Liver Transcriptome Shows Differences between Acute Hypoxia-Tolerant and Intolerant Individuals of Greater Amberjack (Seriola dumerili)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish Sampling and Hypoxia Treatment
2.3. RNA Sequencing
2.4. Differential Expression Analysis and Enrichment Analysis
2.5. Real-Time Quantitative PCR (qRT-RCR) Validation
3. Results
3.1. Sequencing Quality and Annotation Results
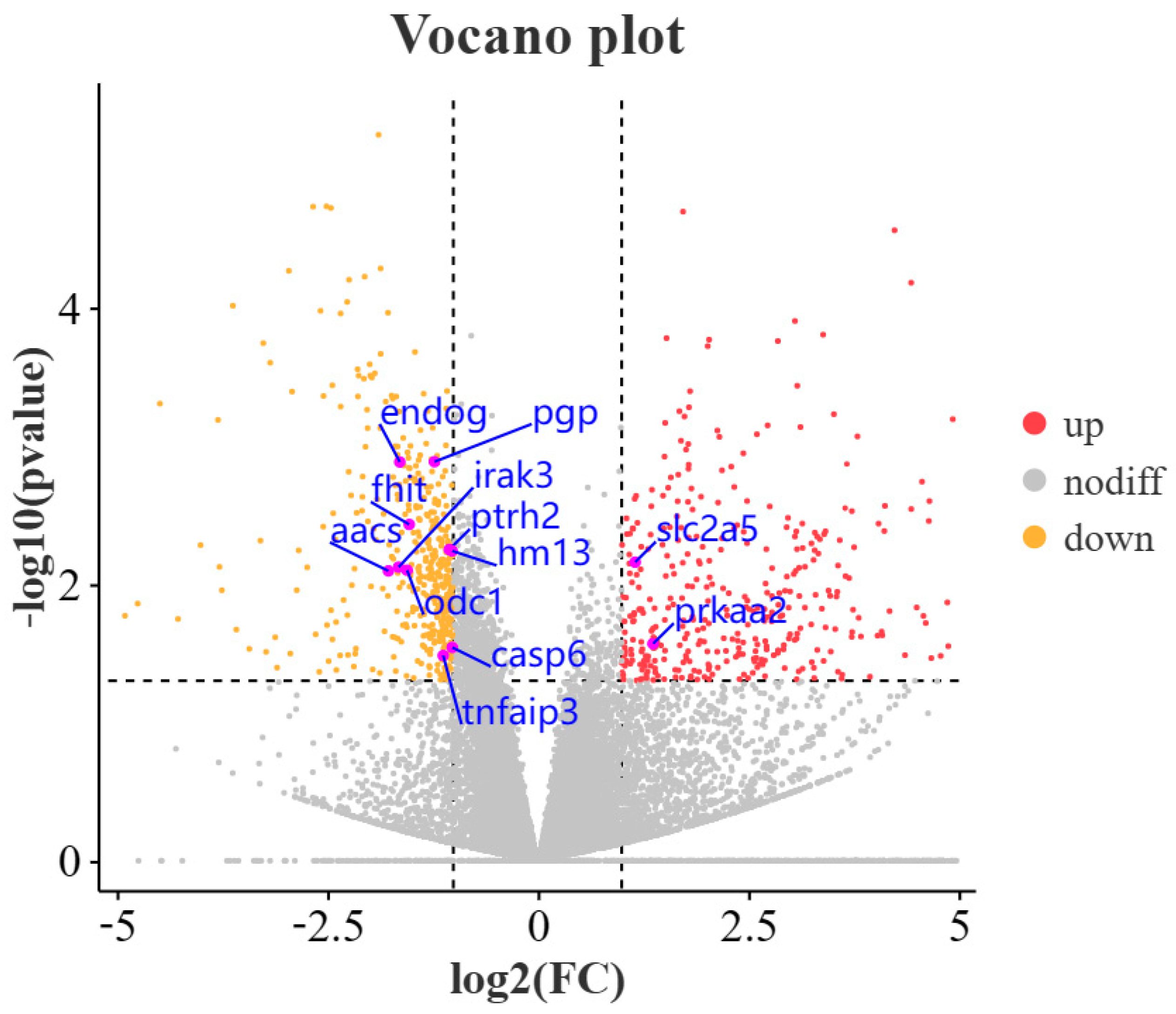
3.2. Differentially Expressed Gene Analysis
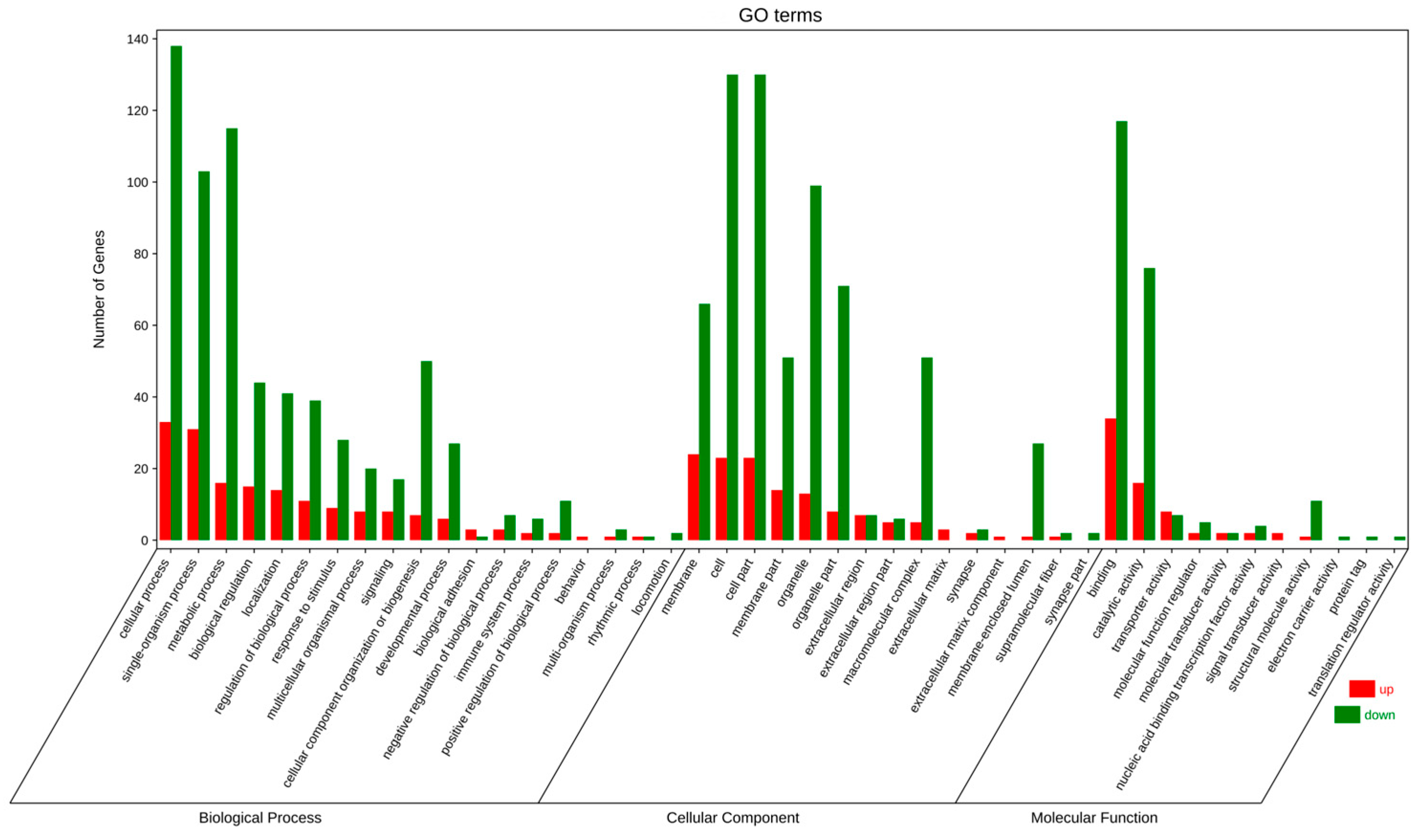
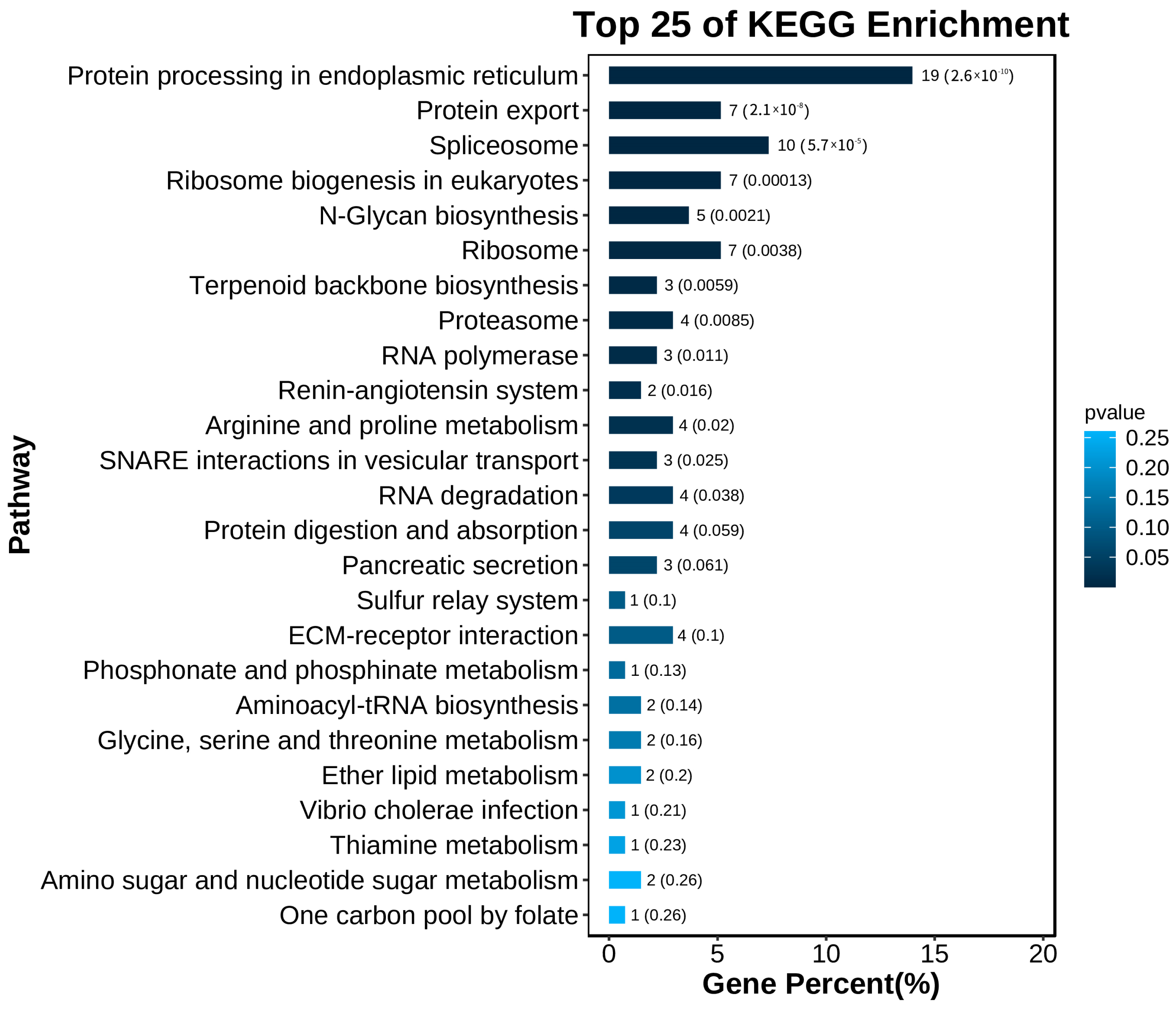
3.3. Overview of Differential Expression Genes and Enrichment Analysis
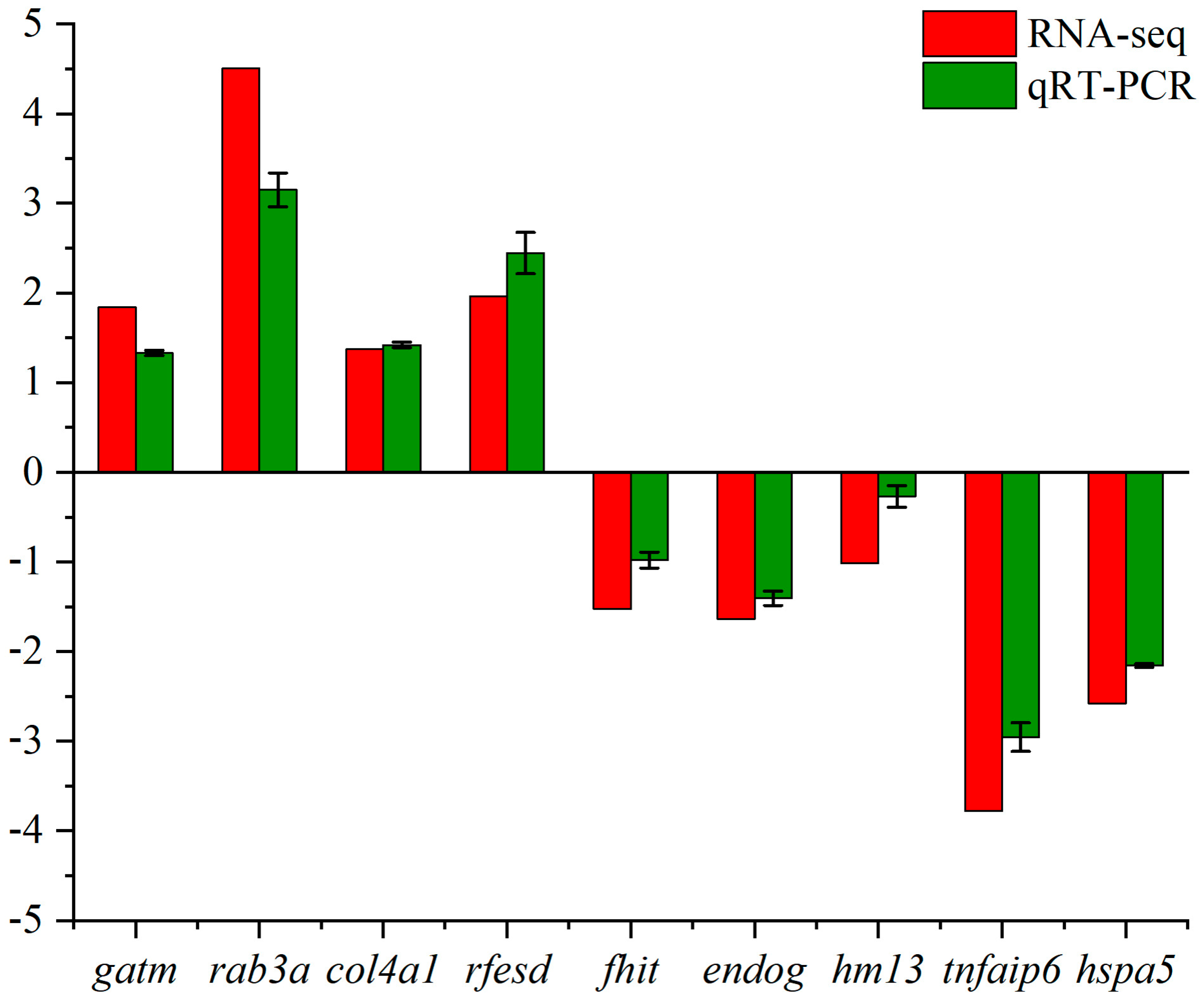
3.4. Validation of RNA-seq Results by qRT-PCR
4. Discussion
4.1. Glucose and Lipid Metabolism
4.2. Antioxidant Effect and Apoptosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz, R.J. Overview of Hypoxia around the World. J. Environ. Qual. 2001, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Diaz Robert, J.; Denise, L.B. Chapter 1 the Hypoxic Environment. In Fish Physiology; Jeffrey, G.R., Anthony, P.F., Colin, J.B., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 1–23. [Google Scholar]
- Levin, L.A.; Breitburg, D.L. Linking coasts and seas to address ocean deoxygenation. Nat. Clim. Change 2015, 5, 401–403. [Google Scholar] [CrossRef]
- Mahfouz, M.E.; Hegazi, M.M.; El-Magd, M.A.; Kasem, E.A. Metabolic and Molecular Responses in Nile Tilapia, Oreochromis Niloticus During Short and Prolonged Hypoxia. Mar. Freshw. Behav. Physiol. 2015, 48, 319–340. [Google Scholar] [CrossRef]
- Vanderplancke, G.; Claireaux, G.; Quazuguel, P.; Huelvan, C.; Corporeau, C.; Mazurais, D.; Zambonino-Infante, J.L. Exposure to Chronic Moderate Hypoxia Impacts Physiological and Developmental Traits of European Sea Bass (Dicentrarchus labrax) Larvae. Fish Physiol. Biochem. 2015, 41, 233–242. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Li, Y.; Pan, Y. Dynamic and systemic regulatory mechanisms in rainbow trout (Oncorhynchus mykiss) in response to acute hypoxia and reoxygenation stress. Aquaculture 2023, 572, 739540. [Google Scholar] [CrossRef]
