Dietary High Glycinin Reduces Growth Performance and Impairs Liver and Intestinal Health Status of Orange-Spotted Grouper (Epinephelus coioides)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Feeding Trial
2.3. Sample Collection
2.4. Proximate Composition Determination
2.5. Determination of Intestinal Permeability and Liver Antioxidant Capacity
2.6. Histological Analysis
2.7. TUNEL Assay
2.8. Intestinal Microbial Diversity
2.9. Determination of Gene Expression
2.10. Statistical Analysis
3. Results
3.1. Growth Performance and Whole-Body Proximate Composition
3.2. Liver Antioxidant Capacity
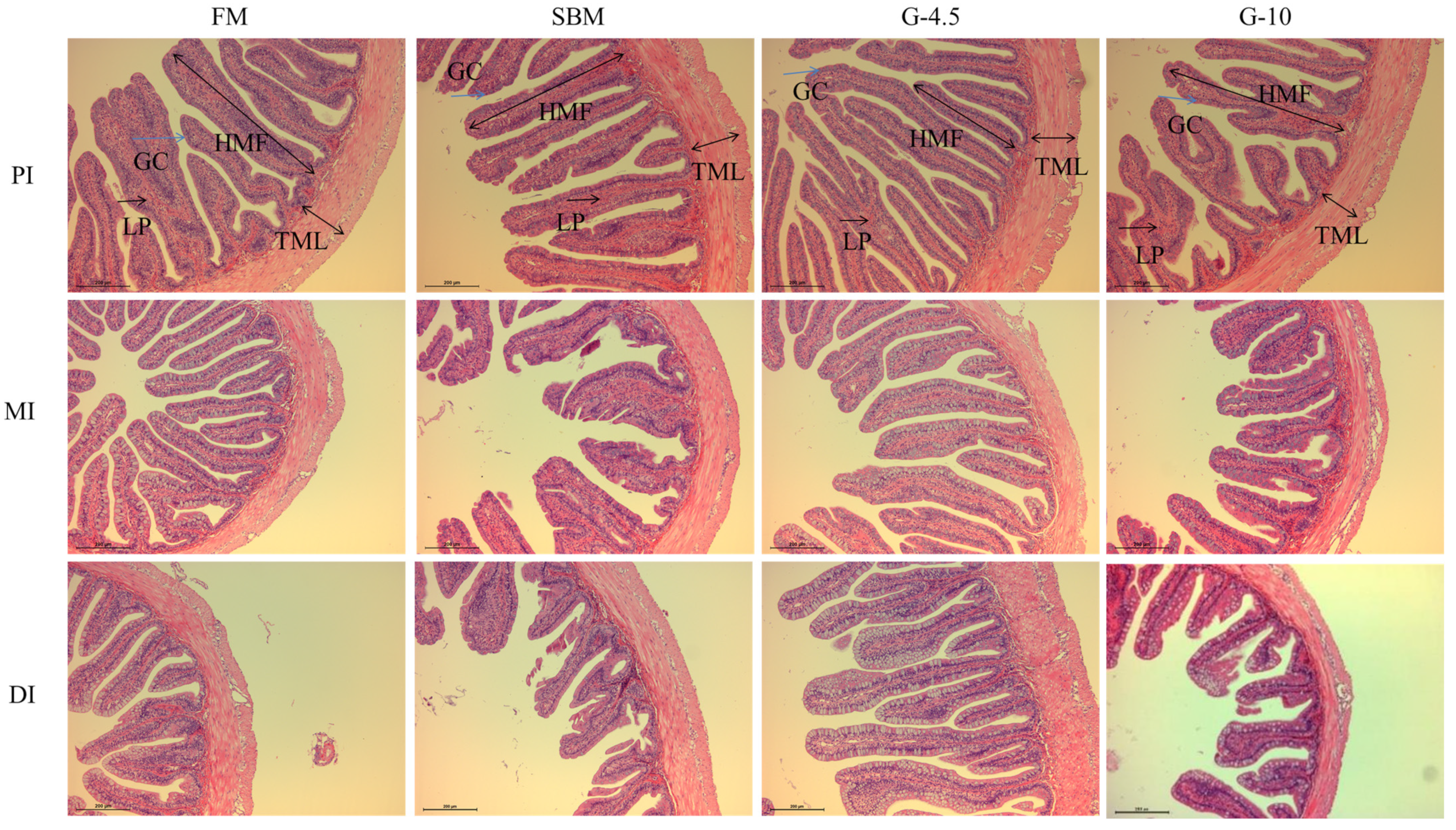
3.3. Intestinal Histological Observation
3.4. Gene Expression Related to Intestinal Tight Junction Function
3.5. Intestinal Mucosal Permeability
3.6. Intestinal Cell Apoptosis
3.7. Expression of Intestinal Inflammatory Factor Genes
3.8. Intestinal Microbial Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Tacon, A.G.J. Trends in Global Aquaculture and Aquafeed Production: 2000–2017. Rev. Fish Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- Yedier, S.; Gümüs, E.; Livengood, E.J.; Chapman, F.A. The relationship between carotenoid type and skin color in the ornamental red zebra cichlid Maylandia estherae. AACL Bioflux. 2014, 7, 207–216. [Google Scholar]
- Zhao, X.; Wang, Y.; Wang, X.; Ye, J. Growth Performance, Plasma Components, and Intestinal Barrier in Grouper (Epinephelus coioides) are Altered by Dietary Fish Meal Replacement with Extruded Soybean Meal. Aquac. Rep. 2021, 21, 100863. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W.; Duan, X.; Feng, L.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.; Tang, L.; Zhou, X. Soybean Glycinin Caused NADPH-Oxidase-Regulated ROS Overproduction and Decreased ROS Elimination Capacity in the Mid and Distal Intestine of Juvenile Grass Carp (Ctenopharyngodon idella). Aquaculture 2020, 516, 734651. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, S.; Cleveland, B.M.; Romano, N.; Lalgudi, R.S.; Benito, M.R.; McGraw, B.; Hardy, R.W. Comparative Evaluation of Processed Soybean Meal (EnzoMealTM) vs. Regular Soybean Meal as a Fishmeal Replacement in Diets of Rainbow Trout (Oncorhynchus mykiss): Effects on Growth Performance and Growth-Related Genes. Aquaculture 2020, 516, 734652. [Google Scholar] [CrossRef]
- Ke, L.; Qin, Y.; Song, T.; Wang, K.; Ye, J. Dietary Sodium Butyrate Administration Alleviates High Soybean Meal-Induced Growth Retardation and Enteritis of Orange-Spotted Groupers (Epinephelus coioides). Front. Mar. Sci. 2022, 9, 1029397. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Ma, Q.; Zhang, B. Dietary Gamma-Aminobutyric Acid Ameliorates Growth Impairment and Intestinal Dysfunction in Turbot (Scophthalmus maximus L.) Fed a High Soybean Meal Diet. Food Funct. 2022, 13, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chang, K.; Chen, J.; Zhao, X.; Gao, S. Dietary Sodium Butyrate Supplementation Attenuates Intestinal Inflammatory Response and Improves Gut Microbiota Composition in Largemouth Bass (Micropterus salmoides) Fed with a High Soybean Meal Diet. Fish Physiol. Biochem. 2021, 47, 1805–1819. [Google Scholar] [CrossRef]
