The Beneficial Effects of Pterostilbene on Post-Thawed Bovine Spermatozoa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
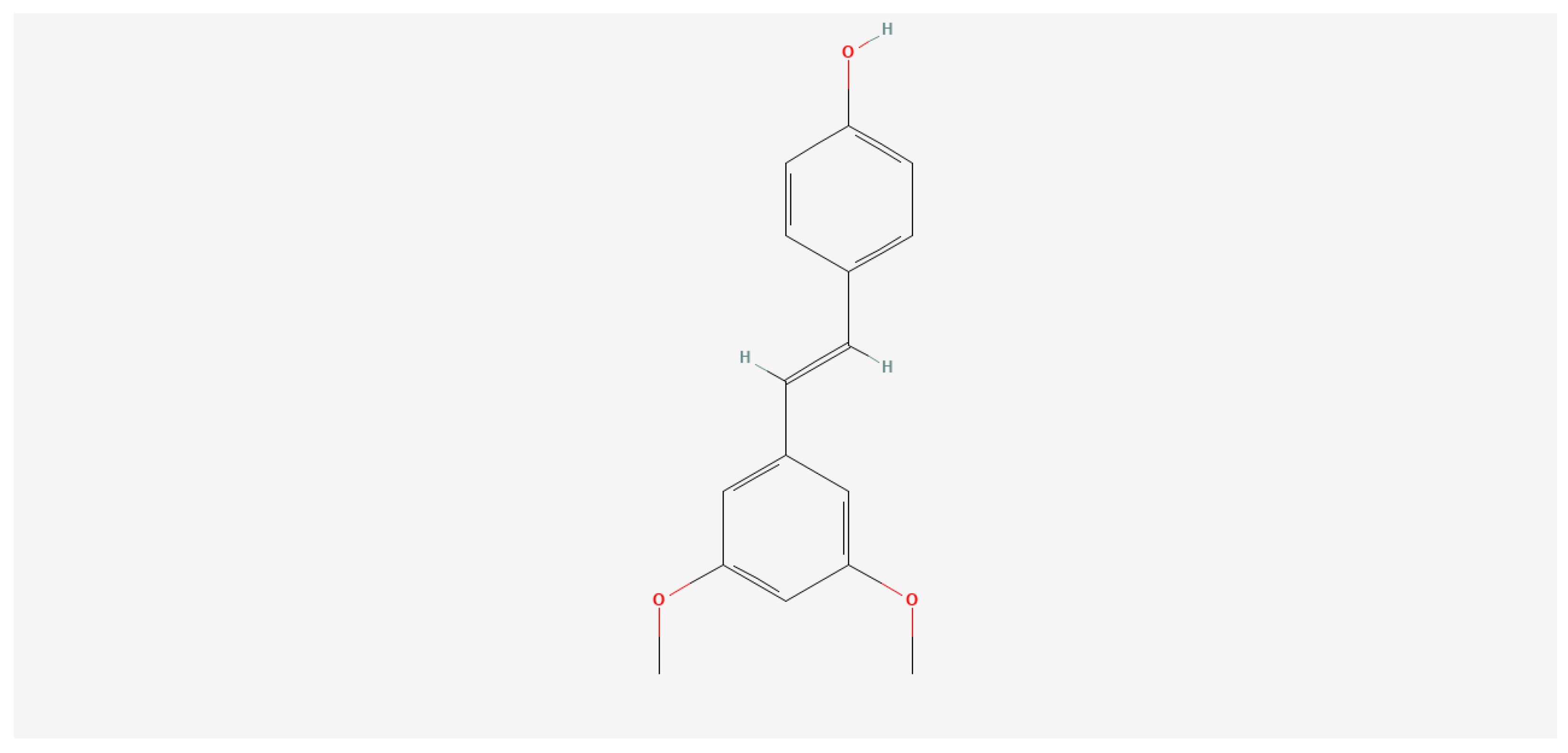
2.1. PT Preparation
2.2. Motility and Viability Assessment
2.3. Quantification of the Superoxide Anion Production (Nitroblue Tetrazolium Test-NBT)
2.4. Capacitation and Acrosome Reaction
2.5. Statistical Analysis
3. Results
3.1. Motility and Viability Assessment
3.2. Superoxide Anion Quantification
3.3. Capacitation and Acrosome Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudière, J. Some chemical and biochemical constraints of oxidative stress in living cells. In New Comprehensive Biochemistry, Free Radical Damage and Its Control; Rice-Evans, C.A., Burdon, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 28, pp. 25–66. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Sikka, S.C.; Rajasekaran, M.; Hellstrom, W.J. Role of oxidative stress and antioxidants in male infertility. J. Androl. 1995, 16, 464–468. [Google Scholar]
- Aitken, R.J.; Clarkson, J.S. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J. Androl. 1998, 9, 367376. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef]
- Correa, J.R.; Pace, M.M.; Zavos, P.M. Relationships among frozen-thawed sperm characteristics assessed via the routine semen analysis, sperm functional tests and fertility of bulls in an artificial insemination program. Theriogenology 1997, 48, 721–731. [Google Scholar] [CrossRef]
- Hoshi, H. In vitro production of bovine embryos and their application for embryo transfer. Theriogenology 2003, 59, 675–685. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2010, 12, 112. [Google Scholar] [CrossRef]
- Pintus, E.; Ros-Santaella, J.L. Impact of Oxidative Stress on Male Reproduction in Domestic and Wild Animals. Antioxidants 2021, 10, 1154. [Google Scholar] [CrossRef]
- Sapanidou, V.; Tsantarliotou, M.P.; Lavrentiadou, S.N. A review of the use of antioxidants in bovine sperm preparation protocols. Anim. Reprod. Sci. 2023, 251, 107215. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int. J. Androl. 1993, 16, 21–25. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Beorlegui, N.; Beconi, M.T. Participation of superoxide anion in the capacitation of cryopreserved bovine sperm. Int. J. Androl. 2003, 26, 109–114. [Google Scholar] [CrossRef]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 10, 667–678. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Pratt, D.E.; Hudson, B.J.F. Natural Antioxidants Not Exploited Commercially. In Food Antioxidants; Hudson, B.J.F., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 171–192. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic Stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef]
