Vitamin D Alleviates Cadmium-Induced Inhibition of Chicken Bone Marrow Stromal Cells’ Osteogenic Differentiation In Vitro
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. SPF- Grade Eggs
2.2. Reagents
2.3. Cell Culture and Treatment
2.4. Real-Time Cell Analyzer (RTCA) Dynamic Monitoring of BMSCs
2.5. ALP Staining and Alizarin Red Staining
2.6. Flow Cytometry Assay
2.7. qRT-PCR
2.8. Immunoblotting
2.9. Immunofluorescence
2.10. Statistical Analysis
3. Results
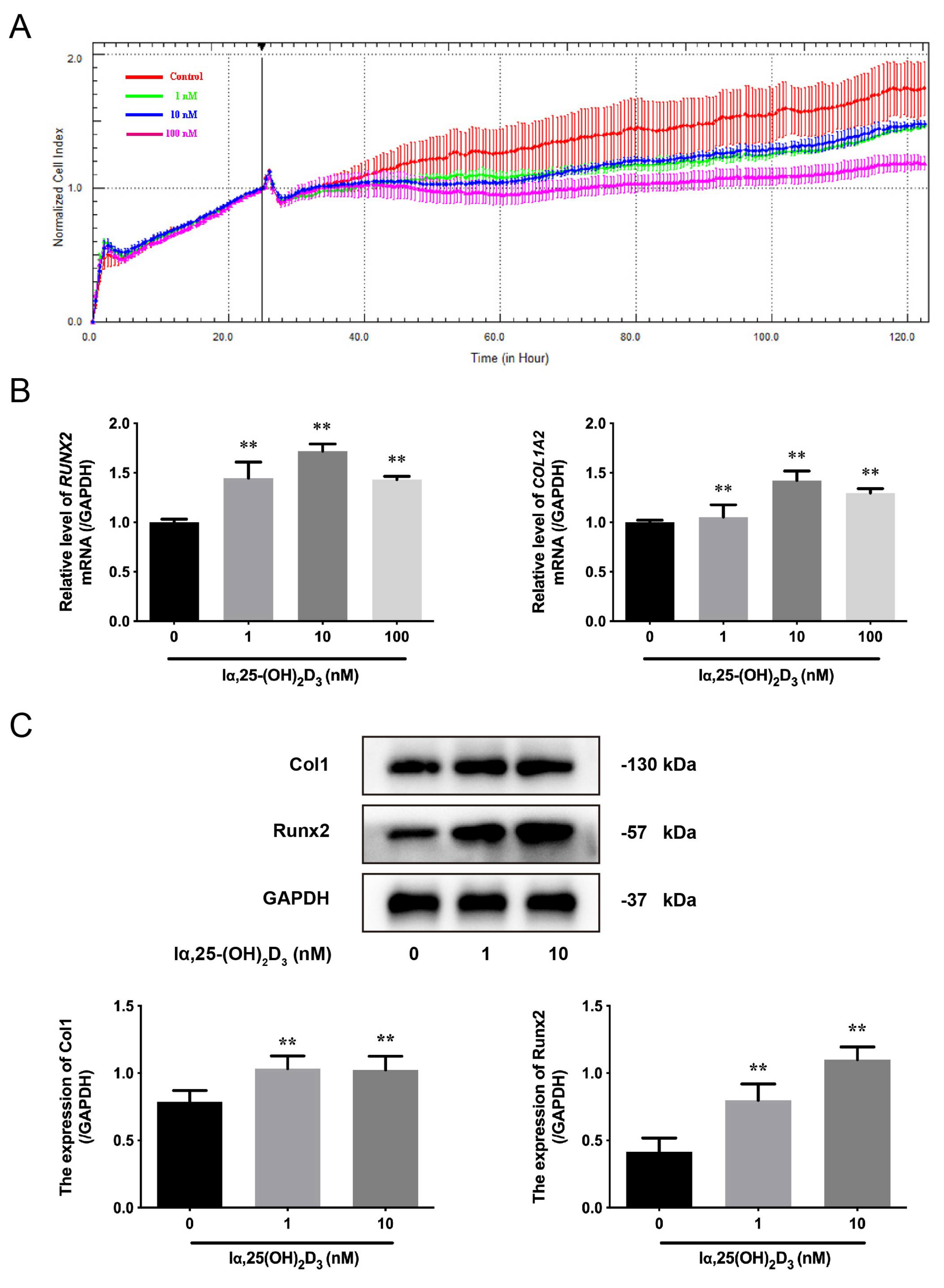
3.1. The Effects of lα, 25-(OH)2D3 on Osteogenic Differentiation of BMSCs from Chickens
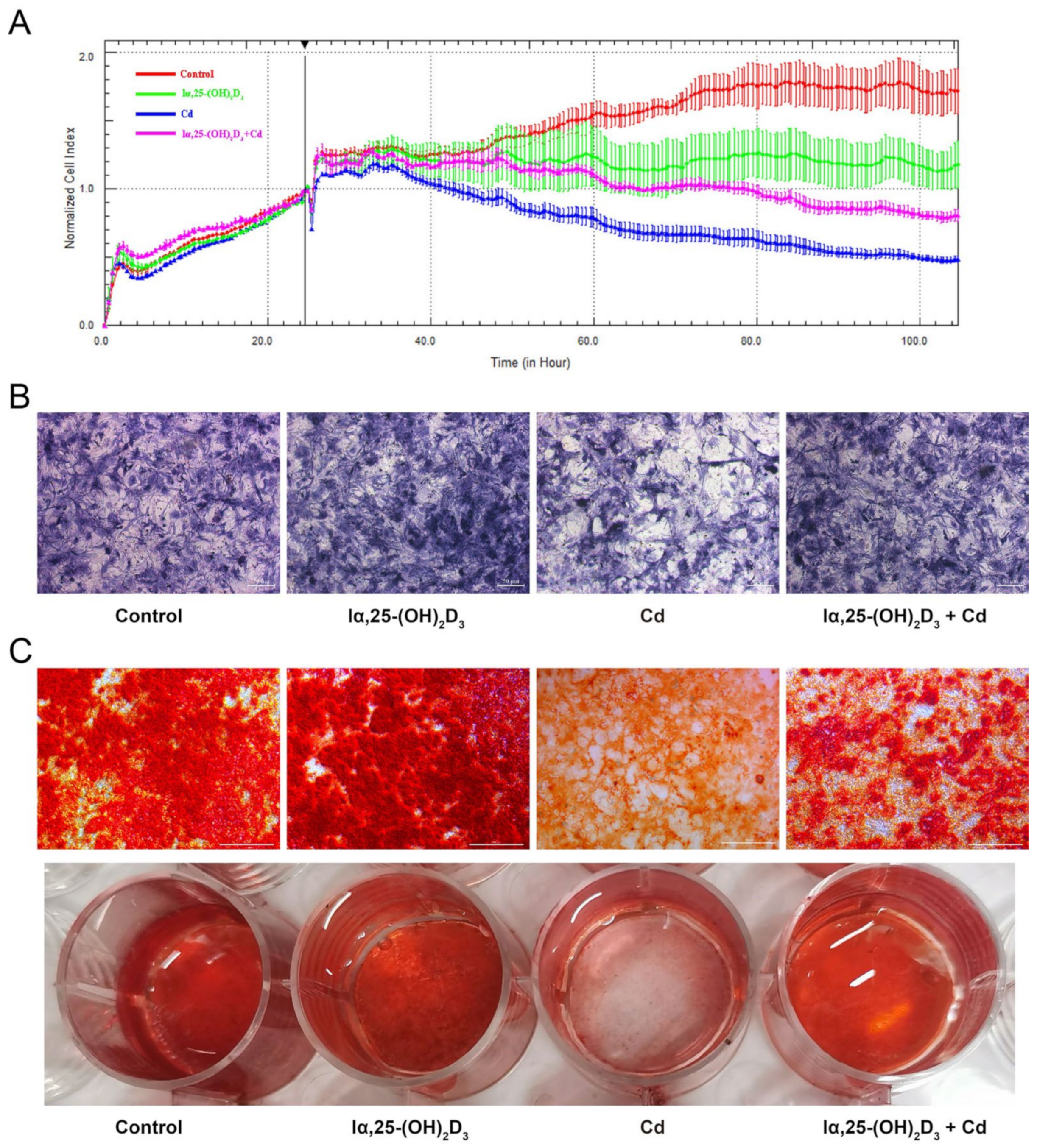
3.2. The Effects of lα, 25-(OH)2D3 on Cd-Inhibited Osteogenic Differentiation of BMSCs from Chickens
3.3. The Distribution of Runx2 during lα, 25-(OH)2D3 on Cd-Induced Osteogenic Differentiation of BMSCs from Chickens
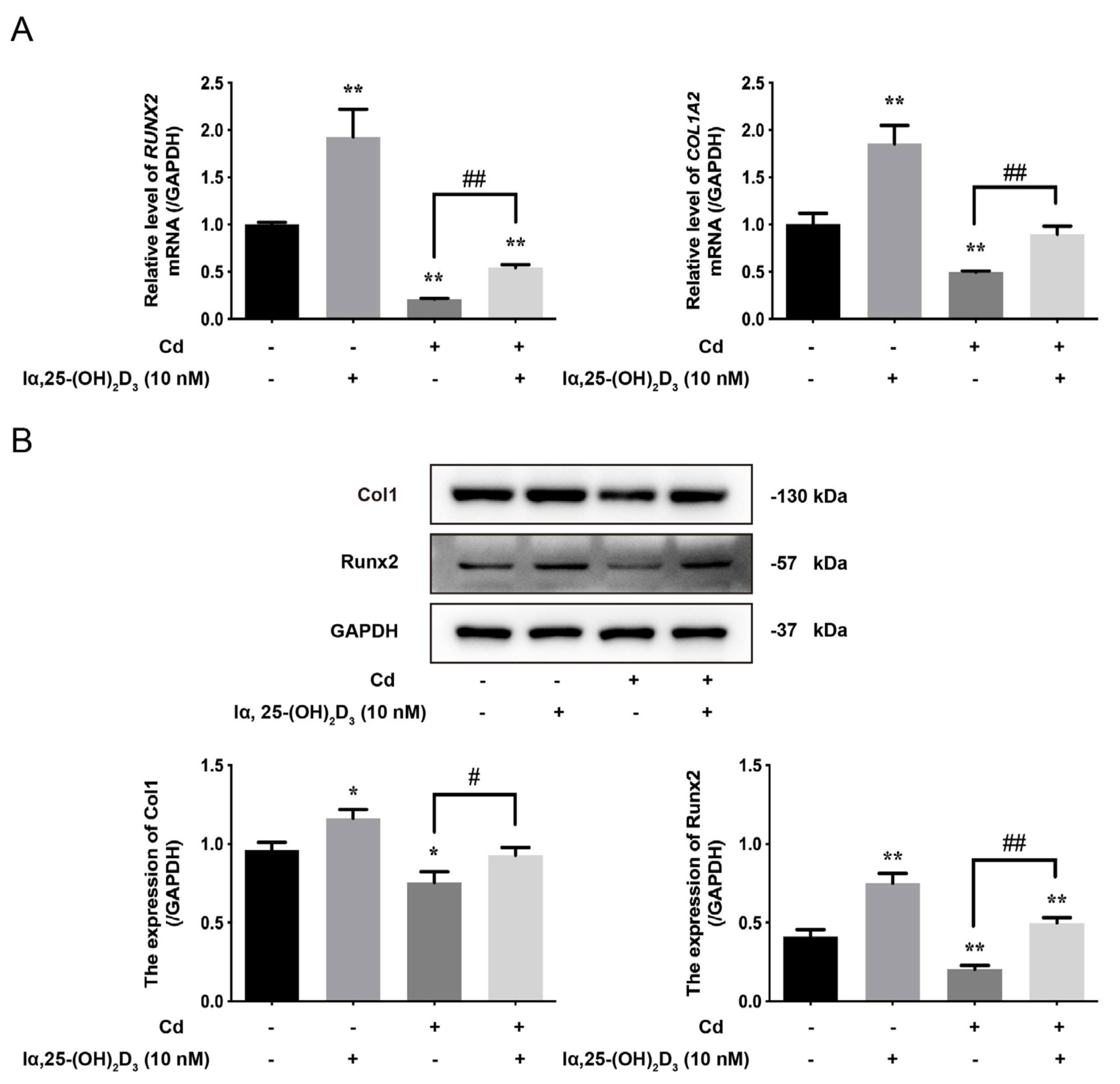
3.4. The Effects of lα, 25-(OH)2D3 on Markers of Cd-Inhibited Osteogenic Differentiation of BMSCs from Chickens
3.5. The Effects of lα, 25-(OH)2D3 on Mitochondrial Membrane Potential and Apoptosis in Cd-Inhibited Osteogenic Differentiation of BMSCs from Chickens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yu, Z.; Fu, L.; Wang, H.; Chen, X.; Zhang, C.; Lv, Z.M.; Xu, D.X. Vitamin D3 inhibits lipopolysaccharide-induced placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Sci. Rep. 2015, 5, 10871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babazadeh, D.; Razavi, S.A.; Abd El-Ghany, W.A.; Cotter, P.F. Vitamin D Deficiency in Farm Animals: A Review. Farm Anim. Health Nutr. 2022, 1, 10–16. [Google Scholar] [CrossRef]
- Warren, M.F.; Livingston, K.A. Implications of Vitamin D Research in Chickens can Advance Human Nutrition and Perspectives for the Future. Curr. Dev. Nutr. 2021, 5, nzab018. