Mastitis in Dairy Cattle: On-Farm Diagnostics and Future Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Diagnosis and Control
2.1. Diagnosis
2.2. Control
3. On-Farm Diagnostic Tools
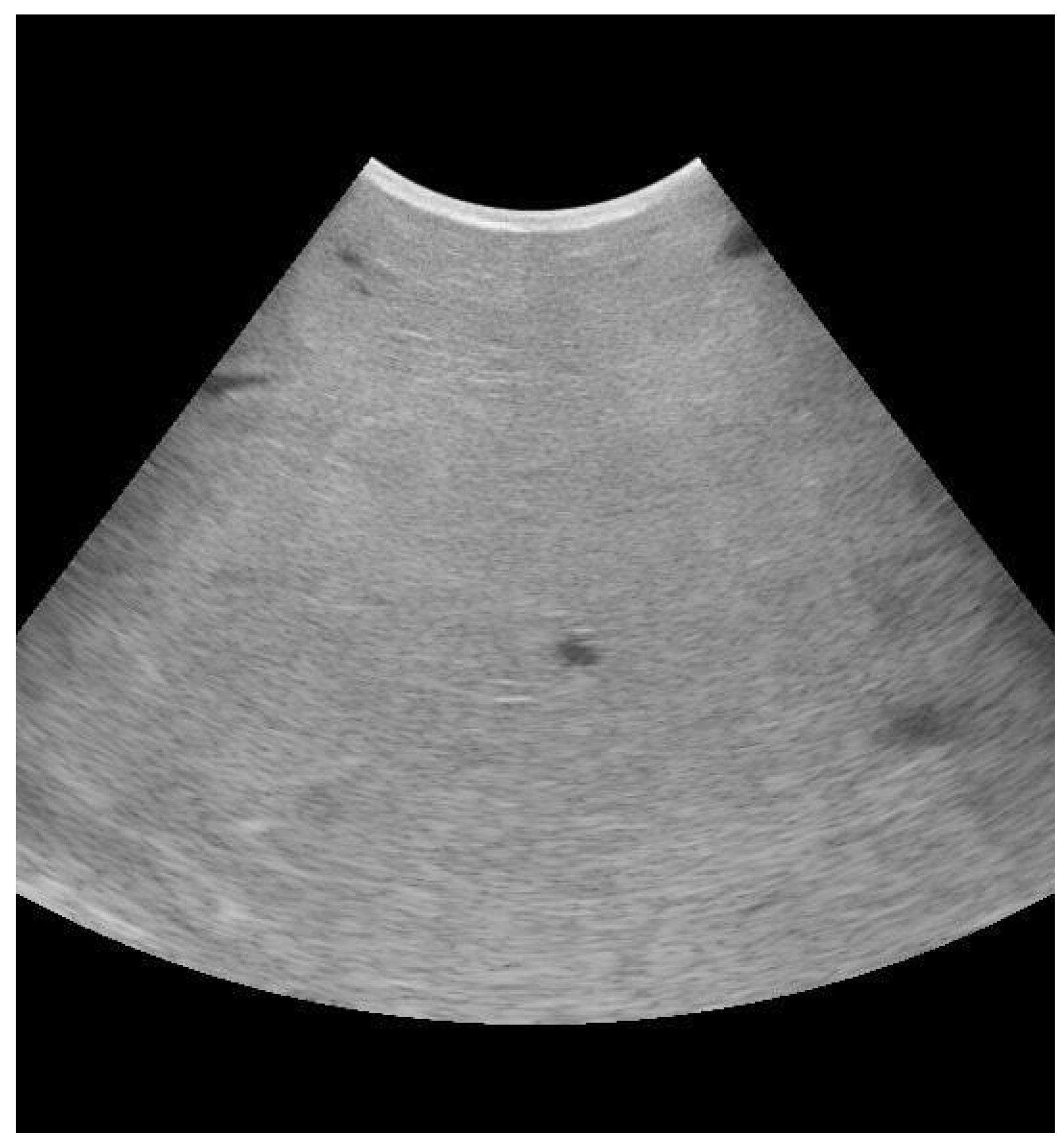
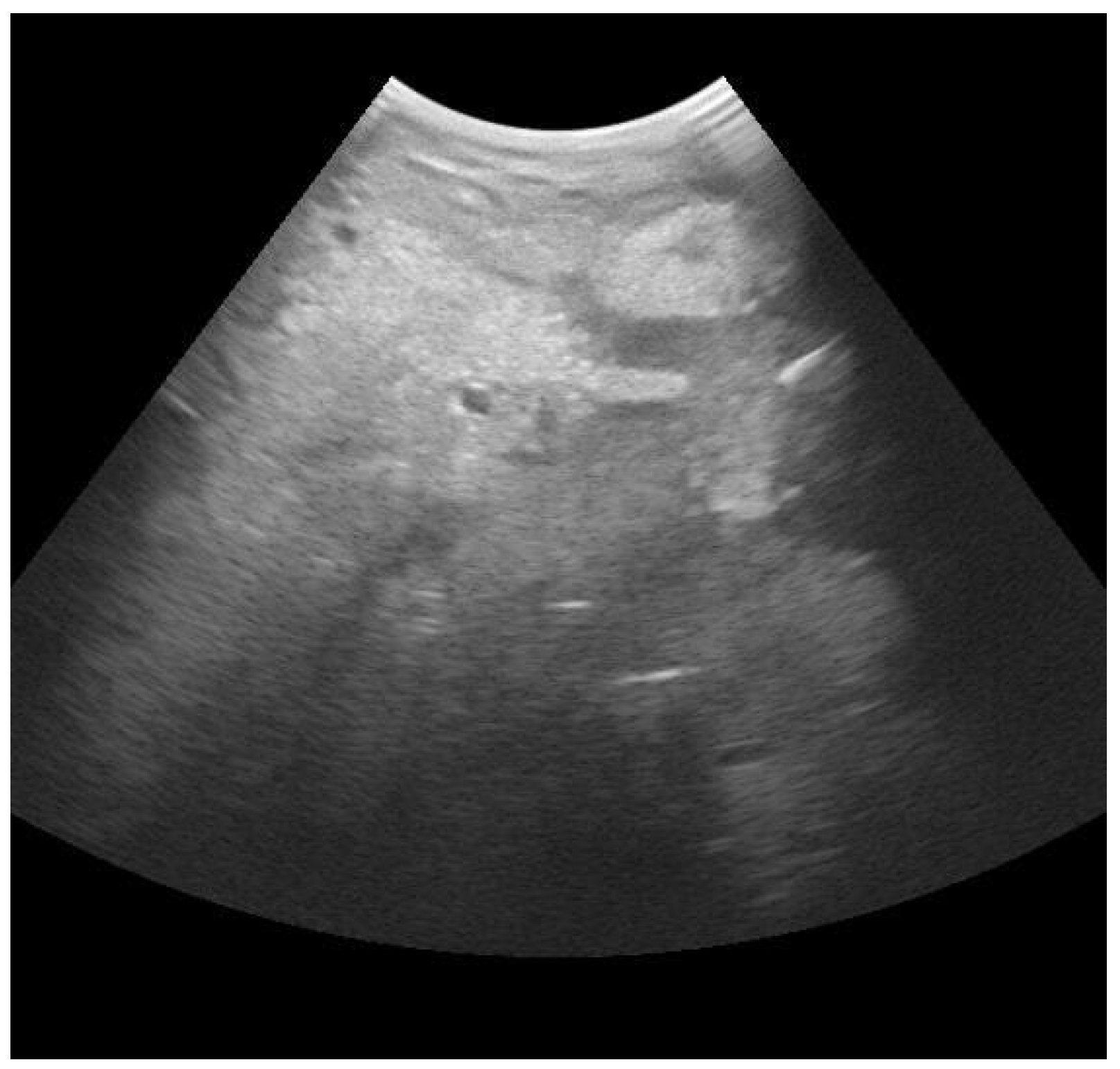
3.1. Mammary Ultrasound
3.2. Blood Gas Analysis
3.3. Electrical Conductivity
3.4. California Mastitis Test
3.5. On-Farm Culture
3.6. Infra-Red Thermography
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamali, H.; Barkema, H.W.; Jacques, M.; Lavallée-Bourget, E.-M.; Malouin, F.; Saini, V.; Stryhn, H.; Dufour, S. Invited Review: Incidence, Risk Factors, and Effects of Clinical Mastitis Recurrence in Dairy Cows. J. Dairy Sci. 2018, 101, 4729–4746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.A.; Shepley, E.; Cue, R.I.; Warner, D.; Dubuc, J.; Vasseur, E. The Hidden Cost of Disease: I. Impact of the First Incidence of Mastitis on Production and Economic Indicators of Primiparous Dairy Cows. J. Dairy Sci. 2021, 104, 7932–7943. [Google Scholar] [CrossRef] [PubMed]
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production Effects Related to Mastitis and Mastitis Economics in Dairy Cattle Herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogeveen, H.; Huijps, K.; Lam, T.J.G.M. Economic Aspects of Mastitis: New Developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Huijps, K.; Lam, T.J.; Hogeveen, H. Costs of Mastitis: Facts and Perception. J. Dairy Res. 2008, 75, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Karns, J.S.; Van Kessel, J.S.; McClusky, B.J.; Perdue, M.L. Incidence of Escherichia Coli O157:H7 and E. Coli Virulence Factors in US Bulk Tank Milk as Determined by Polymerase Chain Reaction. J. Dairy Sci. 2007, 90, 3212–3219. [Google Scholar] [CrossRef] [Green Version]
- Boss, R.; Cosandey, A.; Luini, M.; Artursson, K.; Bardiau, M.; Breitenwieser, F.; Hehenberger, E.; Lam, T.; Mansfeld, M.; Michel, A.; et al. Bovine Staphylococcus Aureus: Subtyping, Evolution, and Zoonotic Transfer. J. Dairy Sci. 2016, 99, 515–528. [Google Scholar] [CrossRef] [Green Version]
- Holko, I.; Tančin, V.; Vršková, M.; Tvarožková, K. Prevalence and Antimicrobial Susceptibility of Udder Pathogens Isolated from Dairy Cows in Slovakia. J. Dairy Res. 2019, 86, 436–439. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. U.S. Code of Federal Regulations, Title 21, Part 54, Title 21–Food and Drugs, Chapter I-Food and Drug Administration, Department of Health and Human Services, Subchapter B-Food for Human Consumption; U.S. Department of Health and Human Services: Silver Spring, MD, USA. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=131 (accessed on 2 August 2023).