- Zhao, L.L.; Sun, J.L.; Liang, J.; Liu, Q.; Luo, J.; Li, Z.Q.; Yan, T.M.; Zhou, J.; Yang, S. Enhancing lipid metabolism and inducing antioxidant and immune responses to adapt to acute hypoxic stress in Schizothorax prenanti. Aquaculture 2020, 519, 734933. [Google Scholar] [CrossRef]
- Cooper, R.U.; Clough, L.M.; Farwell, M.A.; West, T.L. Hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fish Leiostomus xanthurus. J. Exp. Mar. Biol. Ecol. 2002, 279, 1–20. [Google Scholar] [CrossRef]
- Shuang, L.; Chen, S.L.; Ren, C.; Su, X.L.; Xu, X.N.; Zheng, G.D.; Zou, S.M. Effects of Hypoxia and Reoxygenation on Oxidative Stress, Histological Structure, and Apoptosis in a New Hypoxia-Tolerant Variety of Blunt Snout Bream (Megalobrama amblycephala). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 278, 111358. [Google Scholar] [CrossRef]
- Luo, S.-Y.; Liu, C.; Ding, J.; Gao, X.-M.; Wang, J.-Q.; Zhang, Y.-B.; Du, C.; Hou, C.-C.; Zhu, J.-Q.; Lou, B.; et al. Scavenging reactive oxygen species is a potential strategy to protect Larimichthys crocea against environmental hypoxia by mitigating oxidative stress. Zool. Res. 2021, 42, 592–605. [Google Scholar] [CrossRef]
- Prchal, M.; D’Ambrosio, J.; Lagarde, H.; Lallias, D.; Patrice, P.; François, Y.; Poncet, C.; Desgranges, A.; Haffray, P.; Dupont-Nivet, M.; et al. Genome-Wide Association Study and Genomic Prediction of Tolerance to Acute Hypoxia in Rainbow Trout. Aquaculture 2023, 565, 739068. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, X.; Huang, R.; Liu, Q.; Liao, L.; Hu, Y.; He, K.; Zhang, X.; Guo, J.; Chen, S.; et al. Acute hypoxia promotes the liver angiogenesis of largemouth bass (Micropterus salmoides) by HIF—Dependent pathway. Fish Shellfish. Immunol. 