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C.; McCormack, H.A.; McTeir, L.; Fleming, R.H. High vitamin D3 requirements in broilers for bone quality and prevention of tibial dyschondroplasia and interactions with dietary calcium, available phosphorus and vitamin A. Br. Poult. Sci. 2004, 45, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Fritts, C.A.; Waldroup, P.W. Effect of Source and Level of Vitamin D on Live Performance and Bone Development in Growing Broilers1. J. Appl. Poult. Res. 2003, 12, 45–52. [Google Scholar] [CrossRef]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef]
- van Driel, M.; Koedam, M.; Buurman, C.J.; Hewison, M.; Chiba, H.; Uitterlinden, A.G.; Pols, H.A.; van Leeuwen, J.P. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006, 20, 2417–2419. [Google Scholar] [CrossRef]
- Holick, M.F. McCollum Award Lecture, 1994: Vitamin D—New horizons for the 21st century. Am. J. Clin. Nutr. 1994, 60, 619–630. [Google Scholar] [CrossRef]
- Harahap, I.A.; Landrier, J.F.; Suliburska, J. Interrelationship between Vitamin D and Calcium in Obesity and Its Comorbid Conditions. Nutrients 2022, 14, 3187. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Interaction between vitamin D and calcium. Scand. J. Clin. Lab. Investig. 2012, 243, 60–64. [Google Scholar]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Roles of Runx2 in Skeletal Development. Adv. Exp. Med. Biol. 2017, 962, 83–93. [Google Scholar]
- Javed, A.; Chen, H.; Ghori, F.Y. Genetic and transcriptional control of bone formation. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.M., Jr. Perspectives on RUNX genes: An update. Am. J. Med. Genet. A 2009, 149A, 2629–2646. [Google Scholar] [CrossRef]
- Curtis, K.M.; Aenlle, K.K.; Roos, B.A.; Howard, G.A. 24R,25-dihydroxyvitamin D3 promotes the osteoblastic differentiation of human mesenchymal stem cells. Mol. Endocrinol. 2014, 28, 644–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromigué, O.; Marie, P.J.; Lomri, A. Differential effects of transforming growth factor beta2, dexamethasone and 1,25-dihydroxyvitamin D on human bone marrow stromal cells. Cytokine 1997, 9, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, P.; Xing, Y.; Jia, L.; Zhang, Y.; Jia, T.; Wu, X.; Zhao, B.; Xu, X. Effect of 1α, 25-dihydroxyvitamin D3 on the osteogenic