- Ruegg, P. Understanding the Changes in Bulk Tank Somatic Cell Count Monitoring; University of Wisconsin: Madison, WI, USA; Available online: https://outagamie.extension.wisc.edu/files/2010/08/Understanding-the-Changes-in-Bulk-Tank-Somatic-Cell-Count-Monitoring.pdf (accessed on 2 August 2023).
- More, S.J.; Clegg, T.A.; Lynch, P.J.; O’Grady, L. The Effect of Somatic Cell Count Data Adjustment and Interpretation, as Outlined in European Union Legislation, on Herd Eligibility to Supply Raw Milk for Processing of Dairy Products. J. Dairy Sci. 2013, 96, 3671–3681. [Google Scholar] [CrossRef] [Green Version]
- European Union. REGOLAMENTO (CE) N. 853/2004 DEL PARLAMENTO EUROPEO DEL CONSIGLIO 29 Aprile 2004 Stabilisce Norme Specifiche in Materia Di Igiene per gli Alimenti Di Origine Animale; European Union: Brussels, Belgium, 2004; Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:139:0055:0205:it:PDF (accessed on 2 August 2023).
- European Union. REGOLAMENTO (CE) N. 854/2004 DEL PARLAMENTO EUROPEO E DEL CONSIGLIO 29 Aprile 2004 Stabilisce Norme Specifiche per l’organizzazione Di Controlli Ufficiali Sui Prodotti Di Origine Animale al Consumo Umano; European Union: Brussels, Belgium, 2004; Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:226:0083:0127:it:PDF#:~:text=854%2F2004%20del%20Parlamento%20europeo%20e%20del%20Consiglio%20 (accessed on 2 August 2023).
- Neculai-Valeanu, A.S.; Ariton, A.M. Udder Health Monitoring for Prevention of Bovine Mastitis and Improvement of Milk Quality. Bioengineering 2022, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Zigo, F.; Vasil’, M.; Ondrašovičová, S.; Výrostková, J.; Bujok, J.; Pecka-Kielb, E. Maintaining Optimal Mammary Gland Health and Prevention of Mastitis. Front. Vet. Sci. 2021, 8, 607311. [Google Scholar] [CrossRef]
- Abdi, R.D.; Gillespie, B.E.; Ivey, S.; Pighetti, G.M.; Almeida, R.A.; Dego, O.K. Antimicrobial Resistance of Major Bacterial Pathogens from Dairy Cows with High Somatic Cell Count and Clinical Mastitis. Animals 2021, 11, 131. [Google Scholar] [CrossRef]
- Bianchi, R.M.; Schwertz, C.I.; de Cecco, B.S.; Panziera, W.; De Lorenzo, C.; Heck, L.C.; Snel, G.G.M.; Lopes, B.C.; da Silva, F.S.; Pavarini, S.P.; et al. Pathological and Microbiological Characterization of Mastitis in Dairy Cows. Trop. Anim. Health Prod. 2019, 51, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.; Green, M. Use and Interpretation of Somatic Cell Count Data in Dairy Cows; Use and Interpretation of Somatic Cell Count Data in Dairy Cows. Practice 2005, 27, 310–315. [Google Scholar] [CrossRef]
- Harmon, R.J. Symposium: Matitis and Genetic Evaluation for Somatic Cell Count. Physiology of Mastitis and Factors Affecting Somatic Cell Counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Kehrli, M.E.; Shuster, D.E. Factors Affecting Milk Somatic Cells and Their Role in Health of the Bovine Mammary Gland 1. J. Dairy Sci. 1994, 77, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kehrli, M.E.; Harp, J.A. Immunity in the Mammary Gland. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 495–516. [Google Scholar] [CrossRef]
- Gruet, P.; Maincent, P.; Berthelot, X.; Kaltsatos, V. Bovine Mastitis and Intramammary Drug Delivery: Review and Perspectives. Adv. Drug Deliv. Rev. 2001, 50, 245–259. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhang, W.; Chen, S.; Wen, X.; Ran, X.; Wang, H.; Zhao, J.; Qi, Y.; Xue, N. Prevalence of Subclinical Mastitis among Dairy Cattle and Associated Risks Factors in China during 2012–2021: A Systematic Review and Meta-Analysis. Res. Vet. Sci. 2022, 148, 65–73. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in Therapeutic and Managemental Approaches of Bovine Mastitis: A Comprehensive Review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Diagnosis of Bovine Mastitis: From Laboratory to Farm. Trop. Anim. Health Prod. 2018, 50, 1193–1202. [Google Scholar] [CrossRef]
