Evaluation of the Longissimus Thoracis et Lumborum Muscle Quality of Chaka and Tibetan Sheep and the Analysis of Possible Mechanisms Regulating Meat Quality
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Color Measurement and pH Value
2.3. Analysis of Chemical Components and Muscle Fiber Parameters
2.4. Determination of Amino Acids and Fatty Acids
2.5. Determination of Volatile Flavor Components
2.6. RNA-seq Data Analysis
2.7. Non-Targeted Metabolomics Analysis and Metabolite Identification
2.8. Real-Time Quantitative PCR
2.9. Statistical Analysis
3. Results
3.1. Meat Quality of CK Sheep and TB Sheep
3.2. The Composition of Amino Acids and Fatty Acids
3.3. Volatile Flavor Compounds of Meat
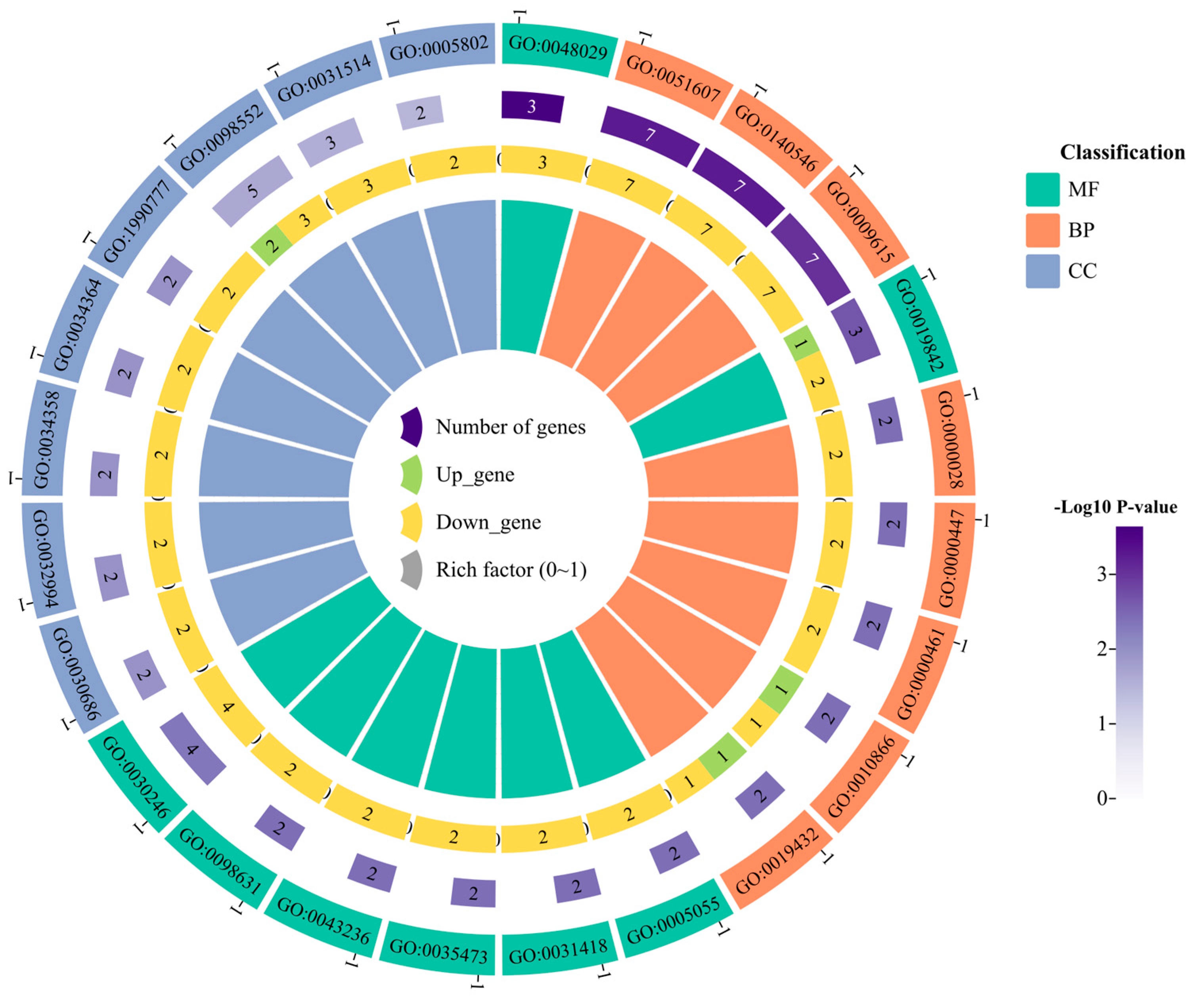
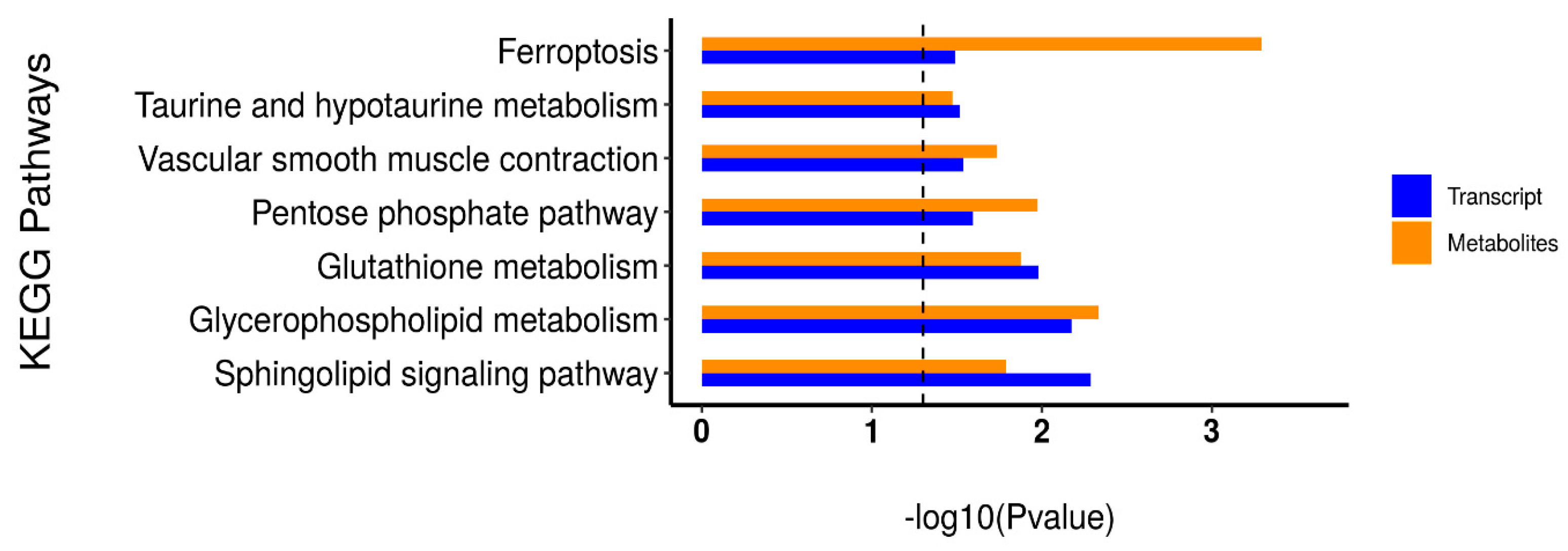
3.4. Differentially Expressed Gene Identification and Functional Enrichment Analysis
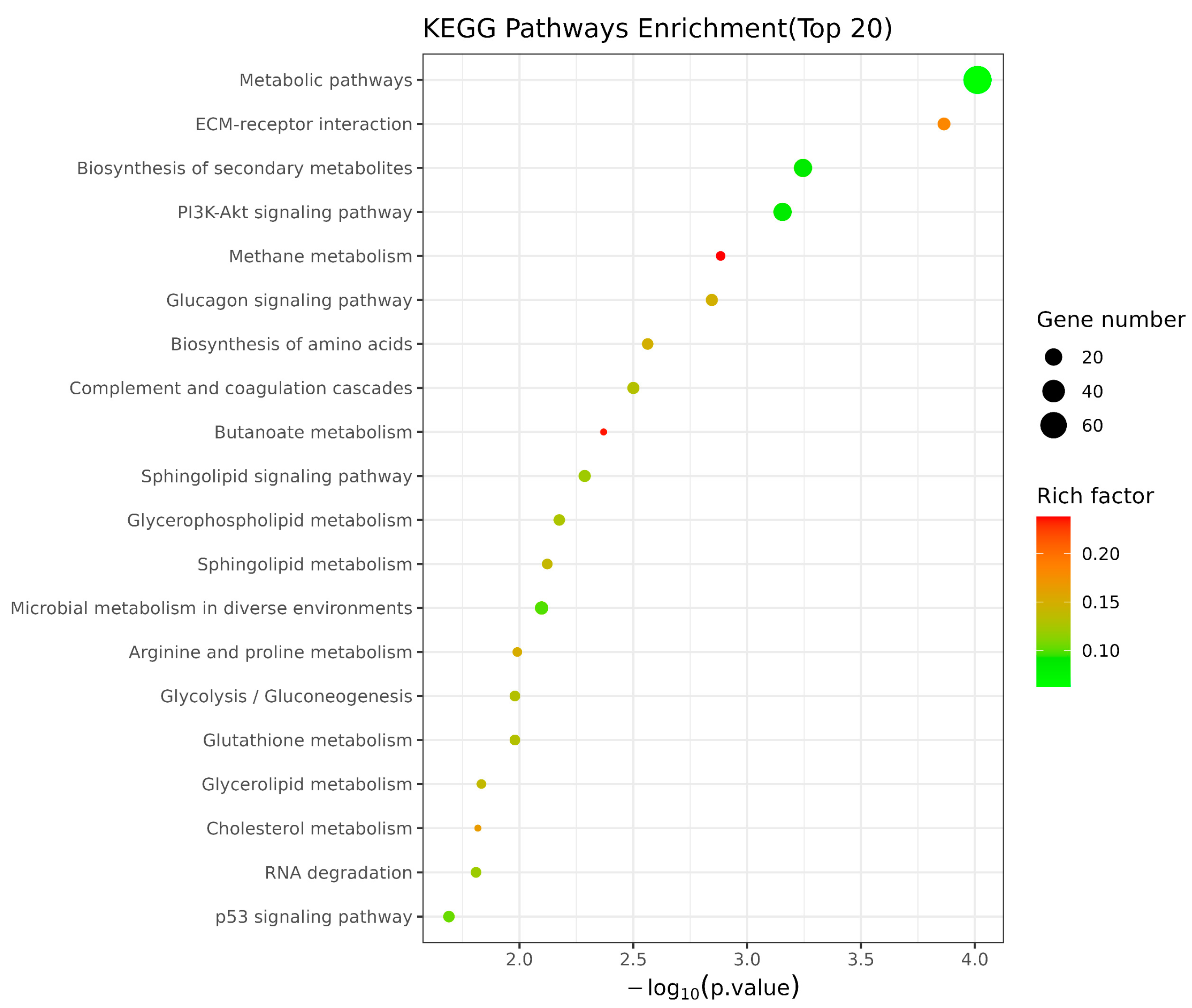
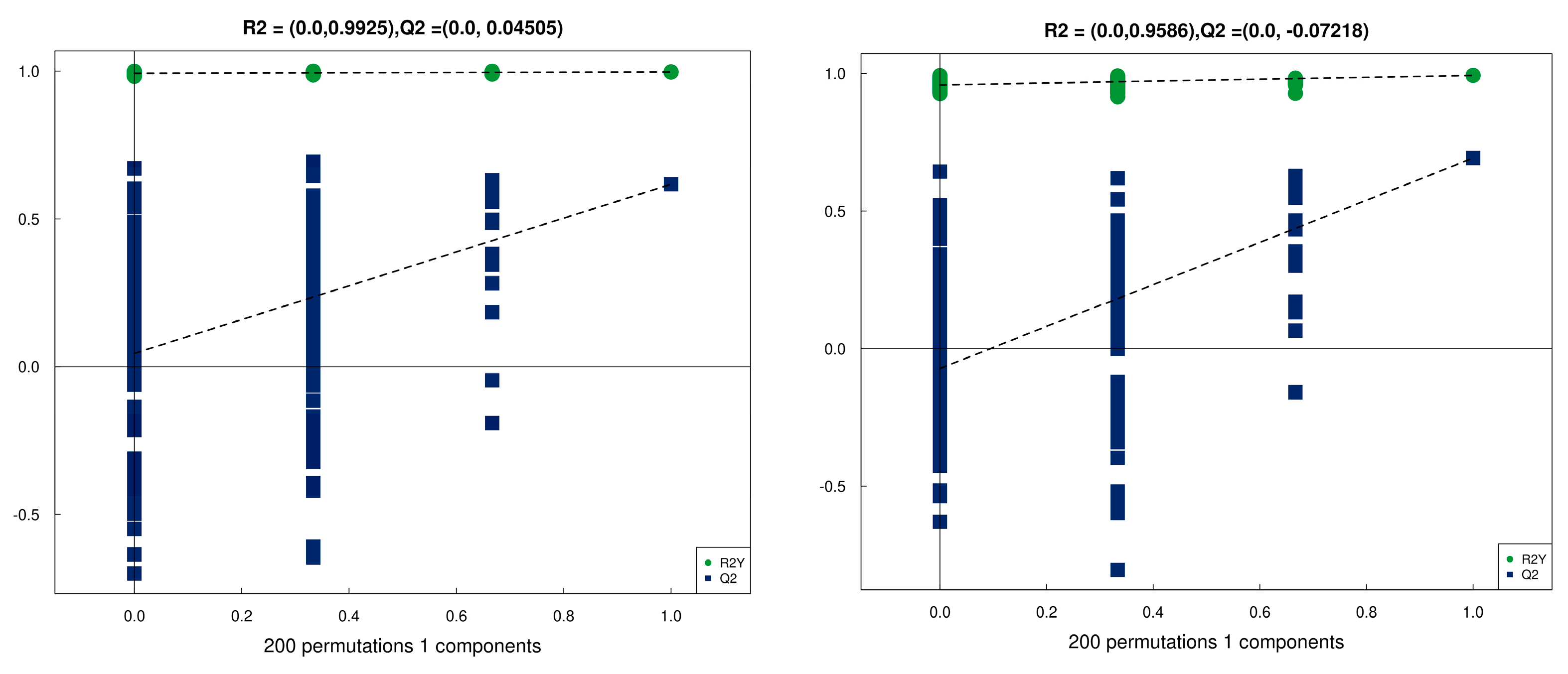
3.5. Metabolomic Profiling Based on UHPLC-Q-TOF MS
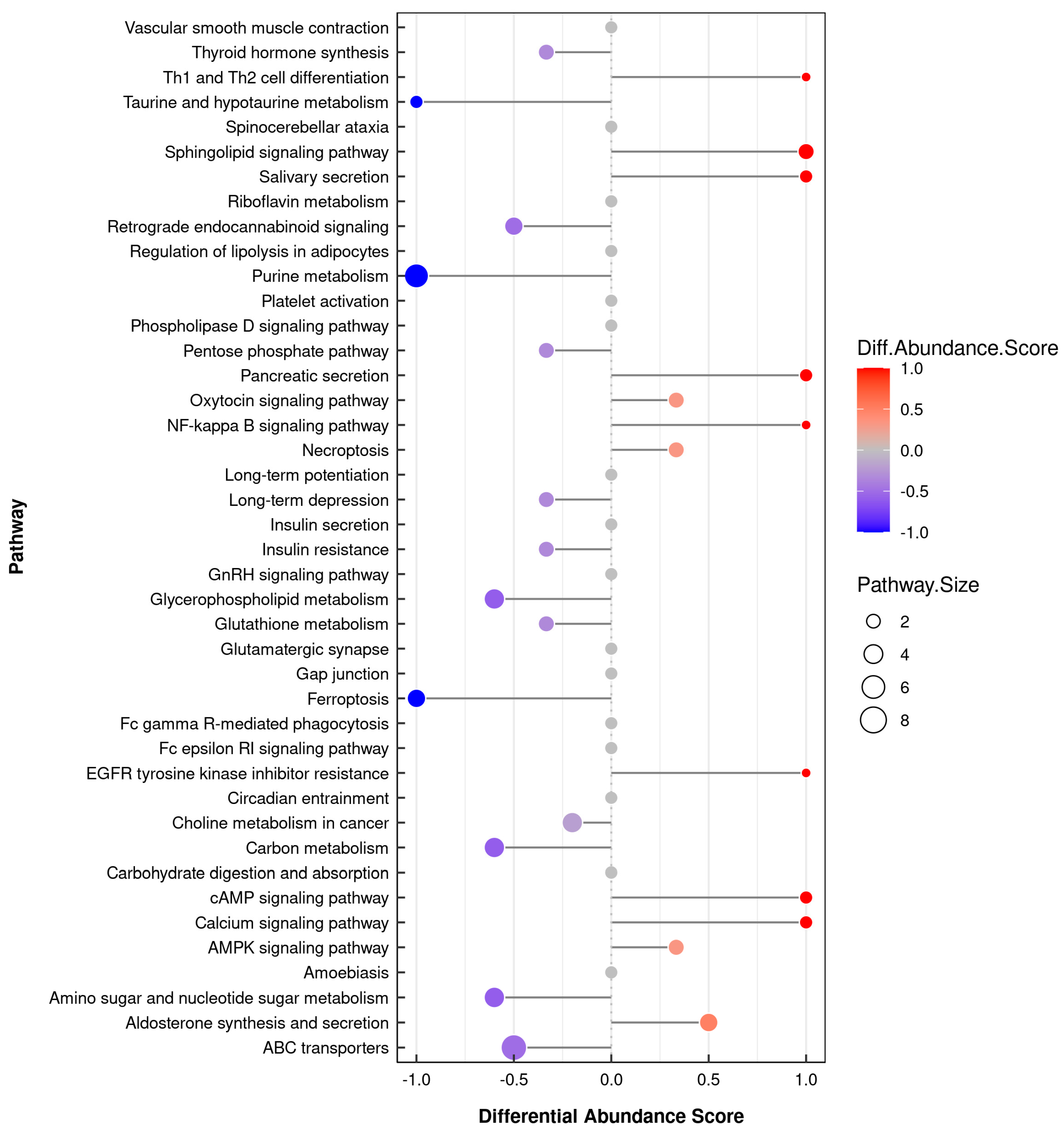
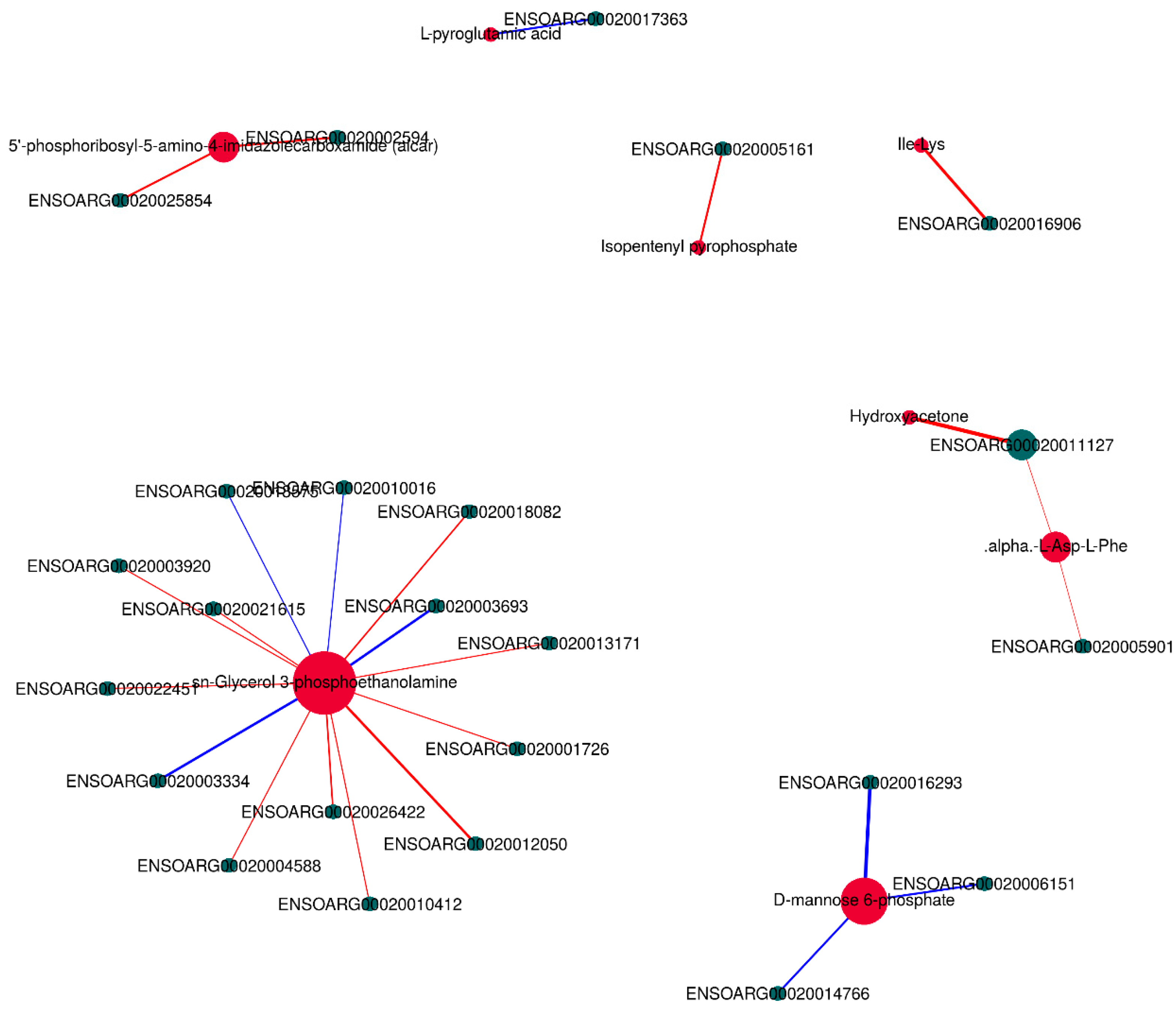
3.6. Integrative Analysis of the Transcriptome and Metabolome
3.7. Correlation Analysis of Meat Quality with DEGs and DMs
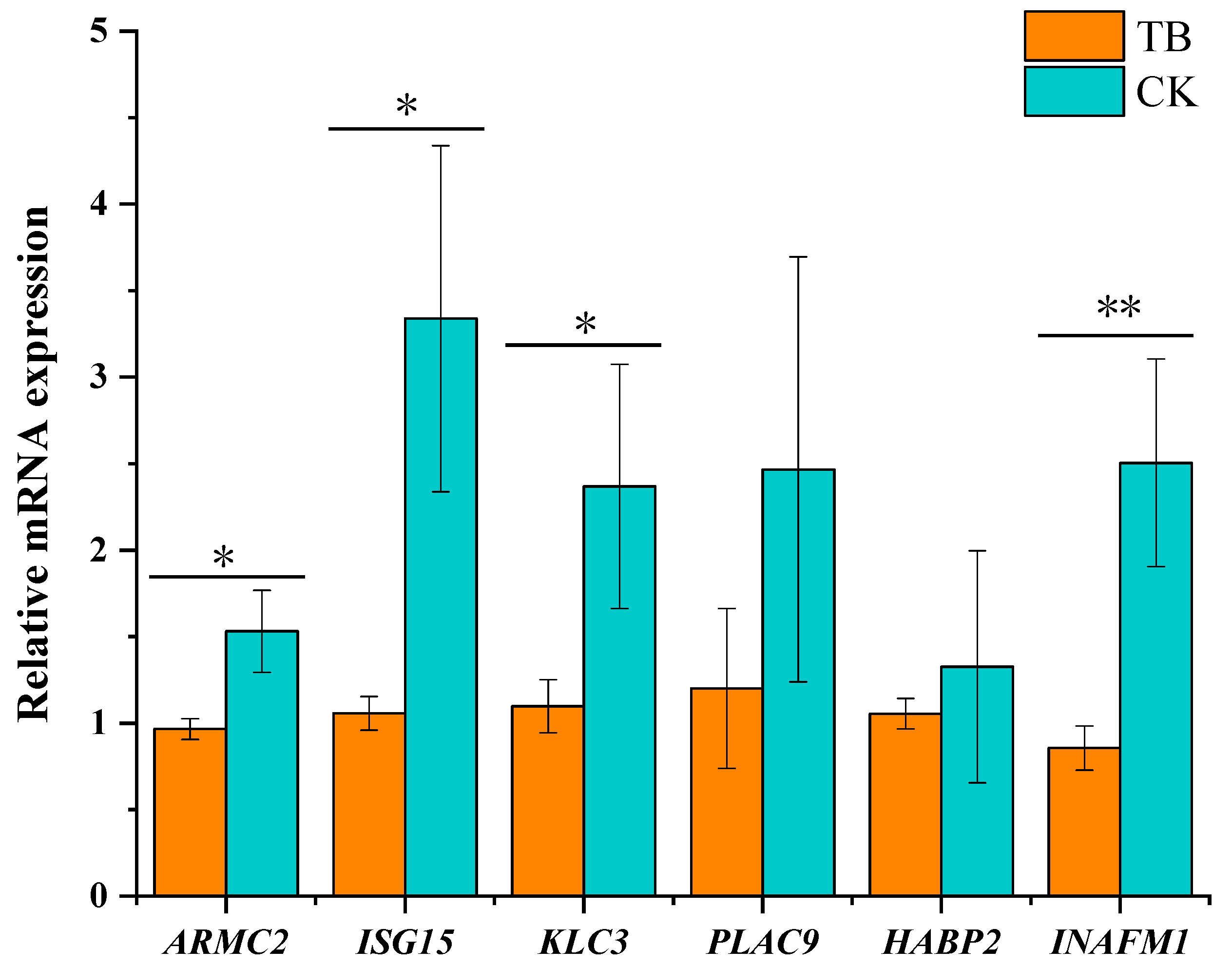
3.8. qRT-PCR Validation of Functional Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, P.; Brown, C.; Arneth, A.; Dias, C.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Could consumption of insects, cultured meat or imitation meat reduce global agricultural land use? Glob. Food Secur. Agric. Policy Econ. Environ. 2017, 15, 22–32. [Google Scholar] [CrossRef]
- Pewan, S.B.; Otto, J.R.; Huerlimann, R.; Budd, A.M.; Mwangi, F.W.; Edmunds, R.C.; Holman, B.W.B.; Henry, M.L.E.; Kinobe, R.T.; Adegboye, O.A.; et al. Genetics of Omega-3 Long-Chain Polyunsaturated Fatty Acid Metabolism and Meat Eating Quality in Tattykeel Australian White Lambs. Genes 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Pleasants, A.B.; Thompson, J.M.; Pethick, D.W. A model relating a function of tenderness, juiciness, flavour and overall liking to the eating quality of sheep meat. Aust. J. Exp. Agric. 2005, 45, 483–489. [Google Scholar] [CrossRef]
- Jacob, R.H.; Pethick, D.W. Animal factors affecting the meat quality of Australian lamb meat. Meat Sci. 2014, 96, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Gao, Z.; Liang, Y.; Zhang, C.; Chen, Z.; Mao, Y.; Huang, B.; Kui, K.; Yang, Z. Longissimus Dorsi Muscle Transcriptomic Analysis of Simmental and Chinese Native Cattle Differing in Meat Quality. Front. Vet. Sci. 2020, 7, 601064. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhong, L.; Luo, H.; Meng, L.; Dong, Y.; Qi, Z.; Wang, H. A comparative analysis of carcass and meat traits, and rumen bacteria between Chinese Mongolian sheep and Dorper x Chinese Mongolian crossbred sheep. Animal 2022, 16, 100503. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, X.; Zhang, Q.; Wei, L.; Liu, H.; Zhao, N.; Hu, L.; Xu, S. Comparative Analysis of the Composition of Fatty Acids and Metabolites between Black Tibetan and Chaka Sheep on the Qinghai-Tibet Plateau. Animals 2022, 12, 2745. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, T.; Zhou, J.; Degen, A.A.; Gou, N.; Li, S.; Bai, Y.; Jing, X.; Wang, W.; Shang, Z. Carcass parameters and meat quality of Tibetan sheep and Small-tailed Han sheep consuming diets of low-protein content and different energy yields. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1010–1023. [Google Scholar] [CrossRef]
- Chen, B.; Yue, Y.; Li, J.; Liu, J.; Yuan, C.; Guo, T.; Zhang, D.; Yang, B.; Lu, Z. Transcriptome-metabolome analysis reveals how sires affect meat quality in hybrid sheep populations. Front. Nutr. 2022, 9, 967985. [Google Scholar] [CrossRef]
- Yu, T.; Tian, X.; Li, D.; He, Y.; Yang, P.; Cheng, Y.; Zhao, X.; Sun, J.; Yang, G. Transcriptome, proteome and metabolome analysis provide insights on fat deposition and meat quality in pig. Food Res. Int. 2023, 166, 112550. [Google Scholar] [CrossRef]
- Wang, X.; Xu, R.; Tong, X.; Zeng, J.; Chen, M.; Lin, Z.; Cai, S.; Chen, Y.; Mo, D. Characterization of different meat flavor compounds in Guangdong small-ear spotted and Yorkshire pork using two-dimensional gas chromatography–time-of-flight mass spectrometry and multi-omics. LWT 2022, 169, 114010. [Google Scholar] [CrossRef]
- Ramanathan, R.; Kiyimba, F.; Gonzalez, J.; Mafi, G.; DeSilva, U. Impact of Up- and Downregulation of Metabolites and Mitochondrial Content on pH and Color of the Longissimus Muscle from Normal-pH and Dark-Cutting Beef. J. Agric. Food Chem. 2020, 68, 7194–7203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Yuan, Z.H.; Li, F.D.; Yue, X.P. Integrating transcriptome and metabolome to identify key genes regulating important muscular flavour precursors in sheep. Animal 2022, 16, 100679. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zheng, C.; Zheng, J.; Zhang, S.; Zhong, Y.; Guo, Q.; Li, F.; Long, C.; Xu, K.; Duan, Y.; et al. Comparisons of carcass traits, meat quality, and serum metabolome between Shaziling and Yorkshire pigs. Anim. Nutr. 2022, 8, 125–134. [Google Scholar] [CrossRef]
- Cloete, J.J.; Hoffman, L.C.; Cloete, S.W. A comparison between slaughter traits and meat quality of various sheep breeds: Wool, dual-purpose and mutton. Meat Sci. 2012, 91, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Hammelman, J.E.; Bowker, B.C.; Grant, A.L.; Forrest, J.C.; Schinckel, A.P.; Gerrard, D.E. Early postmortem electrical stimulation simulates PSE pork development. Meat Sci. 2003, 63, 69–77. [Google Scholar] [CrossRef]