- He, Y.; Ye, G.; Chi, S.; Tan, B.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S. Integrative Transcriptomic and Small RNA Sequencing Reveals Immune-Related miRNAemRNA Regulation Network for Soybean Meal-Induced Enteritis in Hybrid Grouper, Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂. Front. Immunol. 2020, 11, 1502. [Google Scholar] [CrossRef]
- Fuentes-Quesada, J.P.; Viana, M.T.; Rombenso, A.N.; Guerrero-Rentería, Y.; Nomura-Solís, M.; Gomez-Calle, V.; Lazo, J.P.; Mata-Sotres, J.A. Enteritis Induction by Soybean Meal in Totoaba macdonaldi Diets: Effects on Growth Performance, Digestive Capacity, Immune Response and Distal Intestine Integrity. Aquaculture 2018, 495, 78–89. [Google Scholar] [CrossRef]
- Gu, M.; Bai, N.; Zhang, Y.; Krogdahl, Å. Soybean Meal Induces Enteritis in Turbot Scophthalmus maximus at High Supplementation Levels. Aquaculture 2016, 464, 286–295. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Dai, J.; Yang, P.; Xu, W.; Ai, Q.; Zhang, W.; Zhang, Y.; Zhang, Y.; Mai, K. Sodium Butyrate Supplementation in High-Soybean Meal Diets for Turbot (Scophthalmus maximus L.): Effects on Inflammatory Status, Mucosal Barriers and Microbiota in the Intestine. Fish Shellfish Immun. 2019, 88, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Wang, W.; Shao, R.; Liang, S.; Xu, W.; Li, M.; Ai, Q.; Mai, K.; Wan, M. The Effects of Sodium Propionate Supplementation in the Diet with High Soybean Meal on Growth Performance, Intestinal Health, and Immune Resistance to Bacterial Infection in Turbot (Scophthalmus maximus L.). Aquac. Nutr. 2022, 2022, 8952755. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Bai, N.; Xu, W.; Zhou, H.; Zhang, W.; Mai, K. Effects of Dietary β-Conglycinin and Glycinin on Digestive Enzymes Activities, Intestinal Histology and Immune Responses of Juvenile Turbot Scophthalmus maximus. Aquac. Res. 2016, 47, 1001–1008. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important Antinutrients in Plant Feedstuffs for Aquaculture: An Update on Recent Findings Regarding Responses in Salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Wu, J.J.; Cao, C.M.; Ren, D.D.; Zhang, Y.; Kou, Y.N.; Ma, L.Y.; Feng, S.B.; Li, Y.; Wang, X.C. Effects of Soybean Antigen Proteins on Intestinal Permeability, 5-Hydroxytryptamine Levels and Secretory IgA Distribution in the Intestine of Weaned Piglets. Ital. J. Anim. Sci. 2016, 15, 174–180. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhang, Y.; Dong, J.H.; Cao, C.M.; Li, B.; Feng, S.B.; Ding, H.Y.; Ma, L.Y.; Wang, X.C.; Li, Y. Allergens and Intestinal Damage Induced by Soybean Antigen Proteins in Weaned Piglets. Ital. J. Anim. Sci. 2016, 15, 437–445. [Google Scholar] [CrossRef]
- Soumeh, E.A.; Mohebodini, H.; Toghyani, M.; Shabani, A.; Ashayerizadeh, A.; Jazi, V. Synergistic Effects of Fermented Soybean Meal and Mannan-Oligosaccharide on Growth Performance, Digestive Functions, and Hepatic Gene Expression in Broiler Chickens. Poult. Sci. 2019, 98, 6797–6807. [Google Scholar] [CrossRef]
- Hu, J.; Yuan, L.; An, G.; Zhang, J.; Zhao, X.; Liu, Y.; Shan, J.; Wang, Z. Antigenic Activity and Epitope Analysis of β-Conglycinin Hydrolyzed by Pepsin. J. Sci. Food Agr. 2021, 101, 1396–1402. [Google Scholar] [CrossRef]