differentiation of human periodontal ligament stem cells and the underlying regulatory mechanism. Int. J. Mol. Med. 2019, 43, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Zhu, N.; Wang, Y.; Cheng, G. 1,25(OH)2D3 inhibits osteogenic differentiation through activating β-catenin signaling via downregulating bone morphogenetic protein 2. Mol. Med. Rep. 2020, 22, 5023–5032. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. High dose 1,25(OH)2D3 inhibits osteoblast mineralization in vitro. Int. J. Mol. Med. 2012, 29, 934–938. [Google Scholar] [PubMed] [Green Version]
- Wang, J.W.; Zhu, L.; Shi, P.Z.; Wang, P.C.; Dai, Y.; Wang, Y.X.; Lu, X.H.; Cheng, X.F.; Feng, X.M.; Zhang, L. 1,25(OH)2D3 Mitigates Oxidative Stress-Induced Damage to Nucleus Pulposus-Derived Mesenchymal Stem Cells through PI3K/Akt Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 1427110. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Zhao, H.; Song, R.; Zou, H.; Gu, J.; Yuan, Y.; Bian, J.; Zhu, J.; Liu, Z. Cadmium exposure triggers osteoporosis in duck via P2X7/PI3K/AKT-mediated osteoblast and osteoclast differentiation. Sci. Total Environ. 2021, 750, 141638. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- McGrath, S.P. Keeping toxic cadmium out of the food chain. Nat. Food 2022, 3, 569–570. [Google Scholar] [CrossRef]
- Chunhabundit, R. Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Zhu, X.; Shan, D.; Huang, X.; Xu, Q. Metabolomics in liver injury induced by dietary cadmium exposure and protective effect of calcium supplementation. Anal. Biochem. 2022, 641, 114556. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mandalunis, P.M. Effect of cadmium on bone tissue in growing animals. Exp. Toxicol. Pathol. 2016, 68, 391–397. [Google Scholar] [CrossRef]
- Ohta, H.; Ichikawa, M.; Seki, Y. Effects of cadmium intake on bone metabolism of mothers during pregnancy and lactation. Tohoku J. Exp. Med. 2002, 196, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Ou, L.; Wang, H.; Wu, Z.; Wang, P.; Yang, L.; Li, X.; Sun, K.; Zhu, X.; Zhang, R. Effects of cadmium on osteoblast cell line: Exportin 1 accumulation, p-JNK activation, DNA damage and cell apoptosis. Ecotoxicol. Environ. Saf. 2021, 208, 111668. [Google Scholar] [CrossRef]