- Hajji, H.; Joy, M.; Ripoll, G.; Smeti, S.; Mekki, I.; Gahete, F.M.; Mahouachi, M.; Atti, N. Meat physicochemical properties, fatty acid profile, lipid oxidation and sensory characteristics from three North African lamb breeds, as influenced by concentrate or pasture finishing diets. J. Food Compos. Anal. 2016, 48, 102–110. [Google Scholar] [CrossRef]
- Joseph, P.; Suman, S.P.; Rentfrow, G.; Li, S.; Beach, C.M. Proteomics of Muscle-Specific Beef Color Stability. J. Agric. Food Chem. 2012, 60, 3196–3203. [Google Scholar] [CrossRef]
- Liang, Y.; Jiao, D.; Du, X.; Zhou, J.; Degen, A.A.; Ran, F.; Sun, G.; Ji, K.; Wu, X.; Cheng, X.; et al. Effect of dietary Agriophyllum squarrosum on average daily gain, meat quality and muscle fatty acids in growing Tan lambs. Meat Sci. 2023, 201, 109195. [Google Scholar] [CrossRef]
- Sun, L.; Xu, L.; Zhao, L.; Dou, L.; Hou, Y.; Wang, C.; Jin, Y.; Su, L. Dietary L-arginine supplementation influences the muscle fiber characteristics and meat quality of Mongolian sheep through the NO/AMPK/PGC-1α pathway. Food Biosci. 2023, 52, 102446. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, T.; Cao, Y.; Deng, B.; Zhang, J.; Zhao, J. Effects of dietary sea buckthorn pomace supplementation on skeletal muscle mass and meat quality in lambs. Meat Sci. 2020, 166, 108141. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.A.; Wang, Z.; Yang, B.; Sun, S.; Ding, B.; Gui, L.; Simal-Gandara, J.; et al. Effects of different feeding regimes on muscle metabolism and its association with meat quality of Tibetan sheep. Food Chem. 2022, 374, 131611. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Pei, J.; Wu, X.; Kalwar, Q.; Yan, P.; Guo, X. Effect of Gender to Fat Deposition in Yaks Based on Transcriptomic and Metabolomics Analysis. Front. Cell Dev. Biol. 2021, 9, 653188. [Google Scholar] [CrossRef] [PubMed]
- Cividini, A.; Levart, A.; Zgur, S.; Kompan, D. Fatty acid composition of lamb meat from the autochthonous Jezersko-Solcava breed reared in different production systems. Meat Sci. 2014, 97, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Vitor, A.C.M.; Godinho, M.; Francisco, A.E.; Silva, J.; Almeida, J.; Fialho, L.; Soldado, D.; Jeronimo, E.; Scollan, N.D.; Huws, S.A.; et al. Nannochloropsis oceanica microalga feeding increases long-chain omega-3 polyunsaturated fatty acids in lamb meat. Meat Sci. 2023, 197, 109053. [Google Scholar] [CrossRef]
- D’Alessandro, A.G.; Palazzo, M.; Petrotos, K.; Goulas, P.; Martemucci, G. Fatty acid composition of light lamb meat from Leccese and Comisana dairy breeds as affected by slaughter age. Small Rumin. Res. 2015, 127, 36–43. [Google Scholar] [CrossRef]
