Residue Depletion Profile and Estimation of Withdrawal Period for Sulfadimethoxine and Ormetoprim in Edible Tissues of Nile Tilapia (Oreochromis sp.) on Medicated Feed
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Equipment
2.3. Standard Solutions
2.4. Analytical Method for SDM and OMP Determination in Feed
2.5. Analytical Method for SDM and OMP Determination in Fish Fillet
2.6. Medicated Feed
2.7. Animals and Experimental Design
2.8. Depletion Evaluation and Withdrawal Period Estimation
3. Results and Discussion
3.1. Analytical Method Validation
3.2. Drug Feed Incorporation
3.3. Depletion Study and Withdrawal Period Estimation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture (SOFIA 2022): Towards Blue Transformation; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/publications/sofia/2022/en/ (accessed on 25 March 2023).
- Gorito, A.M.; Ribeiro, A.R.L.; Rodrigues, P.; Pereira, M.F.R.; Guimarães, L.; Almeida, C.M.R.; Silva, A.M.T. Antibiotics Removal from Aquaculture Effluents by Ozonation: Chemical and Toxicity Descriptors. Water. Res. 2022, 218, 118497. [Google Scholar] [CrossRef]
- Shinn, A.P.; Avenant-Oldewage, A.; Bondad-Reantaso, M.G.; Cruz-Laufer, A.J.; García-Vásquez, A.; Hernández-Orts, J.S.; Kuchta, R.; Longshaw, M.; Metselaar, M.; Pariselle, A.; et al. A Global Review of Problematic and Pathogenic Parasites of Farmed Tilapia. Rev. Aquac. 2023, 15, 92–153. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. FishStatJ, a Tool for Fishery Statistics Analysis; Berger, T., Sibeni, F., Calderini, F., Eds.; FAO Fisheries Division (NFI): Rome, Italy, 2021. [Google Scholar]
- Hong, S.; Kwon, N.; Kang, H.S.; Jang, E.; Kim, H.; Han, E. Simultaneous Analysis of 21 Sulfonamides, Trimethoprim, Ormetoprim, and Dapsone in Fish and Shrimp Samples by LC-MS/MS Using the QuEChERS Method. Int. J. Environ. Anal. Chem. 2022, 102, 1–12. [Google Scholar] [CrossRef]
- Ojasanya, R.A.; Gardner, I.A.; Groman, D.B.; Saksida, S.; Saab, M.E.; Thakur, K.K. Antimicrobial Susceptibility Profiles of Bacteria Commonly Isolated from Farmed Salmonids in Atlantic Canada (2000–2021). Vet. Sci. 2022, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Habibullah-Al-Mamun, M.; Nagano, I.; Masunaga, S.; Kitazawa, D.; Matsuda, H. Antibiotics, Antibiotic-Resistant Bacteria, and Resistance Genes in Aquaculture: Risks, Current Concern, and Future Thinking. Environ. Sci. Pollut. Res. 2022, 29, 11054–11075. [Google Scholar] [CrossRef] [PubMed]
- Girmatsion, M.; Mahmud, A.; Abraha, B.; Xie, Y.; Cheng, Y.; Yu, H.; Yao, W.; Guo, Y.; Qian, H. Rapid Detection of Antibiotic Residues in Animal Products Using Surface-Enhanced Raman Spectroscopy: A Review. Food Control 2021, 126, 108019. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach-Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of Alternatives to Antibiotic Use in Aquaculture. Rev. Aquac. 2023, 15, 1–31. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Aquaculture Development. 8. Recommendations for Prudent and Responsible Use of Veterinary Medicines in Aquaculture. In FAO Technical Guidelines for Responsible Fisheries; FAO: Rome, Italy, 2019; Available online: https://www.fao.org/documents/card/ru/c/ca7029en/ (accessed on 27 March 2023).