2022, 131, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, F.; Xie, S.; Zhang, L. Acute hypoxia and reoxygenation: Effect on oxidative stress and hypoxia signal transduction in the juvenile yellow catfish (Pelteobagrus fulvidraco). Aquaculture 2020, 531, 735903. [Google Scholar] [CrossRef]
- Fakriadis, I.; Sigelaki, I.; Papadaki, M.; Papandroulakis, N.; Raftopoulos, A.; Tsakoniti, K.; Mylonas, C.C. Control of reproduction of greater amberjack Seriola dumerili reared in aquaculture facilities. Aquaculture 2019, 519, 734880. [Google Scholar] [CrossRef]
- Šegvić-Bubić, T.; Talijančić, I.; Žužul, I.; Žuvić, L.; Grubišić, L.; Izquierdo-Gomez, D. Culture of Seriola dumerili in a Marine Ecosystem: Insights from Genetic and Morphometric Fish Traits and Implications of Escape Events. Estuar. Coast. Shelf Sci. 2022, 278, 108115. [Google Scholar] [CrossRef]
- Papandroulakis, N.; Mylonas, C.; Maingot, E.; Divanach, P. First results of greater amberjack (Seriola dumerili) larval rearing in mesocosm. Aquaculture 2005, 250, 155–161. [Google Scholar] [CrossRef]
- Bordignon, F.; Trocino, A.; Sturaro, E.; Martínez-Llorens, S.; Tomas-Vidal, A.; Xiccato, G.; Berton, M. Fish oil substitution with vegetable oils in diets for greater amberjack (Seriola dumerili): A consequential life cycle assessment approach. Aquaculture 2023, 563, 738903. [Google Scholar] [CrossRef]
- He, Y.; Fu, Z.; Dai, S.; Yu, G.; Ma, Z.; Wang, X. Dietary curcumin supplementation enhances intestinal immunity and gill protection in juvenile greater amberjack (Seriola dumerili). Heliyon 2022, 8, e11887. [Google Scholar] [CrossRef]
- Fernández-Montero, Á.; Torrecillas, S.; Montero, D.; Acosta, F.; Prieto-Álamo, M.-J.; Abril, N.; Jurado, J. Proteomic profile and protease activity in the skin mucus of greater amberjack (Seriola dumerili) infected with the ectoparasite Neobenedenia girellae —An immunological approach. Fish Shellfish. Immunol. 2021, 110, 100–115. [Google Scholar] [CrossRef]