- Kang, J.X. The importance of omega-6/omega-3 fattyacid ratio in cell function—The gene transfer of omega-3 fatty acid desaturase. Omega-6 Omega-3 Essent. Fat. Acid Ratio Sci. Evid. 2003, 92, 23–36. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Importance of the ratio of omega-6/omega-3 essential fatty acids: Evolutionary aspects. Omega-6 Omega-3 Essent. Fat. Acid Ratio Sci. Evid. 2003, 92, 1–22. [Google Scholar]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Jia, W.; Fan, Z.; Shi, Q.; Zhang, R.; Wang, X.; Shi, L. LC-MS-based metabolomics reveals metabolite dynamic changes during irradiation of goat meat. Food Res. Int. 2021, 150, 110721. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Zhang, D.; Liu, Y. Comparison of protein differences between high- and low-quality goat and bovine parts based on iTRAQ technology. Food Chem. 2019, 289, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, T.; Li, Y.; Liu, Y.; Liu, J. An untargeted LC-MS metabolomics approach to the metabolic profiles of bottom cultured scallops (Mizuhopecten yessoensis) subjected to mechanical shock in early post-harvest handling. Aquaculture 2021, 533, 736061. [Google Scholar] [CrossRef]
- Ribeiro de Araujo Cordeiro, A.R.; de Medeiros, L.L.; Alencar Bezerra, T.K.; Bertoldo Pacheco, M.T.; Galvao, M.d.S.; Madruga, M.S. Effects of thermal processing on the flavor molecules of goat by-product hydrolysates. Food Res. Int. 2020, 138, 109758. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, Y.; Pan, D.; Zhou, G.; Wang, Y.; Dang, Y.; He, J.; Li, G.; Cao, J. H-1 NMR-based metabolomics profiling and taste of boneless dry-cured hams during processing. Food Res. Int. 2019, 122, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, K.; Wu, Y.; An, F.; Luo, Z.; Huang, Q.; Wei, S. Exploring the formation mechanism of off-flavor of irradiated yak meat based on metabolomics. Food Chem. X 2022, 16, 100494. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, X.; Zhang, B.; Cui, Y.; Nueraihemaiti, M.; Kou, Q.; Luo, H. Assessment of components related to flavor and taste in Tan-lamb meat under different silage-feeding regimens using integrative metabolomics. Food Chem. X 2022, 14, 100269. [Google Scholar] [CrossRef]
- Ge, Y.; Gai, K.; Li, Z.; Chen, Y.; Wang, L.; Qi, X.; Xing, K.; Wang, X.; Xiao, L.; Ni, H.; et al. HPLC-QTRAP-MS-based metabolomics approach investigates the formation mechanisms of meat quality and flavor of Beijing You chicken. Food Chem. X 2023, 17, 100550. [Google Scholar] [CrossRef]
- Yang, C.; Qiu, M.; Zhang, Z.; Song, X.; Yang, L.; Xiong, X.; Hu, C.; Pen, H.; Chen, J.; Xia, B.; et al. Galacto-oligosaccharides and xylo-oligosaccharides affect meat flavor by altering the cecal microbiome, metabolome, and transcriptome of chickens. Poult. Sci. 2022, 101, 102122. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, F.; Rong, L.; Cai, J.; Yang, S.; Jia, Z.; Li, S. Transcriptomic and Metabolomic Profile Analysis of Muscles Reveals Pathways and Biomarkers Involved in Flavor Differences between Caged and Cage-Free Chickens. Foods 2022, 11, 2890. [Google Scholar] [CrossRef]
