Cytological Quantification of Nodal Mast Cells in Dogs Affected by Non-Neoplastic Condition and Mast Cell Tumor Using Different Sample Preparation Techniques: An Explorative Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection from the Archives and Preliminary Classification into Clinical Categories
2.2. Criteria Applied to Microphotograph Acquisition and Counting
2.3. Statistical Analysis
3. Results
3.1. Selection Workflow and Signalment Data for the Cases Included in the Study
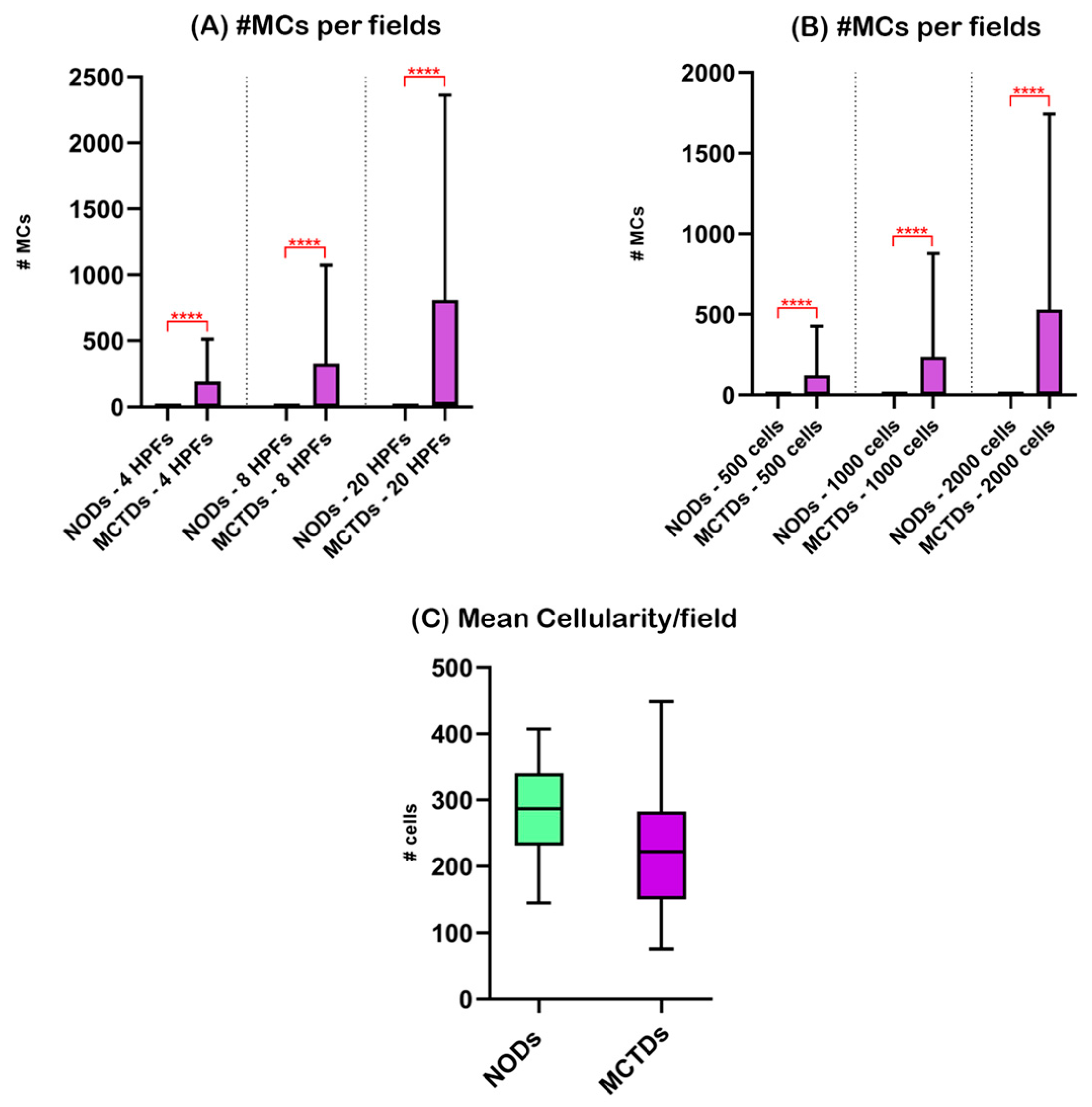
3.2. Nodal Mast Cells in Non-Oncological Dogs (NODs) and Mast Cell Tumor-Bearing Dogs (MCTBDs)
3.3. Nodal Mast Cells in Specimens Obtained from Mast Cell Tumor-Bearing Dogs (MCTBDs) and Classified as “Non Metastatic” (MCT-NM), “Possibly Metastatic” (MCT-PM), and “Metastatic” (MCT-M)
3.4. Nodal Mast Cells in Specimens Obtained from Mast Cell Tumor-Bearing Dogs (MCTBDs) and Sampled via Fine-Needle Aspiration (MCT-F), Scraping Smearing (MCT-S), and Touch Imprinting (MCT-T)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackwood, L.; Murphy, S.; Buracco, P.; De Vos, J.P.; De Fornel-Thibaud, P.; Hirschberger, J.; Kessler, M.; Pastor, J.; Ponce, F.; Savary-Bataille, K.; et al. European Consensus Document on Mast Cell Tumours in Dogs and Cats. Vet. Comp. Oncol. 2012, 10, e1–e29. [Google Scholar] [CrossRef]
- London, C.A.; Thamm, D.H. Mast Cell Tumors. In Small Animal Clinical Oncology; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: St. Louis, MI, USA, 2013; pp. 335–355. ISBN 978-1-4557-4083-3. [Google Scholar]
- Warland, J.; Brioschi, V.; Owen, L.; Dobson, J. Canine Mast Cell Tumours: Decision-Making and Treatment. Practice 2015, 37, 315–332. [Google Scholar] [CrossRef]
- Murphy, S.; Sparkes, A.H.; Blunden, A.S.; Brearley, M.J.; Smith, K.C. Effects of Stage and Number of Tumours on Prognosis of Dogs with Cutaneous Mast Cell Tumours. Vet. Rec. 2006, 158, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Sledge, D.G.; Webster, J.; Kiupel, M. Canine Cutaneous Mast Cell Tumors: A Combined Clinical and Pathologic Approach to Diagnosis, Prognosis, and Treatment Selection. Vet. J. 2016, 215, 43–54. [Google Scholar] [CrossRef]
- Elliott, J.W.; Cripps, P.; Blackwood, L.; Berlato, D.; Murphy, S.; Grant, I.A. Canine Oral Mucosal Mast Cell Tumours. Vet. Comp. Oncol. 2016, 14, 101–111. [Google Scholar] [CrossRef]
- Hillman, L.A.; Garrett, L.D.; de Lorimier, L.-P.; Charney, S.C.; Borst, L.B.; Fan, T.M. Biological Behavior of Oral and Perioral Mast Cell Tumors in Dogs: 44 Cases (1996–2006). J. Am. Vet. Med. Assoc. 2010, 237, 936–942. [Google Scholar] [CrossRef]