- Sun, J.L.; Liu, Y.F.; Jiang, T.; Li, Y.Q.; Song, F.B.; Wen, X.; Luo, J. Golden pompano (Trachinotus blochii) adapts to acute hypoxic stress by altering the preferred mode of energy metabolism. Aquaculture 2021, 542, 736842. [Google Scholar] [CrossRef]
- Liang, Y.S.; Wu, R.X.; Niu, S.F.; Miao, B.B.; Liang, Z.B.; Zhai, Y. Liver Transcriptome Analysis Reveals Changes in Energy Metabolism, Oxidative Stress, and Apoptosis in Pearl Gentian Grouper Exposed to Acute Hypoxia. Aquaculture 2022, 561, 738635. [Google Scholar] [CrossRef]
- Tian, C.; Lin, X.; Saetan, W.; Huang, Y.; Shi, H.; Jiang, D.; Chen, H.; Deng, S.; Wu, T.; Zhang, Y.; et al. Transcriptome analysis of liver provides insight into metabolic and translation changes under hypoxia and reoxygenation stress in silver sillago (Sillago sihama). Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 36, 100715. [Google Scholar] [CrossRef]
- Wang, X.-W.; Xiao, S.-S.; Zhang, R.; Liu, L.; Zhu, H. Physiological changes and transcriptional modulation of HIF-αs in Siberian sturgeon in response to hypoxia. Aquaculture 2021, 545, 737219. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, J.L.; Gu, Y.; Yao, F.C.; Liang, Y.S.; Liu, Y.F.; Zhang, K.X.; Song, F.B.; Zhou, L.; Wang, Z.W.; et al. Hypoxia alters glucose and lipid metabolisms in golden pompano (Trachinotus blochii). Aquaculture 2023, 562, 738747. [Google Scholar] [CrossRef]
- Araki, K.; Aokic, J.-Y.; Kawase, J.; Hamada, K.; Ozaki, A.; Fujimoto, H.; Yamamoto, I.; Usuki, H. Whole Genome Sequencing of Greater Amberjack (Seriola dumerili) for SNP Identification on Aligned Scaffolds and Genome Structural Variation Analysis Using Parallel Resequencing. Int. J. Genom. 2018, 2018, 7984292. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for Rna-Seq Data with Deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, M.; Rees, B.B. Oxygen-Dependent Gene Expression in Fishes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1079–R1090. [Google Scholar] [CrossRef]