- Wu, Q.; Zang, M.; Wang, S.; Zhao, B.; Zhang, S.; Li, S.; Pan, X.; Liu, M.; Fu, X. Changes in flavors profiles of stewed bone-in lamb loin during cooking by DHS/GC-MS combined with electronic bionic systems. Food Biosci. 2023, 53, 102767. [Google Scholar] [CrossRef]
- Ma, X.M.; Wu, J.P.; Li, Y.K.; Liang, X.J.; Casper, D.P.; Ding, W.; Wang, X.Q.; Shi, A.; Shi, X.L.; Ma, L.N.; et al. Transcriptome and metabolome analyses reveal muscle changes in Tan sheep (Ovis aries) at different ages. Livest. Sci. 2022, 255, 104781. [Google Scholar] [CrossRef]
- Du, M.; Ahn, D.U. Volatile substances of Chinese traditional Jinhua ham and Cantonese sausage. J. Food Sci. 2001, 66, 827–831. [Google Scholar] [CrossRef]
- Mottram, D.S.; Edwards, R.A. The role of triglycerides and phospholipids in the aroma of cooked beef. J. Sci. Food Agric. 1983, 34, 517–522. [Google Scholar] [CrossRef]
- Gu, X.; Sun, W.; Yi, K.; Yang, L.; Chi, F.; Luo, Z.; Wang, J.; Zhang, J.; Wang, W.; Yang, T.; et al. Comparison of muscle lipidomes between cattle-yak, yak, and cattle using UPLC-MS/MS. J. Food Compos. Anal. 2021, 103, 104113. [Google Scholar] [CrossRef]
- Whitfield, F.B. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Farmer, L.J. Interactions between Lipids and the Maillard Reaction. In Flavor-Food Interactions; McGorrin, R.J., Leland, J.V., Eds.; American Chemical Society: Washington, DC, USA, 1996; Volume 633, pp. 48–58. [Google Scholar]
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.A.; Gui, L.; Sun, S.; Wang, Z.; Yang, B.; Yuan, Z.; Simal-Gandara, J.; et al. Metabolomics approach reveals high energy diet improves the quality and enhances the flavor of black Tibetan sheep meat by altering the composition of rumen microbiota. Front. Nutr. 2022, 9, 915558. [Google Scholar] [CrossRef]





| Items | CK 1 | TB 2 | SEM 3 | p-Value |
|---|---|---|---|---|
| pH | 6.24 ± 0.055 | 6.50 ± 0.164 | 0.074 | 0.058 |
| L* | 31.67 ± 1.155 | 28.33 ± 1.528 | 0.894 | 0.039 |
| a* | 29.67 ± 2.082 | 24.67 ± 2.517 | 1.400 | 0.057 |
| b* | 13.00 ± 2.000 | 13.00 ± 2.000 | 0.730 | 1.000 |
| MSTR (%) | 71.50 ± 0.700 | 73.07 ± 1.464 | 0.546 | 0.170 |
| Ash (%) | 1.99 ± 0.137 | 2.17 ± 0.033 | 0.053 | 0.101 |
| EE (%) | 10.56 ± 0.367 | 11.95 ± 0.574 | 0.331 | 0.024 |
| CP (%) | 22.63 ± 0.378 | 23.50 ± 0.721 | 0.286 | 0.139 |
| CK 1 | TB 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| total number | 61.67 ± 2.082 | 44.67 ± 7.234 | 4.269 | 0.017 |