- Horta, R.S.; Lavalle, G.E.; Monteiro, L.N.; Souza, M.C.C.; Cassali, G.D.; Araújo, R.B. Assessment of Canine Mast Cell Tumor Mortality Risk Based on Clinical, Histologic, Immunohistochemical, and Molecular Features. Vet. Pathol. 2018, 55, 212–223. [Google Scholar] [CrossRef]
- Marconato, L.; Marchetti, V.; Francione, D.; Masserdotti, C.; Gregori, M.; Leotta, R.; Abramo, F. Morphometrical Approach for Predicting Regional Lymph Node Micrometastatic Load in Canine Mast Cell Tumours: Preliminary Results. Vet. Comp. Oncol. 2008, 6, 162–170. [Google Scholar] [CrossRef]
- Marconato, L.; Stefanello, D.; Kiupel, M.; Finotello, R.; Polton, G.; Massari, F.; Ferrari, R.; Agnoli, C.; Capitani, O.; Giudice, C.; et al. Adjuvant Medical Therapy Provides No Therapeutic Benefit in the Treatment of Dogs with Low-Grade Mast Cell Tumours and Early Nodal Metastasis Undergoing Surgery. Vet. Comp. Oncol. 2020, 18, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Fejös, C.; Troedson, K.; Ignatenko, N.; Zablotski, Y.; Hirschberger, J. Extensive Staging Has No Prognostic Value in Dogs with Low-risk Mast Cell Tumours. Vet. Comp. Oncol. 2022, 20, 265–275. [Google Scholar] [CrossRef]
- Pecceu, E.; Serra Varela, J.C.; Handel, I.; Piccinelli, C.; Milne, E.; Lawrence, J. Ultrasound Is a Poor Predictor of Early or Overt Liver or Spleen Metastasis in Dogs with High-Risk Mast Cell Tumours. Vet. Comp. Oncol. 2020, 18, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, D.; Valenti, P.; Faverzani, S.; Bronzo, V.; Fiorbianco, V.; Pinto da Cunha, N.; Romussi, S.; Cantatore, M.; Caniatti, M. Ultrasound-Guided Cytology of Spleen and Liver: A Prognostic Tool in Canine Cutaneous Mast Cell Tumor. J. Vet. Intern. Med. 2009, 23, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, A.; McManus, P.M.; Hendrick, M.J.; Shofer, F.S.; Sorenmo, K.U. Sensitivity and Specificity of Methods of Assessing the Regional Lymph Nodes for Evidence of Metastasis in Dogs and Cats with Solid Tumors. J. Am. Vet. Med. Assoc. 2001, 218, 1424–1428. [Google Scholar] [CrossRef]
- Krick, E.L.; Billings, A.P.; Shofer, F.S.; Watanabe, S.; Sorenmo, K.U. Cytological Lymph Node Evaluation in Dogs with Mast Cell Tumours: Association with Grade and Survival. Vet. Comp. Oncol. 2009, 7, 130–138. [Google Scholar] [CrossRef]
- Warland, J.; Amores-Fuster, I.; Newbury, W.; Brearley, M.; Dobson, J. The Utility of Staging in Canine Mast Cell Tumours. Vet. Comp. Oncol. 2014, 12, 287–298. [Google Scholar] [CrossRef]