- Liu, W.; Dai, N.; Wang, Y.; Xu, C.; Zhao, H.; Xia, P.; Gu, J.; Liu, X.; Bian, J.; Yuan, Y.; et al. Role of autophagy in cadmium-induced apoptosis of primary rat osteoblasts. Sci. Rep. 2016, 6, 20404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Li, S.; Wang, G.; Zhang, X.; Yuan, Y.; Liu, X.; Bian, J.; Tong, X.; Liu, Z. Cadmium Toxicity on Chondrocytes and the Palliative Effects of 1α, 25-Dihydroxy Vitamin D3 in White Leghorns Chicken’s Embryo. Front. Vet. Sci. 2021, 8, 637369. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Gu, J.; Song, R.; Wang, D.; Sun, Z.; Sui, C.; Zhang, C.; Liu, X.; Bian, J.; Liu, Z. Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J. Cell. Biochem. 2019, 120, 1630–1642. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Zou, H.; Dai, N.; Yao, L.; Zhang, X.; Gao, Q.; Liu, W.; Gu, J.; Yuan, Y.; et al. Osteoprotegerin disrupts peripheral adhesive structures of osteoclasts by modulating Pyk2 and Src activities. Cell Adhes. Migr. 2016, 10, 299–309. [Google Scholar] [CrossRef]
- Tong, X.; Yu, G.; Liu, Q.; Zhang, X.; Bian, J.; Liu, Z.; Gu, J. Puerarin alleviates cadmium-induced oxidative damage to bone by reducing autophagy in rats. Environ. Toxicol. 2022, 37, 720–729. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, C.; Souberbielle, J.C. Vitamin D and multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2017, 14, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Huang, B.; Yang, H.; Li, G.; Zhang, C.; Yang, G.; Lin, F.; Lin, G. TGF-β1 is Involved in Vitamin D-Induced Chondrogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells by Regulating the ERK/JNK Pathway. Cell. Physiol. Biochem. 2017, 42, 2230–2241. [Google Scholar] [CrossRef]