- Miekley, B.; Traulsen, I.; Krieter, J. Principal Component Analysis for the Early Detection of Mastitis and Lameness in Dairy Cows. J. Dairy Res. 2013, 80, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Milner, P.; Page, K.L.; Hillerton, J.E. The Effects of Early Antibiotic Treatment Following Diagnosis of Mastitis Detected by a Change in the Electrical Conductivity of Milk. J. Dairy Sci. 1997, 80, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Schukken, Y.; Wilson, D.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.; Gonzalez Moni, R.; Schukken, Y.H.; Wilson, D.J.; Garrison-tikofsky, L.; Gonzalez, R.N. Monitoring Udder Health and Milk Quality Using Somatic Cell Counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickerson, S.C. Control of Heifer Mastitis: Antimicrobial Treatment-An Overview. Vet. Microbiol. 2009, 134, 128–135. [Google Scholar] [CrossRef]
- Chagunda, M.G.; Larsen, T.; Bjerring, M.; Ingvartsen, K.L. L-Lactate Dehydrogenase and N-Acetyl-b-D-Glucosaminidase Activities in Bovine Milk as Indicators of Non-Specific Mastitis. J. Dairy Res. 2006, 73, 431–440. [Google Scholar] [CrossRef]
- Kennedy, B.W.; Sethar, M.S.; Tong, A.K.W.; Moxley, J.E.; Downey, B.R. Environmental Factors Influencing Test-Day Somatic Cell Counts in Holsteins. J. Dairy Sci. 1982, 65, 275–280. [Google Scholar] [CrossRef]
- Berglund, I.; Pettersson, G.; Svennersten-, K.; Sjaunjä, S. Automatic Milking: Effects on Somatic Cell Count and Teat End-Quality. Livest. Prod. Sci. 2002, 78, 115–124. [Google Scholar] [CrossRef]
- Mijić, P.; Gantner, V.; Bobić, T.; Kuterovac, K. Variation of Somatic Cell Count (SCC) of Dairy Cattle in Conditions of Mediterranean Region in Croatia. EAAP Sci. Ser. 2012, 131, 249–254. [Google Scholar] [CrossRef] [Green Version]
- De Haas, Y.; Veerkamp, R.F.; Barkema, H.W.; Gröhn, Y.T.; Schukken, Y.H. Associations Between Pathogen-Specific Cases of Clinical Mastitis and Somatic Cell Count Patterns. J. Dairy Sci. 2004, 87, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Fang, Z.; Mu, T.; Wang, Z.; Ma, Y.; Ma, Y. Application of Metabolomics in Diagnosis of Cow Mastitis: A Review. Front. Vet. Sci. 2021, 8, 747519. [Google Scholar] [CrossRef] [PubMed]
- Sundekilde, U.K.; Poulsen, N.A.; Larsen, L.B.; Bertram, H.C. Nuclear Magnetic Resonance Metabonomics Reveals Strong Association between Milk Metabolites and Somatic Cell Count in Bovine Milk. J. Dairy Sci. 2013, 96, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Loy, J.D.; Clawson, M.L.; Adkins, P.R.F.; Middleton, J.R. Current and Emerging Diagnostic Approaches to Bacterial Diseases of Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2023, 39, 93–114. [Google Scholar] [CrossRef]
- Giagu, A.; Penati, M.; Traini, S.; Dore, S.; Addis, M.F. Milk Proteins as Mastitis Markers in Dairy Ruminants—A Systematic Review. Vet. Res. Commun. 2022, 1, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Adkins, P.R.F.; Middleton, J.R. Methods for Diagnosing Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [Green Version]
- Bhakat, C.; Mohammad, A.; Mandal, D.K.; Mandal, A.; Rai, S.; Chatterjee, A.; Ghosh, M.K.; Dutta, T.K. Readily Usable Strategies to Control Mastitis for Production Augmentation in Dairy Cattle: A Review. Vet. World 2020, 13, 2364–2370. [Google Scholar] [CrossRef]
- Pyörälä, S. New Strategies to Prevent Mastitis. Reprod. Domest. Anim. 2002, 37, 211–216. [Google Scholar] [CrossRef]