- Jiang, W.D.; Hu, K.; Zhang, J.X.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Soyabean Glycinin Depresses Intestinal Growth and function in Juvenile Jian Carp (Cyprinus carpiovar Jian): Protective Effects of Glutamine. Br. J. Nutr. 2015, 114, 1569–1583. [Google Scholar] [CrossRef]
- He, L.; Han, M.; Qiao, S.; He, P.; Li, D.; Li, N.; Ma, X. Soybean Antigen Proteins and their Intestinal Sensitization Activities. Curr. Protein Pept. Sci. 2015, 16, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, H.; Liu, J.; Yang, P.; Zhang, Y.; Ai, Q.; Xu, W.; Zhang, W.; Mai, K. Dietary Soya Allergen β-Conglycinin Induces Intestinal Inflammatory Reactions, Serum-Specific Antibody Response and Growth Reduction in a Carnivorous Fish Species, Turbot Scophthalmus maximus L. Aquac. Res. 2017, 48, 4022–4037. [Google Scholar] [CrossRef]
- Shan, D.; Yu, H.; Lyu, B.; Fu, H. Soybean β-Conglycinin: Structure Characteristic, Allergenicity, Plasma Lipid-Controlling, Prevention of Obesity and Non-alcoholic Fatty Liver Disease. Curr. Protein Pept. Sci. 2021, 22, 831–847. [Google Scholar] [CrossRef]
- Yin, B.; Liu, H.; Tan, B.; Dong, X.; Chi, S.; Yang, Q.; Zhang, S. MHC II-PI(3)K/Akt/mTOR Signaling Pathway Regulates Intestinal Immune Response Induced by Soy Glycinin in Hybrid Grouper: Protective Effects of Sodium Butyrate. Front. Immunol. 2020, 11, 615980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X.; Feng, L.; Jiang, W.; Wu, P.; Liu, Y.; Kuang, S.; Tang, L.; Zhou, X. Soybean Glycinin Disrupted Intestinal Structural Integrity Related to Aggravation of Apoptosis and Downregulated Transcription of Tight Junction Proteins in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella). Aquaculture 2021, 531, 735909. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Zhu, R.; Yu, Z.; Wang, J.; Duan, J.; Wang, T.; Wu, L. Effects of β-Conglycinin on Growth Performance, Antioxidant Capacity and Intestinal Health in Juvenile Golden Crucian Carp, Carassius auratus. Aquac. Res. 2019, 50, 3231–3241. [Google Scholar] [CrossRef]
- Peng, C.; Ding, X.; Zhu, L.; He, M.; Shu, Y.; Zhang, Y.; Li, Y.; Wang, X.; Feng, S.; Li, J.; et al. β-Conglycinin-Induced Intestinal Porcine Epithelial Cell Damage Via the Nuclear Factor kappaB/Mitogen-Activated Protein Kinase Signaling Pathway. J. Agr. Food Chem. 2019, 67, 9009–9021. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, R.; Li, M.; Yu, Z.; Wang, H.; Quan, Y.; Wu, L. Effects of β-Conglycinin on Growth Performance, Antioxidant Capacity and Immunity in Rhynchocypris lagowskii Dybowski. Aquac. Nutr. 2020, 26, 2059–2073. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Z.; Xie, W.; Peng, C.; Ding, H.; Li, Y.; Feng, S.; Wang, X.; Zhao, C.; Wu, J. 11S Glycinin Up-Regulated NLRP-3-Induced Pyroptosis by Triggering Reactive Oxygen Species in Porcine Intestinal Epithelial Cells. Front. Vet. Sci. 2022, 9, 890978. [Google Scholar] [CrossRef]
- Duan, X.D.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q. Dietary Soybean β-Conglycinin Suppresses Growth Performance and Inconsistently Triggers Apoptosis in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella) in Association with ROS-Mediated MAPK Signalling. Aquac. Nutr. 2019, 25, 770–782. [Google Scholar] [CrossRef]