- Vinarukwong, N.; Lukkana, M.; Berntsen, J.O.; Wongtavatchai, J. Therapeutic Use of Sulfadimethoxine-Ormetoprim for Control of Streptococcus Agalactiae Infection in Nile tilapia (Oreochromis niloticus) Fry. Thai J. Vet. Med. 2018, 48, 367–373. [Google Scholar]
- Du, W.X.; Marshall, M.R.; Wheeler, W.B.; Mathews, M.; Gatlin, D.; Rawles, S.D.; Xu, D.-H.; Rodgers, W.A.; Wei, C.I. Oxytetracycline, Sulfadimethoxine, and Ormetoprim Residues in Channel Catfish by HPLC. J. Food Sci. 1995, 60, 1220–1225. [Google Scholar] [CrossRef]
- Samuelsen, O.B. Absorption, Tissue Distribution, Metabolism and Excretion of Ormetoprim and Sulphadimethoxine in Cod (Gadus morhua) after Oral Administration of Romet30. J. Appl. Ichthyol. 2006, 22, 68–71. [Google Scholar] [CrossRef]
- Health Canada. List of Veterinary Drugs that are Authorized for Sale by Health Canada for Use in Food-Producing Aquatic Animals—Health Canada. 2010. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/legislation-guidelines/policies/list-veterinary-drugs-that-authorized-sale-health-canada-use-food-producing-aquatic-animals.html (accessed on 25 March 2023).
- FDA, Food and Drug Administration. Approved Aquaculture Drugs. 2023. Available online: https://www.fda.gov/animal-veterinary/aquaculture/approved-aquaculture-drugs (accessed on 26 March 2023).
- Bakal, R.S.; Bai, S.A.; Stoskopf, M.K. Pharmacokinetics of Sulfadimethoxine and Ormetoprim in a 5:1 Ratio Following Intraperitoneal and Oral Administration, in the Hybrid Striped Bass (Morone chrysops × Morone saxitalis). J. Vet. Pharmacol. Ther. 2004, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- SINDAN, Sindicato Nacional da Indústria de Produtos Para Saúde Animal—SINDAN. 2023. Available online: https://www.sindan.org.br (accessed on 13 March 2023).
- Li, Q.; Ji, K.; Tang, N.; Li, Y.; Gu, X.; Tang, K. Vortex-Ultrasonic Assisted Dispersive Liquid-Liquid Microextraction for Seven Sulfonamides of Fish Samples Based on Hydrophobic Deep Eutectic Solvent and Simultaneous Detecting with HPLC-PDA. Microchem. J. 2023, 185, 108269. [Google Scholar] [CrossRef]
- Wang, L.; Niu, J.; Wei, P.; Feng, Y.; Ding, M.; He, C.; Ma, Y.; Zhu, Y.; Li, J.; Huang, L.; et al. Rapid Determination of 2,4-Diaminopyrimidine Residues through Sample Pretreatment Using Immunomagnetic Bead Purification along with HPLC–UV. Food Chem. 2022, 376, 131835. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhang, M.; Zhu, J.; Shi, L. Strategies for Studying in Vivo Biochemical Formation Pathways and Multilevel Distributions of Sulfanilamide Metabolites in Food (2012–2022). Food Chem. 2022, 388, 133039. [Google Scholar] [CrossRef]
- Health Canada. List of Maximum Residue Limits (MRLs) for Veterinary Drugs in Foods. 2022. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/maximum-residue-limits-mrls/list-maximum-residue-limits-mrls-veterinary-drugs-foods.html (accessed on 23 March 2023).
- FDA, Food and Drug Administration. CFR—Code of Federal Regulations Title 21. PART 556: Tolerances for Residues of New Animal Drugs in Food. 2023. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=556 (accessed on 28 March 2023).
- European Commission. Commission Regulation (EEC) No. 37/2010. on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Off. J. Eur. Union 2010, L15, 1–72. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:015:0001:0072:en:PDF (accessed on 28 March 2023).
- ANVISA, Agência Nacional de Vigilância Sanitária. Instrução Normativa n° 51, de 19 de Dezembro de 2019. Estabelece a Lista de Limites Máximos de Resíduos (LMR), Ingestão Diária Aceitável (IDA) e Dose de Referência Aguda (DRfA) Para Insumos Farmacêuticos Ativos (IFA) de Medicamentos Veterinários em Alimentos de Origem Animal. [Normative Instruction 51, of December 19, 2019, establishes the list of maximum residue limits (MRL), acceptable daily intake (IDA) and acute reference dose (DRfA) for active pharmaceutical ingredients (IFA) of veterinary medicines in food of animal origin, Brazil]. In Diário Oficial da União; Agencia Nacional de Vigilância Sanitária: Brasília, Brazil, 2019; Edição 249, Seção 1; p. 98. Available online: http://antigo.anvisa.gov.br/documents/10181/5545276/IN_51_2019_COMP.pdf/62c92657-8945-4ac7-b1d3-01147ab90abb (accessed on 1 August 2023).