- Zwarts, I.; van Zutphen, T.; Kruit, J.K.; Liu, W.; Oosterveer, M.H.; Verkade, H.J. Henriette Uhlenhaut, and Johan, W. Jonker. Identification of the Fructose Transporter Glut5 (Slc2a5) as a Novel Target of Nuclear Receptor Lxr. Sci. Rep. 2019, 9, 9299. [Google Scholar] [CrossRef]
- Barone, S.; Fussell, S.L.; Singh, A.K.; Lucas, F.; Xu, J.; Kim, C.; Wu, X.; Yu, Y.; Amlal, H.; Seidler, U.; et al. Slc2a5 (Glut5) Is Essential for the Absorption of Fructose in the Intestine and Generation of Fructose-induced Hypertension. J. Biol. Chem. 2009, 284, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Ton, C.; Stamatiou, D.; Liew, C.-C. Gene expression profile of zebrafish exposed to hypoxia during development. Physiol. Genom. 2003, 13, 97–106. [Google Scholar] [CrossRef]
- Yan, T.; Wu, H.; Xiao, Q.; Fu, H.; Luo, J.; Zhou, J.; Zhao, L.; Wang, Y.; Yang, S.; Sun, J.; et al. Acute hypoxic stress: Effect on blood parameters, antioxidant enzymes, and expression of HIF-1alpha and GLUT-1 genes in largemouth bass (Micropterus salmoides). Fish Shellfish. Immunol. 2017, 67, 449–458. [Google Scholar]
- Hall, J.R.; Richards, R.C.; MacCormack, T.J.; Ewart, K.V.; Driedzic, W.R. Cloning of Glut3 Cdna from Atlantic Cod (Gadus morhua) and Expression of Glut1 and Glut3 in Response to Hypoxia. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2005, 1730, 245–252. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Dong, X.; Wang, H.; Tan, B.; Zhang, S. Molecular Cloning, Characterization and Expression Analysis of Glucose Transporters from Rachycentron Canadum. Aquac. Res. 2019, 50, 2505–2518. [Google Scholar] [CrossRef]
- Mugabo, Y.; Zhao, S.; Seifried, A.; Gezzar, S.; Al-Mass, A.; Zhang, D.; Lamontagne, J.; Attane, C.; Poursharifi, P.; Iglesias, J.; et al. Identification of a mammalian glycerol-3-phosphate phosphatase: Role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc. Natl. Acad. Sci. USA 2016, 113, E430–E439. [Google Scholar] [CrossRef]