- Weishaar, K.M.; Thamm, D.H.; Worley, D.R.; Kamstock, D.A. Correlation of Nodal Mast Cells with Clinical Outcome in Dogs with Mast Cell Tumour and a Proposed Classification System for the Evaluation of Node Metastasis. J. Comp. Pathol. 2014, 151, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Pizzoni, S.; Sabattini, S.; Stefanello, D.; Dentini, A.; Ferrari, R.; Dacasto, M.; Giantin, M.; Laganga, P.; Amati, M.; Tortorella, G.; et al. Features and Prognostic Impact of Distant Metastases in 45 Dogs with de Novo Stage IV Cutaneous Mast Cell Tumours: A Prospective Study. Vet. Comp. Oncol. 2018, 16, 28–36. [Google Scholar] [CrossRef]
- Owen, L.N. (Ed.) Skin—Mastocytoma. In TNM Classification of Tumours in Domestic Animals; World Health Organization: Geneva, Switzerland, 1980; pp. 14–15. [Google Scholar]
- Baginski, H.; Davis, G.; Bastian, R.P. The Prognostic Value of Lymph Node Metastasis with Grade 2 MCTs in Dogs: 55 Cases (2001–2010). J. Am. Anim. Hosp. Assoc. 2014, 50, 89–95. [Google Scholar] [CrossRef]
- Mutz, M.L.; Boudreaux, B.B.; Royal, A.; Merchant, S.; Pucheu-Haston, C.; Griffith, E.H.; Gieger, T.L. Cytologic Comparison of the Percentage of Mast Cells in Lymph Node Aspirate Samples from Clinically Normal Dogs versus Dogs with Allergic Dermatologic Disease and Dogs with Cutaneous Mast Cell Tumors. J. Am. Vet. Med. Assoc. 2017, 251, 421–428. [Google Scholar] [CrossRef]
- Fournier, Q.; Cazzini, P.; Bavcar, S.; Pecceu, E.; Ballber, C.; Elders, R. Investigation of the Utility of Lymph Node Fine-Needle Aspiration Cytology for the Staging of Malignant Solid Tumors in Dogs. Vet. Clin. Pathol. 2018, 47, 489–500. [Google Scholar] [CrossRef]
- Sulce, M.; Marconato, L.; Martano, M.; Iussich, S.; Dentini, A.; Melega, M.; Miniscalco, B.; Riondato, F. Utility of Flow Cytometry in Canine Primary Cutaneous and Matched Nodal Mast Cell Tumor. Vet. J. 2018, 242, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Polton, G.; Stefanello, D.; Morello, E.; Ferrari, R.; Henriques, J.; Tortorella, G.; Benali, S.L.; Bergottini, R.; Vasconi, M.E.; et al. Therapeutic Impact of Regional Lymphadenectomy in Canine Stage II Cutaneous Mast Cell Tumours. Vet. Comp. Oncol. 2018, 16, 580–589. [Google Scholar] [CrossRef]
- Worley, D.R. Incorporation of Sentinel Lymph Node Mapping in Dogs with Mast Cell Tumours: 20 Consecutive Procedures. Vet. Comp. Oncol. 2014, 12, 215–226. [Google Scholar] [CrossRef]
- Krick, E.L.; Kiupel, M.; Durham, A.C.; Thaiwong, T.; Brown, D.C.; Sorenmo, K.U. Investigating Associations Between Proliferation Indices, C-Kit, and Lymph Node Stage in Canine Mast Cell Tumors. J. Am. Anim. Hosp. Assoc. 2017, 53, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, D.; Buracco, P.; Sabattini, S.; Finotello, R.; Giudice, C.; Grieco, V.; Iussich, S.; Tursi, M.; Scase, T.; Di Palma, S.; et al. Comparison of 2- and 3-Category Histologic Grading Systems for Predicting the Presence of Metastasis at the Time of Initial Evaluation in Dogs with Cutaneous Mast Cell Tumors: 386 Cases (2009–2014). J. Am. Vet. Med. Assoc. 2015, 246, 765–769. [Google Scholar] [CrossRef]