- Tiwari, J.; Babra, C.; Kumar Tiwari, H.; Williams, V.; De Wet, S.; Gibson, J.; Paxman, A.; Morgan, E.; Sunagar, R.; Isloor, S.; et al. Trends in Therapeutic and Prevention Strategies for Management of Bovine Mastitis: An Overview. J. Vaccines Vaccin. 2013, 4, 176. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Hossain, M.K.; Paul, S.; Hossain, M.M.; Islam, M.R.; Alam, M.G.S. Bovine Mastitis and Its Therapeutic Strategy Doing Antibiotic Sensitivity Test. Austin J. Vet. Sci. Anim. Husb. 2017, 4, 1030. [Google Scholar]
- Schukken, Y.H.; Deluyker, H.A. Design of Field Trials for the Evaluation of Antibacterial Products for Therapy of Bovine Clinical Mastitis. J. Vet. Pharmacol. Ther. 1995, 18, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Kabera, F.; Roy, J.P.; Afifi, M.; Godden, S.; Stryhn, H.; Sanchez, J.; Dufour, S. Comparing Blanket vs. Selective Dry Cow Treatment Approaches for Elimination and Prevention of Intramammary Infections During the Dry Period: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2021, 8, 688450. [Google Scholar] [CrossRef] [PubMed]
- Natzke, R.P. Elements of Mastitis Control. J. Dairy Sci. 1981, 64, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Keefe, G.; Roy, J.; Stryhn, H.; Dohoo, I.; McKenna, S. Evaluation of Selective Dry Cow Treatment Following On-Farm Culture: Milk Yield and Somatic Cell Count in the Subsequent Lactation. J. Dairy Sci. 2015, 98, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Winder, C.B.; Sargeant, J.M.; Kelton, D.F.; Leblanc, S.J.; Duffield, T.F.; Glanville, J.; Wood, H.; Churchill, K.J.; Dunn, J.; Bergevin, M.D.; et al. Comparative Efficacy of Blanket versus Selective Dry-Cow Therapy: A Systematic Review and Pairwise Meta-Analysis. Anim. Health Res. Rev. 2019, 20, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Hommels, N.M.C.; Ferreira, F.C.; van den Borne, B.H.P.; Hogeveen, H. Antibiotic Use and Potential Economic Impact of Implementing Selective Dry Cow Therapy in Large US Dairies. J. Dairy Sci. 2021, 104, 8931–8946. [Google Scholar] [CrossRef]
- Quintela, L.A.; Barrio, M.; Peña, A.I.; Becerra, J.J.; Cainzos, J.; Herradón, P.G.; Díaz, C. Use of Ultrasound in the Reproductive Management of Dairy Cattle. Reprod. Domest. Anim. 2012, 47, 34–44. [Google Scholar] [CrossRef]
- Hussein, H.A.; Binici, C.; Staufenbiel, R. Comparative Evaluation of Ultrasonography with Clinical Respiratory Score in Diagnosis and Prognosis of Respiratory Diseases in Weaned Dairy Buffalo and Cattle Calves. J. Anim. Sci. Technol. 2018, 60, 29. [Google Scholar] [CrossRef] [Green Version]
- Domecq, J.J.; Skidmore, A.L.; Lloyd, J.W.; Kaneene, J.B. Validation of Body Condition Scores with Ultrasound Measurements of Subcutaneous Fat of Dairy Cows. J. Dairy Sci. 1995, 78, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Fasulkov, I.R. Ultrasonography of the Mammary Gland in Ruminants: A Review. Bulg. J. Vet. Med. 2012, 15, 1–12. [Google Scholar]
- Suzuki, N.; Kurose, T.; Kaneko, S.; Haraguchi, A.; Isobe, N. Outcome Prediction from the First Examination in Clinical Mastitis Using Ultrasonography in Dairy Cows. Anim. Sci. J. 2020, 91, e13452. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya Sunder, H.; Gupta, D.; Singh, R.; Singh, S.; Randhawa, C. Ultrasonographic Changes in Teat and Supramammary Lymph Nodes in Dairy Cows Affected with Clinical Mastitis. Haryana Vet. 2022, 61, 68–71. [Google Scholar]
- Flöck, M.; Winter, P. Diagnostic Ultrasonography in Cattle with Diseases of the Mammary Gland. Vet. J. 2006, 171, 314–321. [Google Scholar] [CrossRef]
- Mourya, A.; Shukla, P.C.; Gupta, D.K.; Sharma, R.K.; Nayak, A.; Tiwari, A.; Singh, B.; Singh, A.P.; Sahi, A.; Jain, A. Ultrasonographic Alteration in Subclinical Mastitis in Cows. J. Entomol. Zool. Stud. 2020, 8, 2058–2063. [Google Scholar]