- Liu, Y.; You, Y.; Lu, J.; Chen, X.; Yang, Z. Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules 2020, 25, 5166. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Chen, Z.P.; Cai, Y.; Phillipson, J.D. Studies on the anti-tumor, anti-bacterial, and wound-healing properties of dragon’s blood. Planta Medica 1994, 60, 541–545. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yáñez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, R.S. Effect of Pterocarpus marsupium on Streptozotocin-induced Oxidative Stress in Kidney of Diabetic Wistar Rats. J. Herbs Spices Med. 2011, 17, 169–182. [Google Scholar] [CrossRef]
- Messina, F.; Guglielmini, G.; Curini, M.; Orsini, S.; Gresele, P.; Marcotullio, M.C. Effect of substituted stilbenes on platelet function. Fitoterapia 2015, 105, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ci, X.; Ma, X.; Yu, Q.; Cui, Y.; Zhen, Y.; Li, S. Pterostilbene Activates the Nrf2-Dependent Antioxidant Response to Ameliorate Arsenic-Induced Intracellular Damage and Apoptosis in Human Keratinocytes. Front. Pharmacol. 2019, 10, 497. [Google Scholar] [CrossRef]
- Li, J.; Deng, R.; Hua, X.; Zhang, L.; Lu, F.; Coursey, T.G.; Pflugfelder, S.C.; Li, D.Q. Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway. Sci. Rep. 2016, 6, 19408. [Google Scholar] [CrossRef]
- Sosa, F.; Romo, S.; Kjelland, M.E.; Álvarez-Gallardo, H.; Pérez-Reynozo, S.; Urbán-Duarte, D.; De La Torre-Sánchez, J.F. Effect of pterostilbene on development, equatorial lipid accumulation and reactive oxygen species production of in vitro-produced bovine embryos. Reprod. Domest. Anim. 2020, 55, 1490–1500. [Google Scholar] [CrossRef]
- Amarnath Satheesh, M.; Pari, L. The antioxidant role of pterostilbene in streptozotocin-nicotinamide-induced type 2 diabetes mellitus in Wistar rats. J. Pharm. Pharmacol. 2006, 58, 1483–1490. [Google Scholar] [CrossRef]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxidative Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef]
- Robb, E.L.; Stuart, J.A. The stilbenes resveratrol, pterostilbene and piceid affect growth and stress resistance in mammalian cells via a mechanism requiring estrogen receptor beta and the induction of Mn-superoxide dismutase. Phytochemistry 2014, 98, 164–173. [Google Scholar] [CrossRef]