- CGAL/SDA/MAPA. Coordenação-Geral de Apoio Laboratorial (CGAL) da Secretaria de Defesa Agropecuária (SDA) do Ministério da Agricultura, Pecuária e Abastecimento (MAPA). In Guia de Validação e Controle de Qualidade: Fármacos em Produtos Para Alimentação Animal e Medicamentos Veterinários; MAPA/ACS: Brasília, Brazil, 2011. [Google Scholar]
- VICH-FDA. International Cooperation on Harmonisation of Technical Requirements for Registration of veterinary Medicinal Products—Food and Drug Administration. Guidance for Industry—Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies; VICH GL49(R); FDA: Rockville, MD, USA, 2015.
- Portela, A.C.V.; Silveira, J.G.F.; Damaceno, M.A.; da Silva, A.F.B.; de Jesus, R.B.; Pilarski, F.; Gadaj, A.; Mooney, M.H.; Paschoal, J.A.R. Food Safety Evaluation for the Use of Albendazole in Fish: Residual Depletion Profile and Withdrawal Period Estimation. Food Addit. Contam. Part A 2020, 37, 596–606. [Google Scholar] [CrossRef]
- Busatto, Z.; da Silva, A.F.B.; de Freitas, O.; Paschoal, J.A.R. LC-MS/MS Methods for Albendazole Analysis in Feed and Its Metabolite Residues in Fish Fillet and a Leaching Study in Feed after an Alternative Procedure for Drug Incorporation. Food Addit. Contam. Part A 2017, 34, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Fais, A.P.; Franco, R.S.B.; da Silva, A.F.B.; de Freitas, O.; Paschoal, J.A.R. LC-MS/MS Methods for Sulfadimethoxine and Ormetoprim Analysis in Feed and Fish Fillet and a Leaching Study for Feed after Alternative Procedures for the Incorporation of Drugs. Food Addit. Contam. Part A 2017, 34, 501–508. [Google Scholar] [CrossRef]
- Fraccarolli-Neto, P.F.; da Silva, A.F.B.; Moro, E.B.; Pilarski, F.; de Freitas, O.; Mooney, M.H.; Paschoal, J.A.R. Emamectin Benzoate in Tilapia: Alternative Method for Drug Incorporation into Feed and Associated Residual Depletion Study. Food Res. Int. 2019, 119, 524–529. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; Banerjee, S. Aqueous Solubility: Methods of Estimation for Organic Compounds; Marcel Dekker: New York, NY, USA, 1992. [Google Scholar]