- Gracey, A.Y.; Lee, T.-H.; Higashi, R.M.; Fan, T. Hypoxia-induced mobilization of stored triglycerides in the euryoxic goby Gillichthys mirabilis. J. Exp. Biol. 2011, 214, 3005–3012. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Qi, C.; Li, E.; Du, Z.; Qin, J.G.; Chen, L. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 2018, 495, 187–195. [Google Scholar] [CrossRef]
- Lai, X.-X.; Zhang, C.-P.; Wu, Y.-X.; Yang, Y.; Zhang, M.-Q.; Qin, W.-J.; Wang, R.-X.; Shu, H. Comparative transcriptome analysis reveals physiological responses in liver tissues of Epinephelus coioides under acute hypoxia stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 43, 101005. [Google Scholar] [CrossRef]
- Carling, D. The Amp-Activated Protein Kinase Cascade—A Unifying System for Energy Control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef]
- Hwang, S.L.; Chang, H.W. Natural Vanadium-Containing Jeju Ground Water Stimulates Glucose Uptake through the Activation of Amp-Activated Protein Kinase in L6 Myotubes. Mol. Cell. Biochem. 2012, 360, 401–409. [Google Scholar] [CrossRef]
- Hasegawa, S.; Noda, K.; Maeda, A.; Matsuoka, M.; Yamasaki, M.; Fukui, T. Acetoacetyl-CoA synthetase, a ketone body-utilizing enzyme, is controlled by SREBP-2 and affects serum cholesterol levels. Mol. Genet. Metab. 2012, 107, 553–560. [Google Scholar] [CrossRef]
- Luo, S.Y.; Wang, J.Q.; Liu, C.; Gao, X.M.; Zhang, Y.B.; Ding, J.; Hou, C.C.; Zhu, J.Q.; Lou, B.; Shen, W.L.; et al. Hif-1α/Hsf1/Hsp70 Signaling Pathway Regulates Redox Homeostasis and Apoptosis in Large Yellow Croaker (Larimichthys crocea) under Environmental Hypoxia. Zool. Res. 2021, 42, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Leveelahti, L.; Rytkönen, K.T.; Renshaw, G.M.C.; Nikinmaa, M. Revisiting redox-active antioxidant defenses in response to hypoxic challenge in both hypoxia-tolerant and hypoxia-sensitive fish species. Fish Physiol. Biochem. 