- Ullah, O.; Li, Z.; Ali, I.; Xu, L.; Liu, H.; Jin, H.Z.; Fang, Y.Y.; Jin, Q.G.; Fang, N. Pterostilbene exerts a protective effect via regulating tunicamycin-induced endoplasmic reticulum stress in mouse preimplantation embryos. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Becerra, M.C.; Eraso, A.J.; Albesa, I. Comparison of oxidative stress induced by ciprofloxacin and pyoverdin in bacteria and in leukocytes to evaluate toxicity. J. Lumin. 2003, 18, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.J.; Susko-Parrish, J.; Winer, M.A.; First, N.L. Capacitation of bovine sperm by heparin. Biol. Reprod. 1988, 38, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.L.; Miller, D.J. Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 1999, 52, 445–449. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Pelzer, K.D.; Kasimanickam, V.; Swecker, W.S.; Thatcher, C.D. Association of classical semen parameters, sperm DNA fragmentation index, lipid peroxidation and antioxidant enzymatic activity of semen in ram-lambs. Theriogenology 2006, 65, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, S.; Sakai, D.; Schol, J.; Sako, K.; Nakamura, Y.; Matsushita, E.; Warita, T.; Hazuki, S.; Nojiri, H.; Sato, M.; et al. N-acetylcysteine attenuates oxidative stress-mediated cell viability loss induced by dimethyl sulfoxide in cryopreservation of human nucleus pulposus cells: A potential solution for mass production. JOR Spine 2020, 5, e1223. [Google Scholar] [CrossRef]
- Tvrdá, E.; Lukáč, N.; Lukáčova, J.; Hashim, F.; Massányi, P. In vitro supplementation of resveratrol to bovine spermatozoa: Effects on motility, viability and superoxide production. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 336–341. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kováčik, A.; Tušimová, E.; Massányi, P.; Lukáč, N. Resveratrol offers protection to oxidative stress induced by ferrous ascorbate in bovine spermatozoa. J. Environ. Sci. Health Part A 2015, 50, 1440–1451. [Google Scholar] [CrossRef]
- Mojica-Villegas, M.A.; Izquierdo-Vega, J.A.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M. Protective Effect of Resveratrol on Biomarkers of Oxidative Stress Induced by Iron/Ascorbate in Mouse Spermatozoa. Nutrients 2014, 6, 489–503. [Google Scholar] [CrossRef]
- Collodel, G.; Federico, M.G.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Τoxicol. 2011, 31, 239–246. [Google Scholar] [CrossRef]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its biologic targets and functional activity. Antioxid. Redox Signal. 2009, 11, 2851–2897. [Google Scholar] [CrossRef]
