von Salis-Soglio, M. Effect of Different Doses of Inhaled Ciclesonide on Lung Function, Clinical Signs Related to Airflow Limitation and Serum Cortisol Levels in Horses with Experimentally Induced Mild to Severe Airway Obstruction. Equine Vet. J. 2019, 51, 779–786. [Google Scholar] [CrossRef]
- Gerber, V.; Straub, R.; Marti, E.; Hauptman, J.; Herholz, C.; King, M.; Imhof, A.; Tahon, L.; Robinson, N.E. Endoscopic Scoring of Mucus Quantity and Quality: Observer and Horse Variance and Relationship to Inflammation, Mucus Viscoelasticity and Volume. Equine Vet. J. 2004, 36, 576–582. [Google Scholar] [CrossRef]
- Koch, C.; Straub, R.; Ramseyer, A.; Widmer, A.; Robinson, N.E.; Gerber, V. Endoscopic Scoring of the Tracheal Septum in Horses and Its Clinical Relevance for the Evaluation of Lower Airway Health in Horses. Equine Vet. J. 2007, 39, 107–112. [Google Scholar] [CrossRef]
- Hoffman, A.M. Bronchoalveolar Lavage: Sampling Technique and Guidelines for Cytologic Preparation and Interpretation. Vet. Clin. North. Am. Equine Pract. 2008, 24, 423–435. [Google Scholar] [CrossRef] [PubMed]
- van Erck, E.; Votion, D.; Art, T.; Lekeux, P. Measurement of Respiratory Function by Impulse Oscillometry in Horses. Equine Vet. J. 2004, 36, 21–28. [Google Scholar] [CrossRef] [PubMed]
- van Erck, E.; Votion, D.M.; Kirschvink, N.; Art, T.; Lekeux, P. Use of the Impulse Oscillometry System for Testing Pulmonary Function during Methacholine Bronchoprovocation in Horses. Am. J. Vet. Res. 2003, 64, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Mazan, M.R.; Hoffman, A.M.; Manjerovic, N. Comparison of Forced Oscillation with the Conventional Method for Histamine Bronchoprovocation Testing in Horses. Am. J. Vet. Res. 1999, 60, 174–180. [Google Scholar]
- Lavoie, J.P.; Cesarini, C.; Lavoie-Lamoureux, A.; Moran, K.; Lutz, S.; Picandet, V.; Jean, D.; Marcoux, M. Bronchoalveolar Lavage Fluid Cytology and Cytokine Messenger Ribonucleic Acid Expression of Racehorses with Exercise Intolerance and Lower Airway Inflammation. J. Vet. Intern. Med. 2011, 25, 322–329. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring Agreement in Method Comparison Studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Nolen-Walston, R.D.; Kuehn, H.; Boston, R.C.; Mazan, M.R.; Wilkins, P.A.; Bruns, S.; Hoffman, A.M. Reproducibility of Airway Responsiveness in Horses Using Flowmetric Plethysmography and Histamine Bronchoprovocation. J. Vet. Intern. Med. 2009, 23, 631–635. [Google Scholar] [CrossRef]
- Dixon, C.E.; Bedenice, D.; Mazan, M.R. Comparison of Flowmetric Plethysmography and Forced Oscillatory Mechanics to Measure Airway Hyperresponsiveness in Horses. Front. Vet. Sci. 2021, 7, 511023. [Google Scholar] [CrossRef]
- Robinson, N.E.; Berney, C.; deFeijter-Rupp, H.L.; Jefcoat, A.M.; Cornelisse, C.J.; Gerber, V.M.; Derksen, F.J. Coughing, Mucus Accumulation, Airway Obstruction, and Airway Inflammation in Control Horses and Horses Affected with Recurrent Airway Obstruction. Am. J. Vet. Res. 2003, 64, 550–557. [Google Scholar] [CrossRef]
- Miskovic, M.; Couëtil, L.L.; Thompson, C.A. Lung Function and Airway Cytologic Profiles in Horses with Recurrent Airway Obstruction Maintained in Low-Dust Environments. J. Vet. Intern. Med. 2007, 21, 1060–1066. [Google Scholar] [CrossRef]