2013, 40, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Prokop, J.W.; Bupp, C.P.; Frisch, A.; Bilinovich, S.M.; Campbell, D.B.; Vogt, D.; Schultz, C.R.; Uhl, K.L.; VanSickle, E.; Rajasekaran, S.; et al. Emerging Role of ODC1 in Neurodevelopmental Disorders and Brain Development. Genes 2021, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Yuhong, L.; Zhengzhong, B.; Feng, T.; Quanyu, Y.; Ge, R.-L. L-arginine Attenuates Hypobaric Hypoxia-Induced Increase in Ornithine Decarboxylase 1. Wilderness Environ. Med. 2017, 28, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Huang, G.; Peng, Y.; Liu, X. Effect of Hypoxia on Growth Performance, Energy Metabolism and Oxidative Stress of Mugil Cephalus. J. Fish. China 2016, 40, 73–82. [Google Scholar]
- Beyaert, R.; Heyninck, K.; Van Huffel, S. A20 and A20-Binding Proteins as Cellular Inhibitors of Nuclear Factor-Κb-Dependent Gene Expression and Apoptosis. Biochem. Pharmacol. 2000, 60, 1143–1151. [Google Scholar] [CrossRef]
- Maria, B.; Maria, M.C.; Antonio, B.; Simona, M.; Rosaria, A.; Andrea, S.; Giulia, M.; Marianna, D.C.; Mario, S. Chemical and biochemical responses to sub−lethal doses of mercury and cadmium in gilthead seabream (Sparus aurata). Chemosphere 2022, 307, 135822. [Google Scholar] [CrossRef]
- Nie, H.; Wang, H.; Jiang, K.; Yan, X. Transcriptome Analysis Reveals Differential Immune Related Genes Expression in Ruditapes philippinarum under Hypoxia Stress: Potential Hif and Nf-Κb Crosstalk in Immune Responses in Clam. BMC Genom. 2020, 21, 318. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, J.; Sun, Y.; Habib, Y.J.; Yang, H.; Zhang, Z.; Wang, Y. Integrative transcriptome analysis and discovery of genes involving in immune response of hypoxia/thermal challenges in the small abalone Haliotis diversicolor. Fish Shellfish. Immunol. 2018, 84, 609–626. [Google Scholar] [CrossRef]