- Ku, C.K.; Kass, P.H.; Christopher, M.M. Cytologic–Histologic Concordance in the Diagnosis of Neoplasia in Canine and Feline Lymph Nodes: A Retrospective Study of 367 Cases. Vet. Comp. Oncol. 2017, 15, 1206–1217. [Google Scholar] [CrossRef]
- Miller, R.L.; Van Lelyveld, S.; Warland, J.; Dobson, J.M.; Foale, R.D. A Retrospective Review of Treatment and Response of High-Risk Mast Cell Tumours in Dogs. Vet. Comp. Oncol. 2016, 14, 361–370. [Google Scholar] [CrossRef]
- Sabattini, S.; Renzi, A.; Marconato, L.; Militerno, G.; Agnoli, C.; Barbiero, L.; Rigillo, A.; Capitani, O.; Tinto, D.; Bettini, G. Comparison between May-Grünwald-Giemsa and Rapid Cytological Stains in Fine-Needle Aspirates of Canine Mast Cell Tumour: Diagnostic and Prognostic Implications. Vet. Comp. Oncol. 2018, 16, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Sapierzyński, R.; Czopowicz, M.; Jagielski, D. Metastatic Lymphadenomegaly in Dogs—Cytological Study. Pol. J. Vet. Sci. 2017, 20, 731–736. [Google Scholar]
- Bookbinder, P.F.; Butt, M.T.; Harvey, H.J. Determination of the Number of Mast Cells in Lymph Node, Bone Marrow, and Buffy Coat Cytologic Specimens from Dogs. J. Am. Vet. Med. Assoc. 1992, 200, 1648–1650. [Google Scholar]
- Bauer, C.L.; Rakich, P.M.; Latimer, K.S.; White, A.; Hensel, P. Assessment and Comparison of Reference Intervals for Mast Cells in Peripheral Lymph Nodes of Normal Dogs and Dogs with Allergic Skin Disease. In Proceedings of the 26th Annual Meeting of the AAVD/ACVD, Abstracts of the North American Veterinary Dermatology Forum, Galveston, TX, USA, 13–16 April 2011; Volume 22, pp. 2–4. [Google Scholar]
- Karaman, S.; Detmar, M. Mechanisms of Lymphatic Metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Balogh, L.; Thuróczy, J.; Andócs, G.; Máthé, D.; Chaudhari, P.; Perge, E.; Biksi, I.; Polyák, A.; Király, R.; Jánoki, G.A. Sentinel Lymph Node Detection in Canine Oncological Patients. Nucl. Med. Rev. 2002, 5, 139–144. [Google Scholar]
- Lurie, D.M.; Seguin, B.; Schneider, P.D.; Verstraete, F.J.; Wisner, E.R. Contrast-Assisted Ultrasound for Sentinel Lymph Node Detection in Spontaneously Arising Canine Head and Neck Tumors. Investig. Radiol. 2006, 41, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, J.L.; Milgram, J.; Worley, D.R.; Dernell, W.S. A Review of Sentinel Lymph Node Evaluation and the Need for Its Incorporation into Veterinary Oncology. Vet. Comp. Oncol. 2009, 7, 81–91. [Google Scholar] [CrossRef]