- Sarvesha, K.; Styanarayana, M.L.; Narayanaswamy, H.D.; Rao, S.; Yathiraj, S.; Isloor, S.; Mukartal, S.Y.; Singh, S.V.; Anuradha, M.E. Haemato-Biochemical Profile and Milk Leukocyte Count in Subclinical and Clinical Mastitis Affected Crossbred Cattle. J. Exp. Biol. Agric. Sci. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Feng, J.; Peng, W.; Hu, Z.; Cai, J.; Liu, J.; Wang, D. Multiple-Vessel-Based Blood Gas Profiles Analysis Revealed the Potential of Blood Oxygen in Mammary Vein as Indicator of Mammary Gland Health Risk of High-Yielding Dairy Cows. Animals 2022, 12, 1484. [Google Scholar] [CrossRef]
- Smith, G.W.; Constable, P.D.; Morin, D.E. Ability of Hematologic and Serum Biochemical Variables to Differentiate Gram-Negative and Gram-Positive Mastitis in Dairy Cows. J. Vet. Intern. Med. 2001, 15, 394–400. [Google Scholar] [CrossRef]
- Rathaur, A.; Bhateshwar, V. Effect of Subclinical Mastitis in Compositional Change in Milk and Blood Parameter of Crossbred Dairy Cow. Int. J. Chem. Stud. 2020, 8, 10–12. [Google Scholar] [CrossRef]
- Sayers, R.G.; Kennedy, A.; Krump, L.; Sayers, G.P.; Kennedy, E. An Observational Study Using Blood Gas Analysis to Assess Neonatal Calf Diarrhea and Subsequent Recovery with a European Commission-Compliant Oral Electrolyte Solution. J. Dairy Sci. 2016, 99, 4647–4655. [Google Scholar] [CrossRef] [Green Version]
- Ider, M.; Naseri, A.; Ok, M.; Uney, K.; Erturk, A.; Durgut, M.K.; Parlak, T.M.; Ismailoglu, N.; Kapar, M.M. Biomarkers in Premature Calves with and without Respiratory Distress Syndrome. J. Vet. Intern. Med. 2021, 35, 2524–2533. [Google Scholar] [CrossRef]
- Bleul, U. Respiratory Distress Syndrome in Calves. Vet. Clin. Food Anim. Pract. 2009, 25, 179–193. [Google Scholar] [CrossRef]
- Pawliński, B.; Gołębiewski, M.; Trela, M.; Witkowska-Piłaszewicz, O. Comparison of Blood Gas Parameters, Ions, and Glucose Concentration in Polish Holstein-Friesian Dairy Cows at Different Milk Production Levels. Sci. Rep. 2023, 13, 1414. [Google Scholar] [CrossRef]
- Qayyum, A.; Khan, J.A.; Hussain, R.; Avais, M.; Ahmad, N.; Khan, M.S. Investigation of Milk and Blood Serum Biochemical Profile as an Indicator of Sub-Clinical Mastitis in Cholistani Cattle. Pak. Vet. J. 2016, 36, 275–279. [Google Scholar]
- Zhang, F.; Nan, X.; Wang, H.; Zhao, Y.; Guo, Y.; Xiong, B. Effects of Propylene Glycol on Negative Energy Balance of Postpartum Dairy Cows. Animals 2020, 10, 1526. [Google Scholar] [CrossRef]
- Gross, J.; Van Dorland, H.A.; Bruckmaier, R.M.; Schwarz, F.J. Performance and Metabolic Profile of Dairy Cows during a Lactational and Deliberately Induced Negative Energy Balance with Subsequent Realimentation. J. Dairy Sci. 2011, 94, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Nielen, M.; Deluyker, H.; Schukken, Y.H.; Brand1, A. Electrical Conductivity of Milk: Measurement, Modifiers, and Meta Analysis of Mastitis Detection Performance. J. Dly. Sci. 1992, 75, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, B.J. Bovine Mastitis: Milk Compositional Changes and Related Diagnostic Tests. J. Dairy Res. 1981, 48, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Norberg, E. Electrical Conductivity of Milk as a Phenotypic and Genetic Indicator of Bovine Mastitis: A Review. Livest. Prod. Sci. 2005, 96, 129–139. [Google Scholar] [CrossRef]
- Lukas, J.M.; Reneau, J.K.; Wallace, R.; Hawkins, D.; Munoz-Zanzi, C. A Novel Method of Analyzing Daily Milk Production and Electrical Conductivity to Predict Disease Onset. J. Dairy Sci. 2009, 92, 5964–5976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janzekovic, M.; Brus, M.; Mursec, B.; Vinis, P.; Stajnko, D.; Cus, F. Mastitis Detection Based on Electric Conductivity of Milk. J. Achiev. Mater. Manuf. Eng. 2009, 34, 39–46. [Google Scholar]