- Peng, C.; Cao, C.; He, M.; Shu, Y.; Tang, X.; Wang, Y.; Zhang, Y.; Xia, X.; Li, Y.; Wu, J. Soybean Glycinin- and β-Conglycinin-Induced Intestinal Damage in Piglets via the p38/JNK/NF-κB Signaling Pathway. J. Agr. Food Chem. 2018, 66, 9534–9541. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yuan, X.; Luo, H.; Shao, J.; Chen, X. High Percentage of Dietary Soybean Meal Inhibited Growth, Impaired Intestine Healthy and Induced Inflammation by TLR-MAPK/NF-κB Signaling Pathway in Large Yellow Croaker (Larimichthys crocea). Aquac. Rep. 2021, 20, 100735. [Google Scholar] [CrossRef]
- Yi, L.; Liu, J.; Yang, H.; Mo, A.; Zhai, Y.; Wang, S.; Yuan, Y. Effects of Dietary Glycinin on Oxidative Damage, Apoptosis and Tight Junction in the Intestine of Juvenile Hybrid Yellow Catfish, Pelteobagrus fulvidraco ♀ × Pelteobaggrus vachelli ♂. Int. J. Mol. Sci. 2022, 23, 11198. [Google Scholar] [CrossRef] [PubMed]
- Yedier, S.; Yalçınkaya, S.K.; Bostancı, D. Exposure to Polypropylene Microplastics via Diet and Water Induces Oxidative Stress in Cyprinus carpio. Aquat. Toxicol. 2023, 259, 106540. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, H.; Guo, M.; Fang, D.; Mei, J.; Xie, J. Analysis of Acute Nitrite Exposure on Physiological Stress Response, Oxidative Stress, Gill Tissue Morphology and Immune Response of Large Yellow Croaker (Larimichthys crocea). Animal 2022, 12, 1791. [Google Scholar] [CrossRef]
- Shapawi, R.; Ching, F.F.; Senoo, S.; Mustafa, S. Nutrition, Growth and Resilience of Tiger Grouper (Epinephelus fuscoguttatus) × Giant Grouper (Epinephelus lanceolatus) Hybrid-A Review. Rev. Aquac. 2019, 11, 1285–1296. [Google Scholar] [CrossRef]
- National Bureau of Statistics. China Fishery Statistics Yearbook. Bureau of Fisheries; China Agriculture Press: Beijing, China, 2022. [Google Scholar]
- Wang, Y.; Wang, L.; Zhang, C.; Song, K. Effects of Substituting Fishmeal with Soybean Meal on Growth Performance and Intestinal Morphology in Orange-Spotted Grouper (Epinephelus coioides). Aquac. Rep. 2017, 5, 52–57. [Google Scholar] [CrossRef]
- He, Y.; Liang, J.; Dong, X.; Liu, H.; Yang, Q.; Zhang, S.; Chi, S.; Tan, B. Soybean β-Conglycinin and Glycinin Reduced Growth Performance and the Intestinal Immune Defense and Altered Microbiome in Juvenile Pearl Gentian Groupers Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂. Anim. Nutr. 2022, 9, 193–203. [Google Scholar] [CrossRef]
- Qin, Y.; He, L.; Wang, Y.; Li, D.; Chen, W.; Ye, J. Growth Performance, Fatty Acid Composition, and Lipid Metabolism are Altered in Groupers (Epinephelus coioides) by Dietary Fish Oil Replacement with Palm Oil. Anim. Nutr. 2022, 8, 102–113. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, H.; Ling, Z.; Ye, J. Microbial Communities Associated with Early Stages of Intensively Reared Orange-Spotted Grouper (Epinephelus coioides). Aquac. Res. 2015, 46, 131–140. [Google Scholar] [CrossRef]
- Anguiano, M.; Pohlenz, C.; Buentello, A.; Gatlin, D.R. The Effects of Prebiotics on the Digestive Enzymes and Gut Histomorphology of Red Drum (Sciaenops ocellatus) and Hybrid Striped Bass (Morone chrysops × M. saxatilis). Br. J. Nutr. 2013, 109, 623–629. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2016. [Google Scholar]
- Niu, X.; Qian, X.; Feng, H.; Yi, K.; Li, D.; Chen, W.; Ye, J. Growth and Metabolic Responses of Grouper Juveniles (Epinephelus coioides) Fed Diets Containing Varying Levels of Leucine. Aquaculture 2021, 534, 736281. [Google Scholar] [CrossRef]