- van den Toorn, L.M.; Prins, J.-B.; Overbeek, S.E.; Hoogsteden, H.C.; de Jongste, J.C. Adolescents in Clinical Remission of Atopic Asthma Have Elevated Exhaled Nitric Oxide Levels and Bronchial Hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2000, 162, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Valli, M.; Ribuffo, V.; Melara, R.; Cappiello, G.; Businarolo, E.; Andreani, A. Relationship between Methacholine Challenge Testing and Exhaled Nitric Oxide in Adult Patients with Suspected Bronchial Asthma. Eur. Ann. Allergy Clin. Immunol. 2014, 46, 109–113. [Google Scholar] [PubMed]
- Wysocka, B.; Kluciński, W. Usefulness of the Assessment of Discharge Accumulation in the Lower Airways and Tracheal Septum Thickening in the Differential Diagnosis of Recurrent Airway Obstruction (RAO) and Inflammatory Airway Disease (IAD) in the Horse. Pol. J. Vet. Sci. 2014, 17, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.E.; Derksen, F.J.; Olszewski, M.A.; Buechner-Maxwell, V.A. The Pathogenesis of Chronic Obstructive Pulmonary Disease of Horses. Br. Vet. J. 1996, 152, 283–306. [Google Scholar] [CrossRef]
- Robinson, N.E.; Vet Med, B.; Wilson, R. Airway Obstruction in the Horse. J. Equine Vet. Sci. 1989, 9, 155–160. [Google Scholar] [CrossRef]
- Fairbairn, S.M.; Lees, P.; Page, C.P.; Cunningham, F.M. Duration of Antigen-induced Hyperresponsiveness in Horses with Allergic Respiratory Disease and Possible Links with Early Airway Obstruction. J. Vet. Pharmacol. Ther. 1993, 16, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Cullimore, A.M.; Secombe, C.J.; Lester, G.D.; Robertson, I.D. Bronchoalveolar Lavage Fluid Cytology and Airway Hyper-Reactivity in Clinically Normal Horses. Aust. Vet. J. 2018, 96, 291–296. [Google Scholar] [CrossRef]
- Wichtel, M.; Gomez, D.; Burton, S.; Wichtel, J.; Hoffman, A. Relationships between Equine Airway Reactivity Measured by Flowmetric Plethysmography and Specific Indicators of Airway Inflammation in Horses with Suspected Inflammatory Airway Disease. Equine Vet. J. 2016, 48, 466–471. [Google Scholar] [CrossRef]
- Richard, E.A.; Depecker, M.; Defontis, M.; Leleu, C.; Fortier, G.; Pitel, P.H.; Couroucé-Malblanc, A. Cytokine Concentrations in Bronchoalveolar Lavage Fluid from Horses with Neutrophilic Inflammatory Airway Disease. J. Vet. Intern. Med. 2014, 28, 1838–1844. [Google Scholar] [CrossRef]
- Brusasco, V.; Crimi, E.; Pellegrino, R. Airway Hyperresponsiveness in Asthma: Not Just a Matter of Airway Inflammation. Thorax 1998, 53, 992–998. [Google Scholar] [CrossRef]
- Crimi, E.; Spanevello, A.; Neri, M.; Ind, P.W.; Rossi, G.A.; Brusasco, V. Dissociation between Airway Inflammation and Airway Hyperresponsiveness in Allergic Asthma. Am. J. Respir. Crit. Care Med. 1998, 17, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Brusasco, V.; Pellegrino, R. Airway Hyperresponsiveness: From Molecules to Bedside—Invited Review: Complexity of Factors Modulating Airway Narrowing in Vivo: Relevance to Assessment of Airway Hyperresponsiveness. J. Appl. Physiol. 2003, 95, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Boushey, H.A.; Holtzman, M.J.; Sheller, J.R.; Nadel, J.A. Bronchial Hyperreactivity. Am. Rev. Respir. Dis. 1980, 121, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.E.; Viel, L. Pulmonary Eosinophilia Associated with Increased Airway Responsiveness in Young Racing Horses. J. Vet. Intern. Med. 1998, 12, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.; Batista, M.; Tilley, P. The Immune Mechanisms of Severe Equine Asthma—Current Understanding and What Is Missing. Animals 2022, 12, 744. [Google Scholar] [CrossRef]