| total area, (mm2) | 0.04 ± 0.001 | 0.04 ± 0.006 | 0.002 | 0.293 |
| density, (n/mm2) | 1426.54 ± 43.804 | 1158.77 ± 109.356 | 67.157 | 0.017 |
| diameter, (mm) | 0.03 ± 0.003 | 0.03 ± 0.001 | 0.0008 | 0.388 |
| Items (%) | CK 1 | TB 2 | SEM 3 | p-Value |
|---|---|---|---|---|
| Asp | 5.72 ± 0.265 | 5.66 ± 0.196 | 0.086 | 0.756 |
| Thr | 2.95 ± 0.173 | 3.02 ± 0.095 | 0.053 | 0.590 |
| Ser | 2.32 ± 0.170 | 2.42 ± 0.040 | 0.049 | 0.407 |
| Glu | 11.53 ± 0.644 | 12.12 ± 0.270 | 0.223 | 0.220 |
| Gly | 3.05 ± 0.040 | 2.88 ± 0.064 | 0.043 | 0.018 |
| Ala | 3.79 ± 0.156 | 3.79 ± 0.086 | 0.046 | 0.976 |
| Cys | 0.34 ± 0.074 | 0.36 ± 0.038 | 0.022 | 0.697 |
| Val | 3.32 ± 0.159 | 3.36 ± 0.153 | 0.057 | 0.807 |
| Met | 1.14 ± 0.150 | 0.92 ± 0.122 | 0.070 | 0.124 |
| Ile | 3.32 ± 0.140 | 3.39 ± 0.137 | 0.053 | 0.570 |
| Leu | 5.38 ± 0.250 | 5.44 ± 0.150 | 0.076 | 0.753 |
| Tyr | 2.13 ± 0.270 | 2.10 ± 0.079 | 0.073 | 0.878 |
| Phe | 3.56 ± 0.255 | 3.49 ± 0.158 | 0.079 | 0.694 |
| His | 2.76 ± 0.157 | 2.64 ± 0.136 | 0.060 | 0.362 |
| Lys | 5.73 ± 0.253 | 5.75 ± 0.203 | 0.084 | 0.933 |
| Arg | 4.37 ± 0.187 | 4.33 ± 0.090 | 0.054 | 0.736 |
| Pro | 2.55 ± 0.064 | 2.58 ± 0.026 | 0.019 | 0.543 |
| TAA | 63.97 ± 2.873 | 64.23 ± 1.535 | 0.843 | 0.894 |
| EAA | 28.17 ± 1.344 | 28.01 ± 0.862 | 0.414 | 0.871 |
| NEAA | 35.80 ± 1.540 | 36.56 ± 0.401 | 0.445 | 0.455 |
| IMP | 1.67 ± 0.060 | 1.79 ± 0.160 | 0.052 | 0.292 |
| Items (%) | CK 1 | TB 2 | SEM 3 | p-Value |
|---|---|---|---|---|
| SFA | 48.83 ± 2.272 | 49.29 ± 2.381 | 0.856 | 0.820 |
| (C4:0) | 0.02 ± 0.018 | 0.03 ± 0.006 | 0.005 | 0.611 |
| (C6:0) | 0.01 ± 0.002 | 0.02 ± 0.009 | 0.003 | 0.233 |
| (C8:0) | 0.13 ± 0.007 | 0.02 ± 0.008 | 0.003 | 0.622 |
| (C10:0) | 0.15 ± 0.006 | 0.19 ± 0.039 | 0.013 | 0.176 |
| (C11:0) | 0.001 ± 0.002 | 0.003 ± 0.0002 | 0.0007 | 0.238 |
| (C12:0) | 0.06 ± 0.0081 | 0.09 ± 0.0193 | 0.007 | 0.139 |
| (C13:0) | 0.01 ± 0.0001 | 0.01 ± 0.008 | 0.002 | 0.260 |
| (C14:0) | 2.10 ± 0.112 | 2.61 ± 0.604 | 0.200 | 0.202 |
| (C15:0) | 0.28 ± 0.006 | 0.34 ± 0.053 | 0.019 | 0.147 |
| (C16:0) | 24.49 ± 0.856 | 27.00 ± 1.611 | 0.732 | 0.076 |
| (C17:0) | 1.03 ± 0.043 | 1.06 ± 0.015 | 0.013 | 0.447 |
| (C18:0) | 20.21 ± 2.359 | 17.52 ± 0.290 | 0.859 | 0.122 |
| (C20:0) | 0.06 ± 0.027 | 0.09 ± 0.011 | 0.011 | 0.122 |
| (C21:0) | 0.25 ± 0.016 | 0.18 ± 0.042 | 0.018 | 0.071 |
| (C22:0) | 0.11 ± 0.021 | 0.07 ± 0.014 | 0.010 | 0.076 |
| (C23:0) | 0.05 ± 0.006 | 0.06 ± 0.029 | 0.008 | 0.560 |
| (C24:0) | 0.02 ± 0.006 | 0.02 ± 0.004 | 0.003 | 0.195 |
| MUFA | 41.72 ± 3.542 | 45.49 ± 3.888 | 1.446 | 0.051 |
| (C14:1) | 0.11 ± 0.003 | 0.16 ± 0.045 | 0.016 | 0.145 |
| (C15:1) | 0.16 ± 0.007 | 0.16 ± 0.036 | 0.009 | 0.859 |
| (C16:1) | 1.29 ± 0.207 | 1.45 ± 0.105 | 0.069 | 0.307 |
| (C17:1) | 0.48 ± 0.057 | 0.54 ± 0.027 | 0.021 | 0.183 |