- Song, T.; Qin, Y.; Ke, L.; Wang, X.; Wang, K.; Sun, Y.; Ye, J. Dietary Lactoferrin Supplementation Improves Growth Performance and Intestinal Health of Juvenile Orange-Spotted Groupers (Epinephelus coioides). Metabolites 2022, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, K.; Ike, F.; Kajita, A.; Yasuno, W.; Yanagiba, M.; Goto, M.; Sakai, K.; Ami, Y.; Kyuwa, S. A Broadly Reactive One-Step SYBR Green I Real-Time RT-PCR Assay for Rapid Detection of Murine Norovirus. PLoS ONE 2014, 9, e98108. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Jahan, H.; Tumpa, I.J.; Qasem, W.A.; Moniruzzaman, M.; Pervin, M.A.; Akter, R.; Omri, A.; Min, T.; Hossain, Z. Evaluation of the Partial Replacement of Dietary Fish Meal with Fermented or Untreated Soybean Meal in Juvenile Silver Barb, Barbonymus gonionotus. Front. Nutr. 2021, 8, 733402. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, X.; Wang, Z.; Wang, K. Effect of Partial Fish Meal Replacement by Soybean Meal on the Growth Performance and Biochemical Indices of Juvenile Japanese Flounder Paralichthys olivaceus. Aquac. Int. 2011, 19, 143–153. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Duan, X.D.; Jiang, W.D.; Feng, L.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Soybean Glycinin Decreased Growth Performance, Impaired Intestinal Health, and Amino Acid Absorption Capacity of Juvenile Grass Carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2019, 45, 1589–1602. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Kong, Y.D.; Zhu, R.; Yu, Z.; Wang, J.Y.; Duan, J.; Wu, L.F. Effects of Glycinin on Growth Performance, Immunity and Antioxidant Capacity in Juvenile Golden Crucian Carp, Cyprinus carpio × Carassius auratus. Aquac. Res. 2020, 51, 465–479. [Google Scholar] [CrossRef]
- Zhu, R.; Li, L.; Li, M.; Yu, Z.; Wang, H.; Quan, Y.; Wu, L. Effects of Dietary Glycinin on the Growth Performance, Immunity, Hepatopancreas and Intestinal Health of Juvenile Rhynchocypris lagowskii Dybowski. Aquaculture 2021, 544, 737030. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Bakke-McKellep, A.M.; Baeverfjord, G. Effects of Graded Levels of Standard Soybean Meal on Intestinal Structure, Mucosal Enzyme Activities, and Pancreatic Response in Atlantic Salmon (Salmo salar L.). Aquac. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Xu, X.; Huang, J.; Ocansey, D.; Xia, Y.; Zhao, Z.; Xu, Z.; Yan, Y.; Zhang, X.; Mao, F. The Emerging Clinical Application of m6A RNA Modification in Inflammatory Bowel Disease and Its Associated Colorectal Cancer. J. Inflamm. Res. 2021, 14, 3289–3306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wu, X.Q.; Zhao, X.Y.; Qu, Z.H.; Quan, Y.N.; Lu, M.H.; Liu, Z.Y.; Wu, L.F. Taurine can Improve Intestinal Function and Integrity in Juvenile Rhynchocypris lagowskii Dybowski Fed High-Dose Glycinin. Fish Shellfish Immun. 2022, 129, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, Z.; Lu, M.; Wu, X.; Zhao, X.; Wang, H.; Quan, Y.; Wu, L. The Protective Role of Vitamin C on Intestinal Damage Induced by High-Dose Glycinin in Juvenile Rhynchocypris lagowskii Dybowski. Fish Shellfish Immun. 2023, 134, 108589. [Google Scholar] [CrossRef] [PubMed]