- Piek, E.; Sleumer, L.S.; van Someren, E.P.; Heuver, L.; de Haan, J.R.; de Grijs, I.; Gilissen, C.; Hendriks, J.M.; van Ravestein-van Os, R.I.; Bauerschmidt, S.; et al. Osteo-transcriptomics of human mesenchymal stem cells: Accelerated gene expression and osteoblast differentiation induced by vitamin D reveals c-MYC as an enhancer of BMP2-induced osteogenesis. Bone 2010, 46, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Song, Y.M.; Baek, S.; Park, Y.H.; Park, J.B. Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells. Medicina 2021, 57, 1271. [Google Scholar] [CrossRef]
- Kim, H.S.; Zheng, M.; Kim, D.K.; Lee, W.P.; Yu, S.J.; Kim, B.O. Effects of 1,25-dihydroxyvitamin D3 on the differentiation of MC3T3-E1 osteoblast-like cells. J. Periodontal Implant Sci. 2018, 48, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnahash, A.Z.; Song, Y.-M.; Min, S.-K.; Lee, H.-J.; Kim, M.-J.; Park, Y.-H.; Park, J.-U.; Park, J.-B. Effects of Connective Tissue Growth Factor on the Cell Viability, Proliferation, Osteogenic Capacity and mRNA Expression of Stem Cell Spheroids. Appl. Sci. 2021, 11, 6572. [Google Scholar] [CrossRef]
- Min, S.K.; Kim, M.; Park, J.B. Insulin-like growth factor 2-enhanced osteogenic differentiation of stem cell spheroids by regulation of Runx2 and Col1 expression. Exp. Ther. Med. 2021, 21, 383. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, H.; Wang, Y. Effects of miR-103 by negatively regulating SATB2 on proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells. PLoS ONE 2020, 15, e0232695. [Google Scholar] [CrossRef]
- Payr, S.; Rosado-Balmayor, E.; Tiefenboeck, T.; Schuseil, T.; Unger, M.; Seeliger, C.; van Griensven, M. Direct comparison of 3D and 2D cultivation reveals higher osteogenic capacity of elderly osteoblasts in 3D. J. Orthop. Surg. Res. 2021, 16, 13. [Google Scholar] [CrossRef]
- Besio, R.; Chow, C.-W.; Tonelli, F.; Marini, J.C.; Forlino, A. Bone biology: Insights from osteogenesis imperfecta and related rare fragility syndromes. FEBS J. 2019, 286, 3033–3056. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Rim, Y.A.; Park, N.; Nam, Y.; Ju, J.H. Restoration of Osteogenesis by CRISPR/Cas9 Genome Editing of the Mutated COL1A1 Gene in Osteogenesis Imperfecta. J. Clin. Med. 2021, 10, 3141. [Google Scholar] [CrossRef]