- Sapanidou, V.G.; Margaritis, I.; Siahos, N.; Arsenopoulos, K.; Dragatidou, E.; Taitzoglou, I.A.; Zervos, I.A.; Theodoridis, A.; Tsantarliotou, M.P. Antioxidant effect of a polyphenol-rich grape pomace extract on motility, viability and lipid peroxidation of thawed bovine spermatozoa. J. Biol. Res. 2014, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Kazantzoglou, G.; Theofanidou, D.; Kakalopoulou, G.; Magiatis, P.; Mitaku, S.; Kouretas, D. Activity of grape extracts from Greek varieties of Vitis vinifera against mutagenicity induced by bleomycin and hydrogen peroxide in Salmonella typhimurium strain TA102. Mutat. Res. 2006, 609, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, F.; Zribi, N.; Ammar-Keskes, L. Antioxidative potential of Quercetin against hydrogen peroxide induced oxidative stress in spermatozoa in vitro. Andrologia 2011, 43, 261–265. [Google Scholar] [CrossRef]
- Sapanidou, V.; Taitzoglou, I.; Tsakmakidis, I.; Kourtzelis, I.; Fletouris, D.; Theodoridis, A.; Lavrentiadou, S.; Tsantarliotou, M. Protective effect of crocetin on bovine spermatozoa against oxidative stress during in vitro fertilization. Andrology 2016, 4, 1138–1149. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992, 13, 368–378. [Google Scholar] [PubMed]
- Aitken, R.J.; Harkiss, D.; Buckingham, D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J. Reprod. Fertil. 1993, 98, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kalo, D.; Zeron, Y.; Roth, Z. Progressive motility—A potential predictive parameter for semen fertilization capacity in bovines. Zygote 2016, 24, 70–82. [Google Scholar] [CrossRef]
- Kathivaran, P.; Kalatharan, J.; Karthikeya, G.; Rengarajan, K.; Kadirvel, G. Objective sperm analysis to assess dairy bull fertility using computer-aided system: A review. Reprod. Domest. Anim. 2011, 46, 165–172. [Google Scholar] [CrossRef]
- Aitken, R.J.; Koppers, A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011, 13, 36–42. [Google Scholar] [CrossRef]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis 1999, 14, 505–512. [Google Scholar] [CrossRef]
- Tvrdá, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Libová, Ľ.; Lukáč, N. Protective Effects of Quercetin on Selected Oxidative Biomarkers in Bovine Spermatozoa Subjected to Ferrous Ascorbate. Reprod. Domest. Anim. 2016, 51, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Weinberg, A.; Hetherington, L.; Villaverde, A.I.; Velkov, T.; Baell, J.; Gordon, C.P. Defining the mechanisms by which the reactive oxygen species by-product, 4-hydroxynonenal, affects human sperm cell function. Biol. Reprod. 2015, 92, 108. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Human Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef] [PubMed]
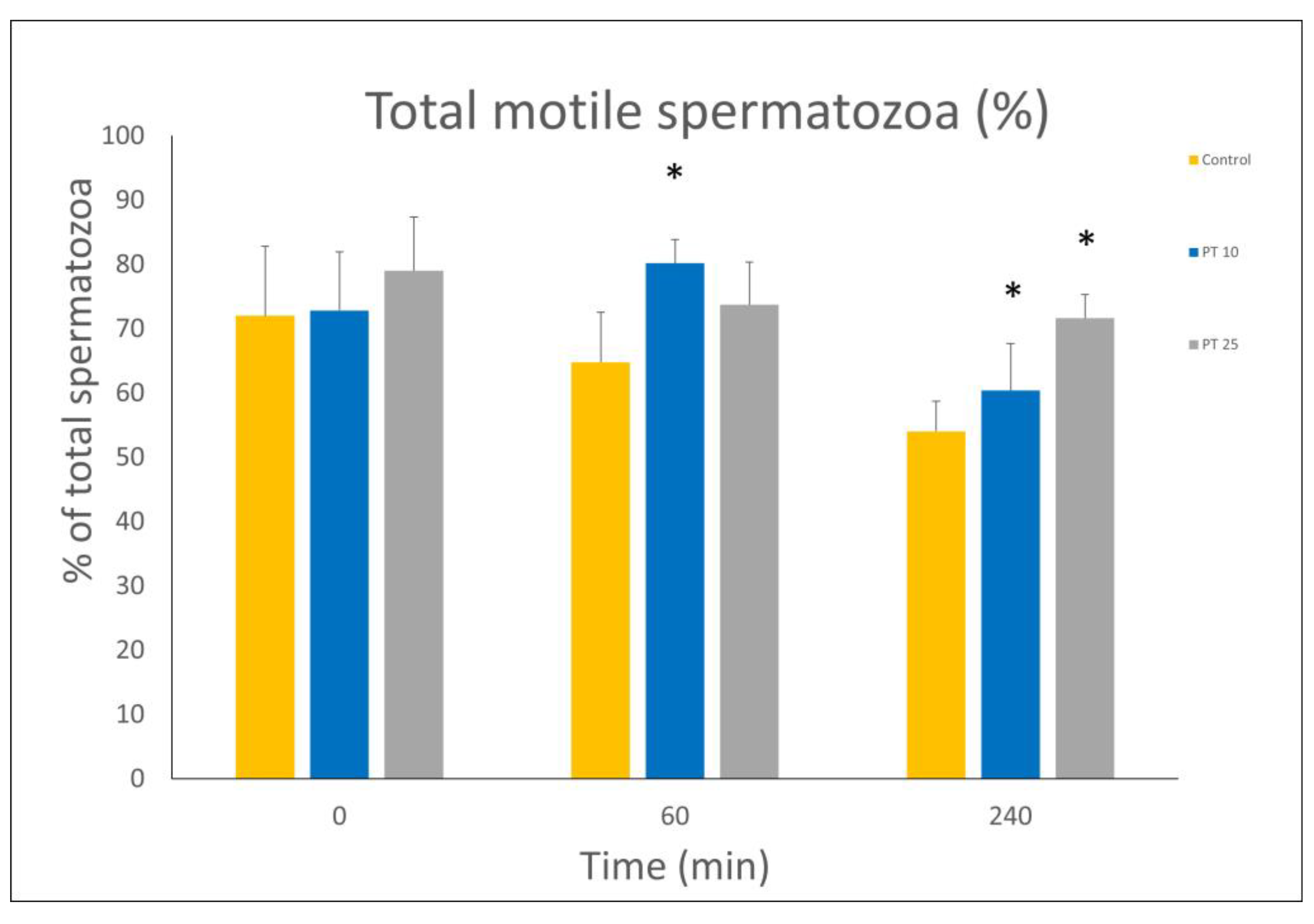





| Time Point | Group | Rapid (%) | Medium (%) | Slow (%) | Total Motile (%) | Static (%) | Progressive Motile (%) |