| Descriptor | Observation | Score |
|---|---|---|
| Respiratory rate (breaths/min) | <16 | 0 |
| 16–20 | 1 | |
| 21–25 | 2 | |
| 26–30 | 3 | |
| >30 | 4 | |
| Nasal discharge | None | 0 |
| Serious | 1 | |
| Mucous | 2 | |
| Mucopurulent | 3 | |
| Nasal flaring | None | 0 |
| Present | 1 | |
| Abdominal lift | None | 0 |
| Mild movement of abdomen and/or thorax and/or anus | 1 | |
| Pronounced movement of abdomen and/or thorax and/or anus | 3 | |
| Tracheal sounds | Normal (tubular sound) | 0 |
| Increase in intensity | 1 | |
| Mucus movement | 3 | |
| Bronchial tones | Normal | 0 |
| Abnormal | 2 | |
| Crackles | None | 0 |
| Present | 2 | |
| Wheezes | None | 0 |
| Present | 2 | |
| Cough | None | 0 |
| Inducible by moderate pressure signal on larynx | 1 | |
| Intermittent | 2 | |
| Paroxysmal | 3 |
| Cytokine | Sense (5′ → 3′) | Antisense (3′ → 5′) |
|---|---|---|
| IFN-γ 1 | CTT GTG CCT CAG CCT CTT CTC CTT C | GCG CTG GAC CTT CAG ATC AT |
| IL-1β 2 | CTT CCA AGA CCT GGA CCT CA | GCC ACA ATG ATT GAC ACG AC |
| IL-8 1 | CTT TCT GCA GCT CTG TGT GAA G | GCA GAC CTC AGC TCC GTT GAC |
| TNF-α 2 | AGC CTC TTC TCC TTC CTC CTT | CAG AGG GTT GAT TGA CTG GAA |
| Parameter | r (95% CI) | p-Value | |
|---|---|---|---|
| WCS | −0.49 (−0.75–−0.10) | 0.01 * | |
| R3 | 0.18 (−0.25–0.56) | 0.39 | |
| Tracheal mucus score | −0.48 (−0.72–−0.02) | 0.04 * | |
| Tracheal septum thickness score | 0.40 (−0.02–0.70) | 0.05 | |
| BALf neutrophilia | −0.42 (−0.71–0.00) | 0.04 * | |
| IFN-γ | Quantification | 0.24 (−0.30–0.67) | 0.36 |
| Gene expression | −0.07 (−0.56–0.45) | 0.79 | |
| IL-1β | Quantification | −0.21 (−0.34–0.65) | 0.44 |
| Gene expression | 0.16 (−0.38–0.62) | 0.56 | |
| IL-8 | Quantification | 0.10 (−0.43–0.58) | 0.70 |
| Gene expression | −0.09 (−0.57–0.44) | 0.74 | |
| TNF-α | Quantification | −0.06 (−0.54–0.63) | 0.85 |
| Gene expression | −0.00 (−0.51–0.51) | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frippiat, T.; Art, T.; Tosi, I. Airway Hyperresponsiveness, but Not Bronchoalveolar Inflammatory Cytokines Profiles, Is Modified at the Subclinical Onset of Severe Equine Asthma. Animals 2023, 13, 2485. https://doi.org/10.3390/ani13152485
Frippiat T, Art T, Tosi I. Airway Hyperresponsiveness, but Not Bronchoalveolar Inflammatory Cytokines Profiles, Is Modified at the Subclinical Onset of Severe Equine Asthma. Animals. 2023; 13(15):2485. https://doi.org/10.3390/ani13152485
Chicago/Turabian StyleFrippiat, Thibault, Tatiana Art, and Irene Tosi. 2023. "Airway Hyperresponsiveness, but Not Bronchoalveolar Inflammatory Cytokines Profiles, Is Modified at the Subclinical Onset of Severe Equine Asthma" Animals 13, no. 15: 2485. https://doi.org/10.3390/ani13152485
APA StyleFrippiat, T., Art, T., & Tosi, I. (2023). Airway Hyperresponsiveness, but Not Bronchoalveolar Inflammatory Cytokines Profiles, Is Modified at the Subclinical Onset of Severe Equine Asthma. Animals, 13(15), 2485. https://doi.org/10.3390/ani13152485