- Leber, B.; Lin, J.; Andrews, D.W. Embedded Together: The Life and Death Consequences of Interaction of the Bcl-2 Family with Membranes. Apoptosis 2007, 12, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Heissmeyer, V.; Krappmann, D.; Hatada, E.N.; Scheidereit, C. Shared Pathways of IκB Kinase-Induced SCF(βTrCP)-Mediated Ubiquitination and Degradation for the NF-κB Precursor p105 and IκBα. Mol. Cell. Biol. 2001, 21, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- McKenney, P.T.; Pamer, E.G. From Hype to Hope: The Gut Microbiota in Enteric Infectious Disease. Cell 2015, 163, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Bereded, N.K.; Abebe, G.B.; Fanta, S.W.; Curto, M.; Waidbacher, H.; Meimberg, H.; Domig, K.J. The Gut Bacterial Microbiome of Nile Tilapia (Oreochromis niloticus) from Lakes across an Altitudinal Gradient. BMC Microbiol. 2022, 22, 87. [Google Scholar] [CrossRef]
- Ren, X.; Ma, H.; Liu, X.; Wu, Y. Effects of Taurine Supplementation on Growth, Feed Utilization, Antioxidant Capacity, and Intestinal Microflora of Largemouth Bass Fed a Low Fish Meal Diet. N. Am. J. Aquac. 2022, 84, 285–294. [Google Scholar] [CrossRef]
- Lin, T.L.; Shu, C.C.; Chen, Y.M.; Lu, J.J.; Wu, T.S.; Lai, W.F.; Tzeng, C.M.; Lai, H.C.; Lu, C.C. Like Cures Like: Pharmacological Activity of Anti-Inflammatory Lipopolysaccharides from Gut Microbiome. Front. Pharmacol. 2020, 11, 554. [Google Scholar] [CrossRef]
- Hakoupian, M.; Ferino, E.; Jickling, G.C.; Amini, H.; Stamova, B.; Ander, B.P.; Alomar, N.; Sharp, F.R.; Zhan, X. Bacterial Lipopolysaccharide is Associated with Stroke. Sci. Rep. 2021, 11, 6570. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, H.; Huang, W. Efficacy of Bifidobacterium Triple Viable Enteric-Coated Capsules Combined with Enteral Nutrition on Patients with Chronic Critical Illness and Influence on Immune and Coagulation Function. Evid. Based Compl. Alt. 2021, 2021, 3718255. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Whon, T.W.; Bae, J. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Pandey, K.; Nautiyal, S. Achromobacter: An Emerging Nosocomial Pathogen. Int. J. Med. Sci. 2019, 7, 3090–3094. [Google Scholar] [CrossRef]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio Anguillarum as a Fish Pathogen: Virulence Factors, Diagnosis and Prevention. J. Fish Dis. 2011, 9, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane Protects Cells against Lipopolysaccharide-Stimulated Inflammation in Murine Macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB Signaling in Inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Mihaly, S.R.; Ninomiya-Tsuji, J.; Morioka, S. TAK1 Control of Cell Death. Cell Death Differ. 2014, 21, 1667–1676. [Google Scholar] [CrossRef]
- Wu, C.; Deng, H.; Li, D.; Fan, L.; Yao, D.; Zhi, X.; Mao, H.; Hu, C. Ctenopharyngodon Idella Tollip Regulates MyD88-Induced NF-κB Activation. Dev. Comp. Immunol. 2021, 123, 104162. [Google Scholar] [CrossRef]
- Oh, H.; Ghosh, S. NF-κB: Roles and Regulation in Different CD4(+) T-Cell Subsets. Immunol. Rev. 2013, 252, 41–51. [Google Scholar]
- Chang, M.; Jin, W.; Chang, J.H.; Xiao, Y.; Brittain, G.C.; Yu, J.; Zhou, X.; Wang, Y.H.; Cheng, X.; Li, P.; et al. The Ubiquitin Ligase Peli1 Negatively Regulates T Cell Activation and Prevents Autoimmunity. Nat. Immunol. 2011, 12, 1002–1009. [Google Scholar] [CrossRef]







| Items | Diets 1 | |||