- Gelb, H.R.; Freeman, L.J.; Rohleder, J.J.; Snyder, P.W. Feasibility of Contrast-Enhanced Ultrasound-Guided Biopsy of Sentinel Lymph Nodes in Dogs. Vet. Radiol. Ultrasound 2010, 51, 628–633. [Google Scholar] [CrossRef]
- Beserra, H.E.O.; Grandi, F.; Dufloth, R.M.; Pinheiro, L.G.P.; Miot, H.A.; Vexenat, S.C.O.R.; Rocha, N.S. Metastasis of Mammary Carcinoma in Bitches: Evaluation of the Sentinel Lymph Node Technique. Adv. Breast Cancer Res. 2016, 05, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Brissot, H.N.; Edery, E.G. Use of Indirect Lymphography to Identify Sentinel Lymph Node in Dogs: A Pilot Study in 30 Tumours. Vet. Comp. Oncol. 2017, 15, 740–753. [Google Scholar] [CrossRef]
- Soultani, C.; Patsikas, M.N.; Karayannopoulou, M.; Jakovljevic, S.; Chryssogonidis, I.; Papazoglou, L.; Papaioannou, N.; Papadopoulou, P.; Pavlidou, K.; Ilia, G.M.; et al. Assessment of Sentinel Lymph Node Metastasis in Canine Mammary Gland Tumors Using Computed Tomographic Indirect Lymphography. Vet. Radiol. Ultrasound 2017, 58, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.; Pozzi, A.; Rohrer Bley, C.; Bacon, N.; Pfammatter, N.S.; Venzin, C. The Role of Sentinel Lymph Node Mapping in Small Animal Veterinary Medicine: A Comparison with Current Approaches in Human Medicine. Vet. Comp. Oncol. 2018, 16, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Chiti, L.E.; Manfredi, M.; Ravasio, G.; De Zani, D.; Zani, D.D.; Giudice, C.; Gambini, M.; Stefanello, D. Biopsy of Sentinel Lymph Nodes after Injection of Methylene Blue and Lymphoscintigraphic Guidance in 30 Dogs with Mast Cell Tumors. Vet. Surg. 2020, 49, 1099–1108. [Google Scholar] [CrossRef]
- Fournier, Q.; Thierry, F.; Longo, M.; Malbon, A.; Cazzini, P.; Bisson, J.; Woods, S.; Liuti, T.; Bavcar, S. Contrast-Enhanced Ultrasound for Sentinel Lymph Node Mapping in the Routine Staging of Canine Mast Cell Tumours: A Feasibility Study. Vet. Comp. Oncol. 2020, 19, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Körner, M.; Suárez, J.; Carozzi, G.; Meier, V.S.; Roos, M.; Rohrer Bley, C. Computed Tomographic-Lymphography as a Complementary Technique for Lymph Node Staging in Dogs with Malignant Tumors of Various Sites. Vet. Radiol. Ultrasound 2018, 59, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.A.; Secrest, S.A.; Wallace, M.L.; Laver, T.; Schmiedt, C.W. Use of Indirect Computed Tomography Lymphangiography to Determine Metastatic Status of Sentinel Lymph Nodes in Dogs with a Pre-Operative Diagnosis of Melanoma or Mast Cell Tumour. Vet. Comp. Oncol. 