- VICH-FDA. Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food-Producing Animals: Marker-Residue-Depletion Studies to Establish Product Withdrawal Periods. In International Cooperation on Harmonisation of Technical Requirements for Registration of veterinary Medicinal Products—Food and Drug Administration; VICH GL48; FDA: Rockville, MD, USA, 2015. [Google Scholar]
- VICH-FDA. Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Human Food-Producing Animals: Marker Residue Depletion Studies to Establish Product Withdrawal Periods in Aquatic Species. In International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products—Food and Drug Administration; VICH GL57; FDA: Rockville, MD, USA, 2019. [Google Scholar]
- Baralla, E.; Varoni, M.V.; Nieddu, M.; Demontis, M.P.; Merella, P.; Burreddu, C.; Garippa, G.; Boatto, G. Determination of Praziquantel in Sparus Aurata L. after Administration of Medicated Animal Feed. Animals 2020, 10, 528. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-Based Composite Films and Their Application in Food Packaging: A Review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Julinta, R.B.; Abraham, T.J.; Roy, A.; Singha, J.; Bardhan, A.; Sar, T.K.; Patil, P.K.; Kumar, K.A. Safety of Emamectin Benzoate Administered in Feed to Nile Tilapia Oreochromis niloticus (L.). Environ. Toxicol. Pharmacol. 2020, 75, 103348. [Google Scholar] [CrossRef] [PubMed]
- Cantelmo, O.A.; Pezzato, L.E.; Barros, M.M.; Pezzato, A.C. Características físicas de dietas para peixes confeccionadas com diferentes aglutinantes. Acta Scientiarum 2002, 24, 949–955. [Google Scholar] [CrossRef][Green Version]