|---|---|---|---|---|
| FM | SBM | G-4.5 | G-10 | |
| Ingredients | ||||
| Fish meal 2 | 520 | 220 | 520 | 520 |
| Casein | 109.2 | 105.1 | 72.3 | 27.2 |
| Gelatin | 27.3 | 26.3 | 18.1 | 6.8 |
| Soybean meal | 0 | 470 | 0 | 0 |
| Fish oil | 8.2 | 35.2 | 8.2 | 8.2 |
| Soybean oil | 35 | 35 | 35 | 35 |
| Soybean lecithin | 20 | 20 | 20 | 20 |
| Glycinin | 0 | 0 | 45 | 100 |
| Corn starch | 245.1 | 54.2 | 247.2 | 248.6 |
| Vitamin mix 3 | 4 | 4 | 4 | 4 |
| Mineral mix 4 | 5 | 5 | 5 | 5 |
| Stay-C 35% | 0.2 | 0.2 | 0.2 | 0.2 |
| Sodium alginate | 10 | 10 | 10 | 10 |
| Ca(H2PO4)2 | 15 | 15 | 15 | 15 |
| Nutrient level of diets (analyzed values) | ||||
| Dry matter | 917.3 | 912.5 | 919.1 | 921.2 |
| Crude protein | 479.3 | 481.2 | 482.7 | 479..1 |
| Crude lipid | 117.7 | 115.9 | 113.4 | 114.7 |
| Ash | 155 | 101.4 | 138.6 | 138.9 |
| Gross energy (kJ/g) | 19.27 | 20.06 | 19.53 | 19.61 |
| Genes | Forward (5′-3′) | Reverse (5′-3′) | Amplicon Size (bp) | E-Value (%) | Accession No. |
|---|---|---|---|---|---|
| caspase-3 | GATGCTGCTGCTGCTATGC | GCCGTCAGTGCCGTATATTATC | 189 | 99 | XM_033633485 |
| caspase8 | TGCCTTGGTGGTATGCGTGCT | GGTGAAGGGCGAGGTCAGTTCT | 100 | 98 | XM_033649666.1 |
| caspase9 | GCCTGTGGAGGAGGTGAAAGAGA | GCTGCTGGATGACATCGGAATGG | 114 | 103 | XM_033629367.1 |
| bcl-2 | GTGCGTGGAGTGCGTTGAGAA | CGCTCCCATCCTCTTTGGCTCT | 120 | 99 | KY321170.1 |
| bcl-xL | AGTAACGGCTTGCTGGTCAA | GCTGTGGTAGGCTGTGTCA | 192 | 98 | MH513638.1 |
| occludin | GGCTACGGTGATCGTGTTGTGT | CCGCCTCCATAACCTCCTCCAT | 174 | 101 | XM_033622283.1 |
| claudin3 | GCATTGACGACGAGGCATCCAA | GCCGACCAGGAGACAGGAATGA | 100 | 106 | MK782153.1 |
| ZO-1 | CGGCAGATCAGCAATGGCAACC | TGGTTCAGGCAGCGGAGGTAAC | 165 | 95 | MK809396.1 |
| NF-κB1 (P50) | CTTACATTCGCCGCCTCAGT | TGCAACAACGCCTTCAAACC | 158 | 103 | JX856139.1 |
| RelA (P65) | TCTTCTCAGTCCAGCCCAAGGT | GGTGGTAGAGGAGCAGGAGGAT | 167 | 96 | EU219847.1 |
| MyD88 | GCATTGACGACGAGGCATCCAA | GCCGACCAGGAGACAGGAATGA | 100 | 98 | JF271883 |
| IKK-α | TGGCTGAGAGCGAACAAGTCCT | AGCAGAGGCGGCACTGAAGAT | 151 | 99 | KM669150.1 |
| TAK1 | TCTCAAGGGAGCAACGACAC | GCAGGCAGACTCTCAACACT | 122 | 104 | JX856141 |
| IL-8 | AAGTTTGCCTTGACCCCGAA | TGAAGCAGATCTCTCCCGGT | 101 | 94 | FJ913064.1 |
| IL-1β | GCAACTCCACCGACTGATGA | ACCAGGCTGTTATTGACCCG | 107 | 116 | EF582837.1 |
| TNF-a | GGATCTGGCGCTACTCAGAC | CGCCCAGATAAATGGCGTTG | 135 | 91 | FJ009049.1 |
| IL-10 | GTCCACCAGCATGACTCCTC | AGGGAAACCCTCCACGAATC | 124 | 99 | KJ741852.1 |
| TGF-β1 | GCTTACGTGGGTGCAAACAG | ACCATCTCTAGGTCCAGCGT | 112 | 102 | GQ503351.1 |
| β-actin | GATCTGGCATCACACCTTCT | CATCTTCTCCCTGTTGGCTT | 104 | AY510710.2 |
| Items | Diets 2 | |||
|---|---|---|---|---|
| FM | SBM | G-4.5 | G-10 | |
| Growth performance | ||||
| IBW (g/fish) 3 | 8.32 ± 0.13 | 8.19 ± 0.26 | 8.27 ± 0.09 | 7.94 ± 0.23 |
| FBW (g/fish) 3 | 61.52 ± 2.43 c | 43.91 ± 1.11 a | 63.96 ± 0.65 c | 57.50 ± 0.55 b |
| WG (%) 3 | 639.3 ± 18.0 bc | 436.9 ± 15.3 a | 673.9 ± 9.0 c | 625.4 ± 14.6 b |
| SGR (%/d) 3 | 3.57 ± 0.04 bc | 3.00 ± 0.05 a | 3.66 ± 0.02 c | 3.54 ± 0.04 b |
| FE (%) 3 | 110 ± 3 | 107 ± 5 | 115 ± 3 | 105 ± 2 |
| FR (%/d) | 2.24 ± 0.03 ab | 2.14 ± 0.04 a | 2.21 ± 0.04 ab | 2.19 ± 0.03 ab |
| Survival (%) 3 | 100 | 100 | 100 | 100 |
| HSI (%) 4 | 3.02 ± 0.13 b | 2.44 ± 0.04 a | 2.57 ± 0.14 a | 2.39 ± 0.09 a |