| (C18:1n9t) | ND | ND | - | - |
| (C18:1n9c) | 39.41 ± 3.330 | 42.90 ± 4.054 | 1.463 | 0.461 |
| (C20:1) | 0.11 ± 0.019 | 0.13 ± 0.032 | 0.011 | 0.513 |
| (C22:1n9) | 0.02 ± 0.013 | 0.01 ± 0.005 | 0.004 | 0.846 |
| (C24:1) | 0.14 ± 0.019 | 0.10 ± 0.032 | 0.013 | 0.150 |
| PUFA | 3.97 ± 0.568 | 5.71 ± 0.630 | 0.228 | 0.047 |
| (C18:2n6t) | ND | 0.94 ± 0.514 | - | - |
| (C18:2n6c) | 3.30 ± 0.439 | 2.53 ± 0.029 | 0.205 | 0.040 |
| (C18:3n6) | 0.54 ± 0.146 | 0.58 ± 0.181 | 0.061 | 0.770 |
| (C18:3n3) | 0.15 ± 0.018 | 0.12 ± 0.041 | 0.013 | 0.383 |
| (C20:2) | ND | 0.01 ± 0.012 | - | - |
| (C20:3n3) | 0.26 ± 0.020 | 0.22 ± 0.028 | 0.012 | 0.131 |
| (C20:4n6) | 0.08 ± 0.012 | 0.06 ± 0.017 | 0.008 | 0.079 |
| (C20:3n6) | 0.87 ± 0.037 | 0.63 ± 0.144 | 0.065 | 0.052 |
| (C22:2) | 0.02 ± 0.0023 | 0.01 ± 0.0062 | 0.002 | 0.208 |
| (C20:5n3) | 0.65 ± 0.111 | 0.56 ± 0.076 | 0.041 | 0.285 |
| (C22:6n3) | 0.11 ± 0.035 | 0.04 ± 0.014 | 0.018 | 0.039 |
| UFA | 47.69 ± 2.985 | 51.20 ± 3.798 | 1.366 | 0.042 |
| SFA/UFA | 0.97 ± 0.103 | 1.03 ± 0.118 | 0.043 | 0.535 |
| PUFA/SFA | 0.12 ± 0.006 | 0.17 ± 0.018 | 0.005 | 0.629 |
| ω-3PUFA | 1.17 ± 0.154 | 0.94 ± 0.145 | 0.074 | 0.140 |
| ω-6PUFA | 4.78 ± 0.555 | 4.74 ± 0.501 | 0.193 | 0.927 |
| ω-6/ω-3PUFA | 4.15 ± 0.738 | 5.06 ± 0.462 | 0.303 | 0.144 |
| Items (%) | CK 1 | TB 2 | SEM 3 | p-Value |
|---|---|---|---|---|
| Aldehydes | 62.94 ± 5.997 | 40.58 ± 2.158 | 5.264 | 0.04 |
| Pentanal | 0.24 ± 0.051 | 0.34 ± 0.122 | 0.041 | 0.261 |
| 2-Thiophene formaldehyde | 0.21 ± 0.059 | 0.34 ± 0.082 | 0.384 | 0.095 |
| Hexanal | 4.28 ± 0.144 | 2.58 ± 0.354 | 0.393 | 0.002 |
| Heptanal | 4.67 ± 0.879 | 1.65 ± 0.195 | 0.714 | 0.004 |
| Octanal | 3.65 ± 0.445 | 1.66 ± 0.693 | 0.493 | 0.014 |
| Nonanal | 13.78 ± 0.677 | 3.26 ± 0.335 | 2.361 | <0.001 |
| Methylthiopropionaldehyde | ND | 0.89 ± 0.305 | - | - |
| Decanal | 1.39 ± 0.300 | 1.25 ± 0.099 | 0.087 | 0.471 |
| Benzaldehyde | 21.24 ± 3.623 | 18.49 ± 1.625 | 1.196 | 0.296 |
| (E)-2-Decenal | 2.17 ± 0.089 | 1.57 ± 0.833 | 0.255 | 0.283 |
| 2-Undecenal | 3.72 ± 0.498 | 1.54 ± 0.180 | 0.506 | 0.002 |
| 2-Phenyl-2-butylenaldehyde | 0.79 ± 0.055 | 0.50 ± 0.070 | 0.688 | 0.005 |
| (E,E)-2,4-heptadienal | 0.73 ± 0.305 | 0.19 ± 0.023 | 1.431 | 0.039 |
| Pentadecanal | 1.26 ± 0.350 | 1.02 ± 0.241 | 0.123 | 0.372 |
| Hexadecanal | 9.35 ± 0.291 | 5.54 ± 0.390 | 0.861 | <0.001 |
| Alcohols | 6.60 ± 0.801 | 8.30 ± 0.131 | 0.433 | 0.022 |
| 1-Pentanol | 0.78 ± 0.078 | 1.07 ± 0.262 | 0.095 | 0.143 |
| 1-Hexanol | 0.44 ± 0.099 | 0.57 ± 0.101 | 0.047 | 0.185 |
| 2-Furanmethanol | 0.37 ± 0.097 | 0.46 ± 0.145 | 0.049 | 0.422 |
| 1-Octen-3-ol | 1.55 ± 0.387 | 2.51 ± 0.351 | 0.255 | 0.033 |
| 1-Heptanol | 0.93 ± 0.119 | 1.32 ± 0.315 | 0.123 | 0.115 |