- Milner, N.P.; Johnson, M.R.; Perry, K.J. Determination of Sulfadimethoxine and Ormetoprim Residues in Channel Catfish Fillets After Treatment with Romet and Evaluation of a Commercially Available Rapid Diagnostic Test for Drug Residues in Fish Fillets. J. AOAC Int. 1994, 77, 875–881. [Google Scholar] [CrossRef]
- Samuelsen, O.B.; Ervik, A.; Wennevik, V. Absorption, Tissue Distribution, Metabolism and Excretion of Ormetoprim and Sulphadimethoxine in Atlantic Salmon (Salmo salar) after Intravenous and Oral Administration of Romet30. Xenobiotica 1995, 25, 1169–1180. [Google Scholar] [CrossRef]
- Kosoff, R.E.; Chen, C.Y.; Wooster, G.A.; Getchell, R.G.; Clifford, A.; Craigmill, A.L.; Bowser, P.R. Sulfadimethoxine and Ormetoprim Residues in Three Species of Fish after Oral Dosing in Feed. J. Aquat. Anim. Health 2007, 19, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, J.A.R.; Quesada, S.P.; Gonçalves, L.U.; Cyrino, J.E.P.; Reyes, F.G.R. Depletion Study and Estimation of the Withdrawal Period for Enrofloxacin in Pacu (Piaractus mesopotamicus). J. Vet. Pharmacol. Ther. 2013, 36, 594–602. [Google Scholar] [CrossRef]
- Cao, C.; Liu, Y.; Zhang, G.; Dong, J.; Xu, N.; Zhou, S.; Yang, Y.; Yang, Q.; Ai, X. Temperature-Dependent Residue Depletion Regularities of Tiamulin in Nile tilapia (Oreochromis niloticus) Following Multiple Oral Administrations. Front. Vet. Sci. 2021, 8, 679657. [Google Scholar] [CrossRef]
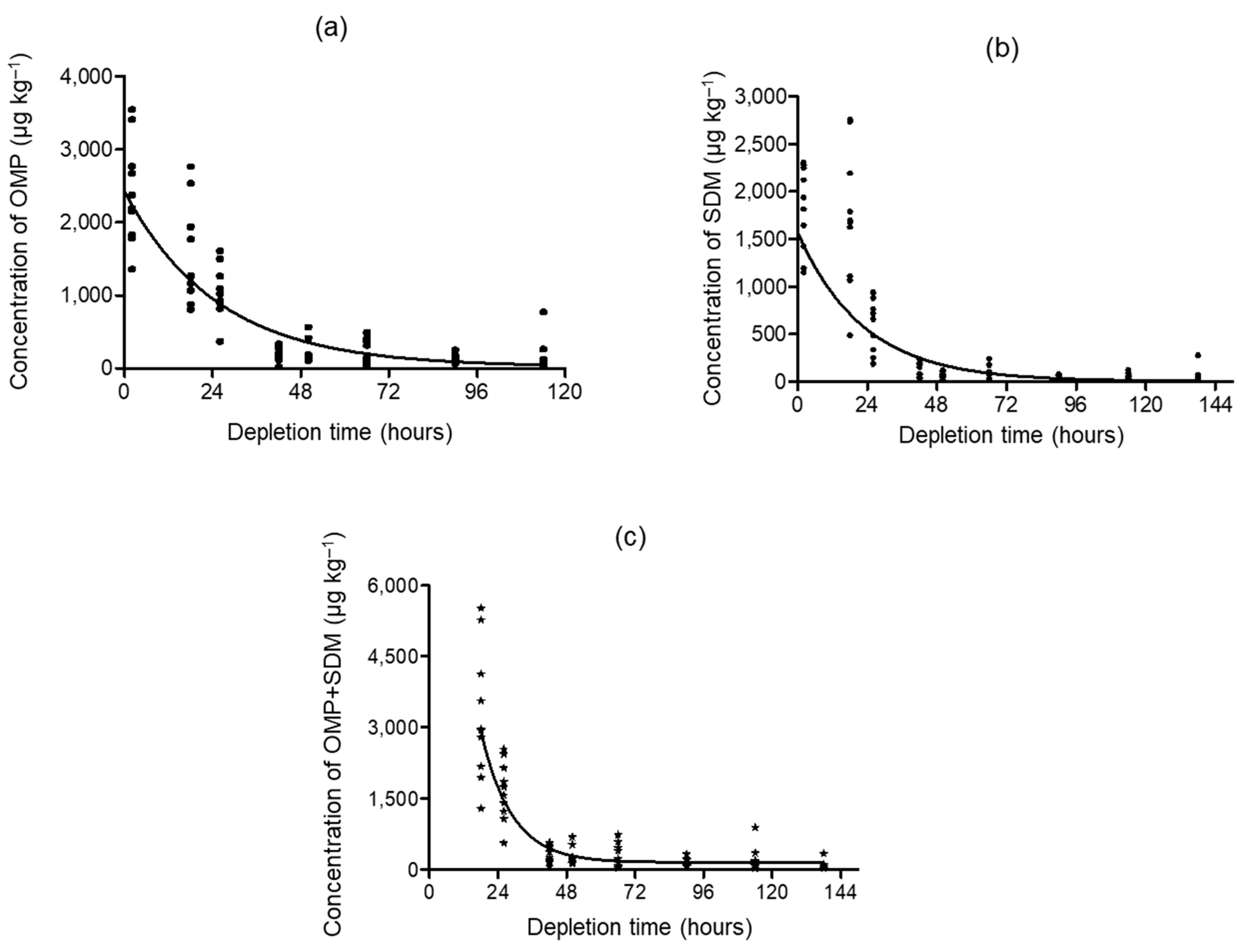
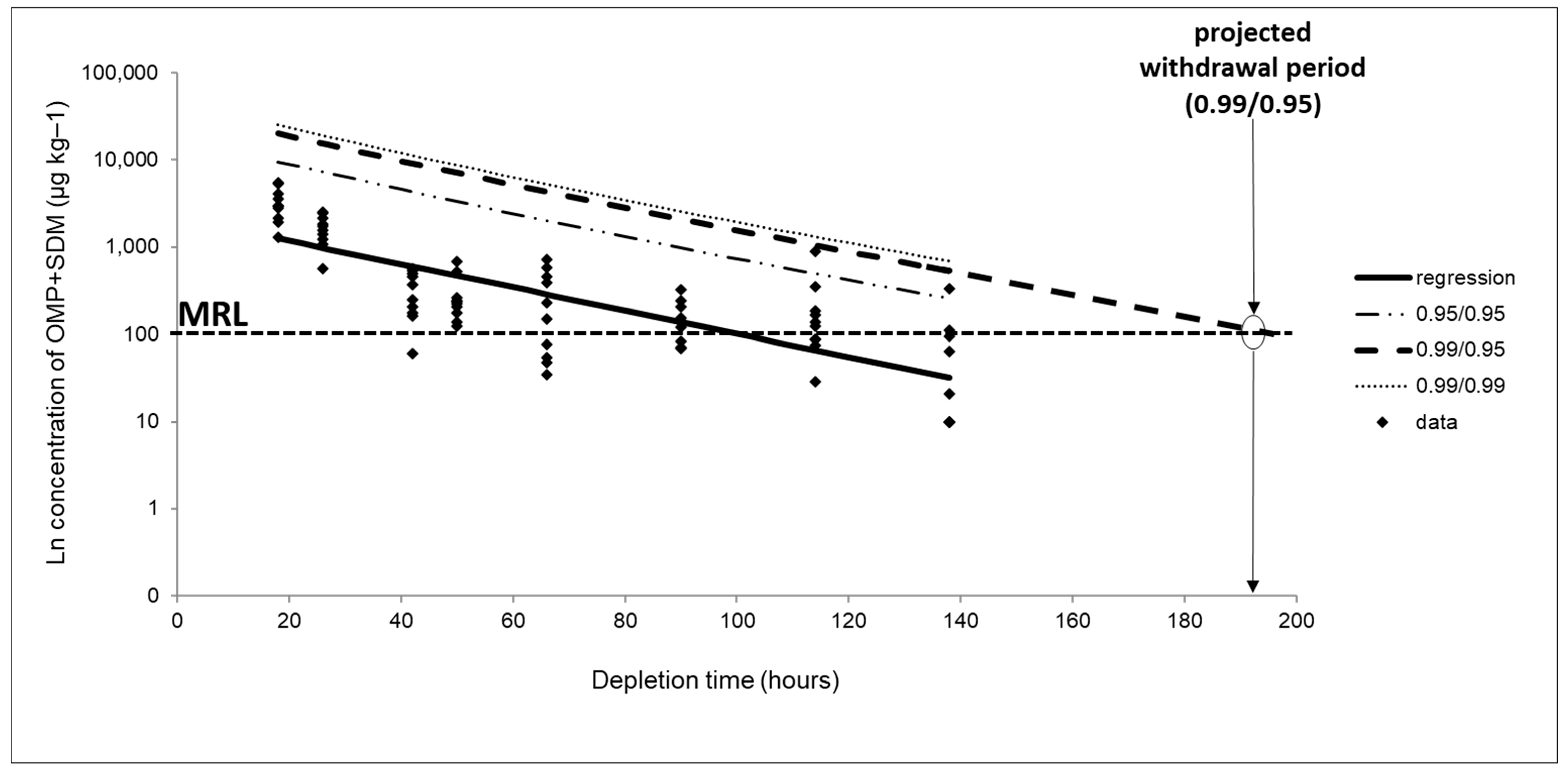
| Sampling Time (h) | Range Concentration (µg kg−1) | Mean Concentration (µg kg−1, n = 10) | |||
|---|---|---|---|---|---|
| OMP | SDM | OMP | SDM | Sum | |
| 18 | 808–2765 | 485–2756 | 1545 | 1714 | 3259 |
| 26 | 373–1607 | 189–940 | 1042 | 616 | 1658 |
| 42 | 23–336 | 37–238 | 202 | 130 | 331 |
| 50 | 110–564 | 16–491 | 218 | 65 | 283 |
| 66 | 35–121 | 10–241 | 207 | 75 | 281 |
| 90 | 69–250 | 10–78 | 121 | 37 | 159 |
| 114 | 29–771 | 10–124 | 176 | 42 | 218 |