- Augusciak-Duma, A.; Witecka, J.; Sieron, A.L.; Janeczko, M.; Pietrzyk, J.J.; Ochman, K.; Galicka, A.; Borszewska-Kornacka, M.K.; Pilch, J.; Jakubowska-Pietkiewicz, E. Mutations in the COL1A1 and COL1A2 genes associated with osteogenesis imperfecta (OI) types I or III. Acta Biochim. Pol. 2018, 65, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Delaney, J.; Franceschetti, T.; Bilic-Curcic, I.; Kalinovsky, J.; Lorenzo, J.A.; Grcevic, D.; Rowe, D.W.; Kalajzic, I. Immature osteoblast lineage cells increase osteoclastogenesis in osteogenesis imperfecta murine. Am. J. Pathol. 2010, 176, 2405–2413. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Whitfield, G.K.; Hsieh, J.C.; Thompson, P.D.; Barthel, T.K.; Bartik, L.; Egan, J.B.; Wu, Y.; Kubicek, J.L.; et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J. Steroid Biochem. Mol. Biol. 2010, 121, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Wei, Q.; Lv, Y.; Xue, J.; Zhang, B.; Sun, Q.; Xiao, T.; Huang, R.; Wang, P.; Dai, X.; et al. Wnt/β-Catenin Pathway Is Involved in Cadmium-Induced Inhibition of Osteoblast Differentiation of Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 2,4,5-Trimethoxyldalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/β-catenin pathway. Cell Death Dis. 2015, 6, e1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Fu, B.; Tian, S.; Liu, C.; Li, Q.; Liu, J. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021, 263, 128346. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Li, S.; Baiyun, R.; Lv, Z.; Li, J.; Han, D.; Zhao, W.; Yu, L.; Deng, N.; Liu, Z.; Zhang, Z. Exploring the kidney hazard of exposure to mercuric chloride in mice:Disorder of mitochondrial dynamics induces oxidative stress and results in apoptosis. Chemosphere 2019, 234, 822–829. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yan, J.; Zou, X.-L.; Guo, K.-J.; Zhao, Y.; Meng, C.-Y.; Yin, F.; Guo, L. Bone marrow mesenchymal stem cells repair cadmium-induced rat testis injury by inhibiting mitochondrial apoptosis. Chem. Biol. Interact. 2017, 271, 39–47. [Google Scholar] [CrossRef]