- Fernando, R.S.; Spahr, S.L.; Jaster, E.H. Comparison of Electrical Conductivity of Milk with Other Indirect Methods for Detection of Subclinical Mastitis. J. Dairy Sci. 1985, 68, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.S.; Rindsig, R.B.; Spahr, S.L. Electrical Conductivity of Milk for Detection of Mastitis. J. Dairy Sci. 1982, 65, 659–664. [Google Scholar] [CrossRef]
- Woolford, M.W.; Williamson, J.H.; Henderson, H.V. Changes in Electrical Conductivity and Somatic Cell Count between Milk Fractions from Quarters Subclinically Infected with Particular Mastitis Pathogens. J. Dairy Res. 1998, 65, 187–198. [Google Scholar] [CrossRef]
- Ebrahimie, E.; Ebrahimi, F.; Ebrahimi, M.; Tomlinson, S.; Petrovski, K.R. A Large-Scale Study of Indicators of Sub-Clinical Mastitis in Dairy Cattle by Attribute Weighting Analysis of Milk Composition Features: Highlighting the Predictive Power of Lactose and Electrical Conductivity. J. Dairy Res. 2018, 85, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Swinkels, J.; Leach, K.; Breen, J.; Payne, B.; White, V.; Green, M.; Bradley, A. Randomized Controlled Field Trial Comparing Quarter and Cow Level Selective Dry Cow Treatment Using the California Mastitis Test. J. Dairy Sci. 2021, 104, 9063–9081. [Google Scholar] [CrossRef]
- Tanni, N.S.; Islam, M.S.; Kabir, M.; Parvin, S.; Ehsan, M.A.; Islam, M.T. Evaluation of Sodium Lauryl Sulfate for the Development of Cow-Side Mastitis Screening Test. Vet. World 2021, 14, 2290. [Google Scholar] [CrossRef]
- Marshall, R.T.; Edmondson, J.E.; Steevens, B. Using the California Mastitis Test|MU Extension. Available online: https://extension.missouri.edu/publications/g3653 (accessed on 4 July 2023).
- Hoque, M.N.; Das, Z.C.; Talukder, A.K.; Alam, M.S.; Rahman, A.N.M.A. Different Screening Tests and Milk Somatic Cell Count for the Prevalence of Subclinical Bovine Mastitis in Bangladesh. Trop. Anim. Health Prod. 2015, 47, 79–86. [Google Scholar] [CrossRef]
- Kaşikçi, G.; Çetin, Ö.; Barış Bingöl, E.; Can Gündüz, M. Relations between Electrical Conductivity, Somatic Cell Count, California Mastitis Test and Some Quality Parameters in the Diagnosis of Subclinical Mastitis in Dairy Cows. Turk. J. Vet. Anim. Sci. 2012, 36, 49–55. [Google Scholar] [CrossRef]
- Dingwell, R.T.; Leslie, K.E.; Schukken, Y.H.; Sargeant, J.M.; Timms, L.L. Evaluation of the California Mastitis Test to Detect an Intramammary Infection with a Major Pathogen in Early Lactation Dairy Cows. Can. Vet. J. 2003, 44, 413–416. [Google Scholar] [PubMed]
- Poutrel, B.; Rainard, P. California Mastitis Test Guide of Selective Dry Cow Therapy. J. Dairy Sci. 1981, 64, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Sanford, C.J.; Keefe, G.P.; Sanchez, J.; Dingwell, R.T.; Barkema, H.W.; Leslie, K.E.; Dohoo, I.R. Test Characteristics from Latent-Class Models of the California Mastitis Test. Prev. Vet. Med. 2006, 77, 96–108. [Google Scholar] [CrossRef]
- Bhutto, A.L.; Murray, R.D.; Woldehiwet, Z. California Mastitis Test Scores as Indicators of Subclinical Intra-Mammary Infections at the End of Lactation in Dairy Cows. Res. Vet. Sci. 2012, 92, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Bisinotto, R.S.; Decter, D.H.; Bicalho, R.C. Evaluation of an On-Farm Culture System (Accumast) for Fast Identification of Milk Pathogens Associated with Clinical Mastitis in Dairy Cows. PLoS ONE 2016, 11, e0155314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lago, A.; Godden, S.; Bey, R.; Ruegg, P.; Leslie, K. The Selective Treatment of Clinical Mastitis Based on On-Farm Culture Results: I. Effects on Antibiotic Use, Milk Withholding Time, and Short-Term Clinical and Bacteriological Outcomes. J. Dairy Sci. 2011, 94, 4441–4456. [Google Scholar] [CrossRef] [Green Version]