| 138 | <LOQ–59 | <LOQ–278 | <LOQ | <LOQ | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas, L.V.P.; da Mota Júnior, C.A.A.; Damaceno, M.A.; Silveira, J.G.F.; Portela, A.C.V.; Campanharo, S.C.; da Silva, A.F.B.; Assane, I.M.; Pilarski, F.; Sasanya, J.J.; et al. Residue Depletion Profile and Estimation of Withdrawal Period for Sulfadimethoxine and Ormetoprim in Edible Tissues of Nile Tilapia (Oreochromis sp.) on Medicated Feed. Animals 2023, 13, 2499. https://doi.org/10.3390/ani13152499
de Freitas LVP, da Mota Júnior CAA, Damaceno MA, Silveira JGF, Portela ACV, Campanharo SC, da Silva AFB, Assane IM, Pilarski F, Sasanya JJ, et al. Residue Depletion Profile and Estimation of Withdrawal Period for Sulfadimethoxine and Ormetoprim in Edible Tissues of Nile Tilapia (Oreochromis sp.) on Medicated Feed. Animals. 2023; 13(15):2499. https://doi.org/10.3390/ani13152499
Chicago/Turabian Stylede Freitas, Lucas Victor Pereira, Carlos Augusto Alvarenga da Mota Júnior, Marina Alves Damaceno, Juliana Grell Fernandes Silveira, Ana Carolina Vellosa Portela, Sarah Chagas Campanharo, Agnaldo Fernando Baldo da Silva, Inácio Mateus Assane, Fabiana Pilarski, James Jacob Sasanya, and et al. 2023. "Residue Depletion Profile and Estimation of Withdrawal Period for Sulfadimethoxine and Ormetoprim in Edible Tissues of Nile Tilapia (Oreochromis sp.) on Medicated Feed" Animals 13, no. 15: 2499. https://doi.org/10.3390/ani13152499
APA Stylede Freitas, L. V. P., da Mota Júnior, C. A. A., Damaceno, M. A., Silveira, J. G. F., Portela, A. C. V., Campanharo, S. C., da Silva, A. F. B., Assane, I. M., Pilarski, F., Sasanya, J. J., & Paschoal, J. A. R. (2023). Residue Depletion Profile and Estimation of Withdrawal Period for Sulfadimethoxine and Ormetoprim in Edible Tissues of Nile Tilapia (Oreochromis sp.) on Medicated Feed. Animals, 13(15), 2499. https://doi.org/10.3390/ani13152499