2020, 18, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Thamm, D.H.; Turek, M.M.; Vail, D.M. Outcome and prognostic factors following adjuvant prednisone/vinblastine chemotherapy for high-risk canine mast cell tumour: 61 cases. J. Vet. Med. Sci. 2006, 68, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Vos, J.H.; van den Ingh, T.S.; van Mil, F.N. Non-exfoliative canine cytology: The value of fine needle aspiration and scraping cytology. Vet. Q. 1989, 11, 222–231. [Google Scholar] [CrossRef]
- Cohen, M.; Bohling, M.W.; Wright, J.C.; Welles, E.A.; Spano, J.S. Evaluation of sensitivity and specificity of cytologic examination: 269 cases (1999–2000). J. Am. Vet. Med. Assoc. 2003, 222, 964–967. [Google Scholar] [CrossRef]


| Cytological Criteria | Interpretation |
|---|---|
| No MCs observed; OR >50% small lymphocytes with a mixed population of prolymphocytes, lymphoblasts, plasma cells, and/or few to moderate numbers of macrophages, neutrophils, and eosinophils, and/or rare individual MCs | Non-metastatic (MCT-NM) (former “normal” + “reactive lymphoid hyperplasia”) |
| 2–3 incidences of MCs aggregated in couples or triplets | Possibly metastatic (MCT-PM) |
| >3 incidences of MCs aggregated in couples or triplets and/or 2–5 aggregates composed by >3 MCs; OR effacement of lymphoid tissue by MCs, and/or aggregated, poorly differentiated MCs (pleomorphism, anisocytosis, anisokaryosis, and/or decreased or variable granulation), and/or >5 aggregates composed by >3 MCs | Metastatic (MCT-M) (former “probable metastasis” + “certain metastasis”) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzzi, G.; Gambini, M.; Recordati, C.; Grieco, V.; Stefanello, D.; Ferrari, R.; Zamboni, C.; Manfredi, M.; Giudice, C. Cytological Quantification of Nodal Mast Cells in Dogs Affected by Non-Neoplastic Condition and Mast Cell Tumor Using Different Sample Preparation Techniques: An Explorative Study. Animals 2023, 13, 2634. https://doi.org/10.3390/ani13162634
Buzzi G, Gambini M, Recordati C, Grieco V, Stefanello D, Ferrari R, Zamboni C, Manfredi M, Giudice C. Cytological Quantification of Nodal Mast Cells in Dogs Affected by Non-Neoplastic Condition and Mast Cell Tumor Using Different Sample Preparation Techniques: An Explorative Study. Animals. 2023; 13(16):2634. https://doi.org/10.3390/ani13162634
Chicago/Turabian StyleBuzzi, Giulia, Matteo Gambini, Camilla Recordati, Valeria Grieco, Damiano Stefanello, Roberta Ferrari, Clarissa Zamboni, Martina Manfredi, and Chiara Giudice. 2023. "Cytological Quantification of Nodal Mast Cells in Dogs Affected by Non-Neoplastic Condition and Mast Cell Tumor Using Different Sample Preparation Techniques: An Explorative Study" Animals 13, no. 16: 2634. https://doi.org/10.3390/ani13162634