- Lago, A.; Godden, S.M.; Bey, R.; Ruegg, P.L.; Leslie, K. The Selective Treatment of Clinical Mastitis Based on On-Farm Culture Results: II. Effects on Lactation Performance, Including Clinical Mastitis Recurrence, Somatic Cell Count, Milk Production, and Cow Survival. J. Dairy Sci. 2011, 94, 4457–4467. [Google Scholar] [CrossRef]
- Perry, J.D. A Decade of Development of Chromogenic Culture Media for Clinical Microbiology in an Era of Molecular Diagnostics. Clin. Microbiol. Rev. 2017, 30, 449–479. [Google Scholar] [CrossRef] [Green Version]
- Granja, B.M.; Fidelis, C.E.; Garcia, B.L.N.; dos Santos, M.V. Evaluation of Chromogenic Culture Media for Rapid Identification of Microorganisms Isolated from Cows with Clinical and Subclinical Mastitis. J. Dairy Sci. 2021, 104, 9115–9129. [Google Scholar] [CrossRef]
- Borchardt, S.; Heuwieser, W. Comparison of Immediate Blanket Treatment versus a Delayed Pathogen-Based Treatment Protocol for Clinical Mastitis Using an On-Farm Culture Test at a Commercial German Dairy Farm. Antibiotics 2022, 11, 368. [Google Scholar] [CrossRef]
- Garcia, B.L.N.; Fidelis, C.E.; Freu, G.; Granja, B.d.M.; dos Santos, M.V. Evaluation of Chromogenic Culture Media for Rapid Identification of Gram-Positive Bacteria Causing Mastitis. Front. Vet. Sci. 2021, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzner, M.; Sauter-Louis, C.; Seemueller, A.; Petzl, W.; Klee, W. Infrared Thermography of the Udder Surface of Dairy Cattle: Characteristics, Methods, and Correlation with Rectal Temperature. Vet. J. 2014, 199, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Colak, A.; Polat, B.; Okumus, Z.; Kaya, M.; Yanmaz, L.E.; Hayirli, A. Short Communication: Early Detection of Mastitis Using Infrared Thermography in Dairy Cows. J. Dairy Sci. 2008, 91, 4244–4248. [Google Scholar] [CrossRef] [Green Version]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Jayaprakash, G.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Das, D.N.; Kataktalware, M.A.; Prakash, M.A.; et al. Infrared Thermography: A Potential Noninvasive Tool to Monitor Udder Health Status in Dairy Cows. Vet. World 2016, 9, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Machado, N.A.F.; Da Costa, L.B.S.; Barbosa-Filho, J.A.D.; De Oliveira, K.P.L.; De Sampaio, L.C.; Peixoto, M.S.M.; Damasceno, F.A. Using Infrared Thermography to Detect Subclinical Mastitis in Dairy Cows in Compost Barn Systems. J. Therm. Biol. 2021, 97, 102881. [Google Scholar] [CrossRef]
- Khakimov, A.R.; Pavkin, D.Y.; Yurochka, S.S.; Astashev, M.E.; Dovlatov, I.M. Development of an Algorithm for Rapid Herd Evaluation and Predicting Milk Yield of Mastitis Cows Based on Infrared Thermography. Appl. Sci. 2022, 12, 6621. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommasoni, C.; Fiore, E.; Lisuzzo, A.; Gianesella, M. Mastitis in Dairy Cattle: On-Farm Diagnostics and Future Perspectives. Animals 2023, 13, 2538. https://doi.org/10.3390/ani13152538
Tommasoni C, Fiore E, Lisuzzo A, Gianesella M. Mastitis in Dairy Cattle: On-Farm Diagnostics and Future Perspectives. Animals. 2023; 13(15):2538. https://doi.org/10.3390/ani13152538
Chicago/Turabian StyleTommasoni, Chiara, Enrico Fiore, Anastasia Lisuzzo, and Matteo Gianesella. 2023. "Mastitis in Dairy Cattle: On-Farm Diagnostics and Future Perspectives" Animals 13, no. 15: 2538. https://doi.org/10.3390/ani13152538
APA StyleTommasoni, C., Fiore, E., Lisuzzo, A., & Gianesella, M. (2023). Mastitis in Dairy Cattle: On-Farm Diagnostics and Future Perspectives. Animals, 13(15), 2538. https://doi.org/10.3390/ani13152538