| 1-Octanol | 1.86 ± 0.211 | 1.89 ± 0.297 | 0.094 | 0.893 |
| (E)-2-Octene-1-ol | 0.67 ± 0.72 | 0.44 ± 0.154 | 0.068 | 0.079 |
| Ketones | 1.72 ± 0.180 | 3.34 ± 0.264 | 0.372 | 0.001 |
| 2-Pentadecanone | 0.58 ± 0.085 | 0.65 ± 0.145 | 0.462 | 0.511 |
| 3-Hexanone | 0.13 ± 0.045 | 0.75 ± 0.232 | 0.152 | 0.011 |
| 2,3-Octanedione | 0.78 ± 0.082 | 0.37 ± 0.056 | 0.095 | 0.002 |
| Hydroxyacetone | ND | 1.21 ± 0.078 | - | - |
| 6-Methyl-5-hepten-2-one | 0.23 ± 0.061 | 0.36 ± 0.042 | 0.035 | 0.035 |
| Hydrocarbons | 3.78 ± 0.700 | 5.80 ± 2.471 | 0.802 | 0.246 |
| Heptylbenzene | 0.99 ± 0.125 | 1.92 ± 0.405 | 0.234 | 0.020 |
| Xylene | 0.76 ± 0.371 | 0.89 ± 0.396 | 0.143 | 0.699 |
| Octylbenzene | 1.17 ± 0.154 | 1.68 ± 0.055 | 0.122 | 0.006 |
| Nonylbenzene | 0.86 ± 0.146 | 1.64 ± 0.331 | 0.198 | 0.020 |
| Esters | 0.47 ± 0.129 | 1.98 ± 0.572 | 0.370 | 0.011 |
| 2-Ethylethyl acetate | ND | 0.24 ± 0.127 | - | - |
| Methyl diethyldithiocarbamate | 0.47 ± 0.129 | 1.73 ± 0.446 | 0.308 | 0.009 |
| Others | 9.94 ± 1.023 | 17.29 ± 4.920 | 2.095 | 0.064 |
| 2-Pentyl-furan | 1.50 ± 0.280 | 4.76 ± 1.006 | 0.777 | 0.006 |
| 2-Acetyl thiazole | 0.15 ± 0.025 | 0.58 ± 0.125 | 0.100 | 0.005 |
| 2- (pentenyl) furan | 1.40 ± 0.627 | 1.21 ± 0.362 | 0.192 | 0.673 |
| 2-Acetyl pyrrole | 1.49 ± 0.148 | 3.26 ± 0.180 | 0.400 | <0.001 |
| Acetoin | 3.60 ± 0.246 | 1.69 ± 0.242 | 0.436 | 0.001 |
| Dimethyl disulfide | 0.18 ± 0.030 | 0.20 ± 0.173 | 0.010 | 0.374 |
| Dimethyl trisulfide | 0.27 ± 0.021 | 0.25 ± 0.087 | 0.024 | 0.719 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Liu, H.; Wang, X.; Zhang, Q.; Guo, T.; Hu, L.; Xu, S. Evaluation of the Longissimus Thoracis et Lumborum Muscle Quality of Chaka and Tibetan Sheep and the Analysis of Possible Mechanisms Regulating Meat Quality. Animals 2023, 13, 2494. https://doi.org/10.3390/ani13152494
Xu X, Liu H, Wang X, Zhang Q, Guo T, Hu L, Xu S. Evaluation of the Longissimus Thoracis et Lumborum Muscle Quality of Chaka and Tibetan Sheep and the Analysis of Possible Mechanisms Regulating Meat Quality. Animals. 2023; 13(15):2494. https://doi.org/10.3390/ani13152494
Chicago/Turabian StyleXu, Xianli, Hongjin Liu, Xungang Wang, Qian Zhang, Tongqing Guo, Linyong Hu, and Shixiao Xu. 2023. "Evaluation of the Longissimus Thoracis et Lumborum Muscle Quality of Chaka and Tibetan Sheep and the Analysis of Possible Mechanisms Regulating Meat Quality" Animals 13, no. 15: 2494. https://doi.org/10.3390/ani13152494
APA StyleXu, X., Liu, H., Wang, X., Zhang, Q., Guo, T., Hu, L., & Xu, S. (2023). Evaluation of the Longissimus Thoracis et Lumborum Muscle Quality of Chaka and Tibetan Sheep and the Analysis of Possible Mechanisms Regulating Meat Quality. Animals, 13(15), 2494. https://doi.org/10.3390/ani13152494