|---|---|---|---|---|---|---|---|
| 0 min | Control | 45.13 ± 2.07 | 22.63 ± 8.70 | 4.21 ± 2.86 | 71.98 ± 10.81 | 29.88 ± 8.44 | 22.61 ± 2.24 |
| ΡT 10 | 47.25 ± 5.45 | 20.13 ± 3.09 | 5.42 ± 3.10 | 72.80 ± 9.13 | 27.18 ± 9.11 | 21.77 ± 3.71 | |
| ΡT 25 | 51.2 ± 6.37 | 23.26 ± 5.63 | 4.53 ± 1.71 | 79 ± 8.31 | 21 ± 8.31 | 25.31 ± 2.88 | |
| 60 min | Control | 30.18 ± 4.51 | 24.83 ± 5.43 | 9.73 ± 3.78 | 64.75 ± 7.79 | 33.6 ± 9.1 | 18.46 ± 2.87 |
| ΡT 10 | 49.8 ± 7.43 * | 20.95 ± 10.39 | 9.4 ± 7.89 | 80.15 ± 3.7 * | 19.86 ± 3.72 * | 22.68 ± 3.85 | |
| ΡT 25 | 45.96 ± 3.95 * | 24.26 ± 4.56 | 3.45 ± 1.80 | 73.68 ± 6.61 | 26.31 ± 6.61 | 27.11 ± 2.79 * | |
| 240 min | Control | 21.7 ± 3.16 | 22.44 ± 6.96 | 15.57 ± 6.52 | 54 ± 5.15 | 43.27 ± 8.58 | 18.95 ± 2.38 |
| ΡT 10 | 28.48 ± 4.64 * | 24.05 ± 4.97 | 7.83 ± 4.15 | 60.36 ± 7.28 * | 39.65 ± 7.28 * | 19.65 ± 3.13 * | |
| ΡT 25 | 32.95 ± 3.92 * | 28.05 ± 4.22 | 10.61 ± 3.11 | 71.61 ± 3.69 * | 28.73 ± 3.73 * | 24.96 ± 3.06 * |
| Time Point | Group | VCL (μm/s) | VSL (μm/s) | VAP (μm/s) | ALH |
|---|---|---|---|---|---|
| 0 min | Control | 62.43 ± 6.92 | 20.06 ± 2.29 | 34.41 ± 2.16 | 3.26 ± 0.62 |
| ΡT 10 | 58.03 ± 9.27 | 18.67 ± 2.05 | 30.73 ± 4.8 | 3.03 ± 0.28 | |
| ΡT 25 | 65.2 ± 9.39 | 20.03 ± 1.36 | 36.13 ± 1.87 | 3.27 ± 0.52 | |
| 60 min | Control | 54 ± 2.53 | 20.02 ± 3.67 | 32.75 ± 3.2 | 2.98 ± 0.29 |
| ΡT 10 | 59.48 ± 6.36 | 25.33 ± 7.72 | 37.7 ± 6.09 | 2.76 ± 0.28 | |
| ΡT 25 | 62.51 ± 4.64 | 24.9 ± 2.09 | 38.66 ± 2.85 | 2.95 ± 0.34 | |
| 240 min | Control | 53.75 ± 10.51 | 24.78 ± 7.73 | 34.92 ± 7.1 | 2.42 ± 0.23 |
| ΡT 10 | 48.95 ± 11.46 | 19.88 ± 7.55 | 31.55 ± 9.4 | 2.88 ± 0.47 | |
| ΡT 25 | 51.35 ± 10.99 | 21.31 ± 7.39 | 32.98 ± 7.79 | 3.01 ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapanidou, V.; Tsantarliotou, M.; Lavrentiadou, S.; Tzekaki, E.; Efraimidis, I.; Lialiaris, T.; Asimakopoulos, B. The Beneficial Effects of Pterostilbene on Post-Thawed Bovine Spermatozoa. Animals 2023, 13, 2713. https://doi.org/10.3390/ani13172713
Sapanidou V, Tsantarliotou M, Lavrentiadou S, Tzekaki E, Efraimidis I, Lialiaris T, Asimakopoulos B. The Beneficial Effects of Pterostilbene on Post-Thawed Bovine Spermatozoa. Animals. 2023; 13(17):2713. https://doi.org/10.3390/ani13172713
Chicago/Turabian StyleSapanidou, Vasiliki, Maria Tsantarliotou, Sophia Lavrentiadou, Elena Tzekaki, Ioannis Efraimidis, Theodoros Lialiaris, and Byron Asimakopoulos. 2023. "The Beneficial Effects of Pterostilbene on Post-Thawed Bovine Spermatozoa" Animals 13, no. 17: 2713. https://doi.org/10.3390/ani13172713