| Proximate composition (%) | ||||
| Moisture | 67.61 ± 0.27 a | 71.08 ± 0.51 c | 68.62 ± 0.80 ab | 70.53 ± 0.35 c |
| Crude protein | 18.08 ± 0.47 | 17.48 ± 0.18 | 18.35 ± 0.07 | 17.63 ± 0.13 |
| Crude lipid | 6.58 ± 0.20 bc | 5.75 ± 0.16 a | 6.81 ± 0.10 c | 6.24 ± 0.13 ab |
| Ash | 4.66 ± 0.10 c | 4.30 ± 0.09 ab | 4.43 ± 0.07 bc | 4.18 ± 0.07 a |
| Items | Diets 2 | |||
|---|---|---|---|---|
| FM | SBM | G-4.5 | G-10 | |
| T-AOC (mmol/g) | 0.38 ± 0.01 b | 0.25 ± 0.06 a | 0.37 ± 0.02 b | 0.28 ± 0.04 ab |
| GSH-Px (U/mg prot) | 62.02 ± 1.13 b | 54.80 ± 0.71 a | 62.75 ± 2.61 b | 58.82 ± 1.66 ab |
| CAT (U/mg prot) | 7.02 ± 1.53 | 7.39 ± 1.00 | 8.37 ± 1.52 | 7.87 ± 0.89 |
| SOD (U/mg prot) | 204.43 ± 3.02 | 190.61 ± 8.22 | 198.85 ± 7.08 | 197.89 ± 9.97 |
| MDA (nmol/mg prot) | 3.08 ± 0.43 a | 6.27 ± 0.45 b | 3.17 ± 0.39 a | 4.10 ± 0.32 a |
| Items 3 | Diets 2 | ||||
|---|---|---|---|---|---|
| FM | SBM | G-4.5 | G-10 | ||
| PI | HMF (μm) | 449.89 ± 19.40 | 421.35 ± 22.14 | 455.80 ± 20.34 | 428.75 ± 14.84 |
| TML (μm) | 93.50 ± 3.90 | 71.47 ± 10.56 | 90.37 ± 15.90 | 84.44 ± 7.67 | |
| NFM (unit) | 40.00 ± 0.58 | 37.67 ± 1.76 | 41.33 ± 1.86 | 41.33 ± 1.86 | |
| MI | HMF (μm) | 370.86 ± 25.48 | 323.06 ± 12.57 | 384.08 ± 34.42 | 354.67 ± 23.82 |
| TML (μm) | 73.91 ± 2.64 b | 61.47 ± 4.50 a | 72.84 ± 4.29 b | 69.72 ± 4.13 ab | |
| NFM (unit) | 28.67 ± 0.33 | 29.00 ± 0.58 | 29.00 ± 1.15 | 31.33 ± 1.76 | |
| DI | HMF (μm) | 341.18 ± 9.57 b | 279.84 ± 15.43 a | 342.93 ± 10.73 b | 305.48 ± 9.41 a |
| TML (μm) | 77.67 ± 1.67 b | 64.79 ± 3.59 a | 76.41 ± 2.38 b | 71.46 ± 3.65 ab | |
| NFM (unit) | 31.33 ± 0.33 | 32.00 ± 1.00 | 33.67 ± 1.86 | 34.00 ± 2.31 | |
| Items | Diets 2 | |||
|---|---|---|---|---|
| FM | SBM | G-4.5 | G-10 | |
| OTUs | 245.00 ± 80.16 | 228.33 ± 113.87 | 166.33 ± 51.94 | 255.67 ± 113.91 |
| Ace | 473.72 ± 12.88 | 476.39 ± 85.51 | 514.60 ± 80.80 | 339.86 ± 29.76 |
| Chao1 | 457.24 ± 28.78 | 380.55 ± 131.81 | 493.16 ± 93.57 | 253.60 ± 53.60 |
| Shannon | 2.81 ± 0.4 | 3.29 ± 0.16 | 4.36 ± 0.54 | 3.45 ± 0.90 |
| Simpson | 0.70 ± 0.07 | 0.80 ± 0.01 | 0.75 ± 0.14 | 0.75 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Zhao, X.; Yang, L.; Wang, K.; Sun, Y.; Ye, J. Dietary High Glycinin Reduces Growth Performance and Impairs Liver and Intestinal Health Status of Orange-Spotted Grouper (Epinephelus coioides). Animals 2023, 13, 2605. https://doi.org/10.3390/ani13162605
Yin Y, Zhao X, Yang L, Wang K, Sun Y, Ye J. Dietary High Glycinin Reduces Growth Performance and Impairs Liver and Intestinal Health Status of Orange-Spotted Grouper (Epinephelus coioides). Animals. 2023; 13(16):2605. https://doi.org/10.3390/ani13162605
Chicago/Turabian StyleYin, Yanxia, Xingqiao Zhao, Lulu Yang, Kun Wang, Yunzhang Sun, and Jidan Ye. 2023. "Dietary High Glycinin Reduces Growth Performance and Impairs Liver and Intestinal Health Status of Orange-Spotted Grouper (Epinephelus coioides)" Animals 13, no. 16: 2605. https://doi.org/10.3390/ani13162605
APA StyleYin, Y., Zhao, X., Yang, L., Wang, K., Sun, Y., & Ye, J. (2023). Dietary High Glycinin Reduces Growth Performance and Impairs Liver and Intestinal Health Status of Orange-Spotted Grouper (Epinephelus coioides). Animals, 13(16), 2605. https://doi.org/10.3390/ani13162605