- Yin, F.; Meng, C.; Lu, R.; Li, L.; Zhang, Y.; Chen, H.; Qin, Y.; Guo, L. Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy. Neural Regen. Res. 2014, 9, 1665–1671. [Google Scholar]
- Gonzalo, S. Novel roles of 1α,25(OH)2D3 on DNA repair provide new strategies for breast cancer treatment. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 59–64. [Google Scholar] [CrossRef] [Green Version]



| Gene Name | Primer Sequence (5’–3’) | Gene Number | Length (bp) |
|---|---|---|---|
| RUNX2 | Forward: AACCCAAACTTGCCCAACCA | NM_204128.2 | 114 |
| Reverse: AGTACGGCCTCCAAACGGA | |||
| COL1A2 | Forward: ATGGTCCTAGAGGTCTGCGT | NM_001079714.2 | 109 |
| Reverse: AGCCTCTCCAGATGGACCTT | |||
| GAPDH | Forward: GGGTGGTGCTAAGCGTGTTA | NM_204305.2 | 180 |
| Reverse: ACCCTCCACAATGCCAAAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Zhang, Y.; Zhao, Y.; Li, Y.; Li, T.; Zou, H.; Yuan, Y.; Bian, J.; Liu, Z.; Gu, J. Vitamin D Alleviates Cadmium-Induced Inhibition of Chicken Bone Marrow Stromal Cells’ Osteogenic Differentiation In Vitro. Animals 2023, 13, 2544. https://doi.org/10.3390/ani13152544
Tong X, Zhang Y, Zhao Y, Li Y, Li T, Zou H, Yuan Y, Bian J, Liu Z, Gu J. Vitamin D Alleviates Cadmium-Induced Inhibition of Chicken Bone Marrow Stromal Cells’ Osteogenic Differentiation In Vitro. Animals. 2023; 13(15):2544. https://doi.org/10.3390/ani13152544
Chicago/Turabian StyleTong, Xishuai, Ying Zhang, Yutian Zhao, Yawen Li, Tan Li, Hui Zou, Yan Yuan, Jianchun Bian, Zongping Liu, and Jianhong Gu. 2023. "Vitamin D Alleviates Cadmium-Induced Inhibition of Chicken Bone Marrow Stromal Cells’ Osteogenic Differentiation In Vitro" Animals 13, no. 15: 2544. https://doi.org/10.3390/ani13152544
APA StyleTong, X., Zhang, Y., Zhao, Y., Li, Y., Li, T., Zou, H., Yuan, Y., Bian, J., Liu, Z., & Gu, J. (2023). Vitamin D Alleviates Cadmium-Induced Inhibition of Chicken Bone Marrow Stromal Cells’ Osteogenic Differentiation In Vitro. Animals, 13(15), 2544. https://doi.org/10.3390/ani13152544