- ScScherer, H.U.; van der Linden, M.P.; Kurreeman, F.A.; Stoeken-Rijsbergen, G.; le Cessie, S.; Huizinga, T.W.; van der Helm-van Mil, A.H.; Toes, R.E. Association of the 6q23 Region with the Rate of Joint Destruction in Rheumatoid Arthritis. Ann. Rheum. Dis. 2010, 69, 567. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Xu, L.; Li, W.; Shang, R.; Chen, J.; Hu, F.; Wang, S.; Liu, Q.; Wu, C.; Zhou, R.; et al. Comparative analysis of liver transcriptomes associated with hypoxia tolerance in the gynogenetic blunt snout bream. Aquaculture 2020, 523, 735163. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hernandez, L.D.; Galán, J.E.; Janeway, C.A.; Medzhitov, R.; Flavell, R.A. Ruslan Medzhitov, and Richard, A. Flavell. Irak-M Is a Negative Regulator of Toll-Like Receptor Signaling. Cell 2002, 110, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Z.; Wang, Z.; Zhang, X.; Dai, X.; Zhou, G.; Ding, Q. Histocompatibility Minor 13 (Hm13), Targeted by Mir-760, Exerts Oncogenic Role in Breast Cancer by Suppressing Autophagy and Activating Pi3k-Akt-Mtor Pathway. Cell Death Dis. 2022, 13, 728. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, X.; Chaggan, C.; Liao, Z.; In Wong, K.; He, F.; Singh, S.; Loomba, R.; Karin, M.; Joseph, L.; et al. An Ampk–Caspase-6 Axis Controls Liver Damage in Nonalcoholic Steatohepatitis. Science 2020, 367, 652–660. [Google Scholar] [CrossRef]
- Jan, Y.; Matter, M.; Pai, J.T.; Chen, Y.L.; Pilch, J.; Komatsu, M.; Ong, E.; Fukuda, M.; Ruoslahti, E. A Mitochondrial Protein, Bit1, Mediates Apoptosis Regulated by Integrins and Groucho/Tle Corepressors. Cell 2004, 116, 751–762. [Google Scholar] [CrossRef]
- Semba, S.; Trapasso, F.; Fabbri, M.; McCorkell, K.A.; Volinia, S.; Druck, T.; Iliopoulos, D.; Pekarsky, Y.; Ishii, H.; Garrison, P.N.; et al. Fhit Modulation of the Akt-Survivin Pathway in Lung Cancer Cells: Fhit-Tyrosine 114 (Y114) Is Essential. Oncogene 2006, 25, 2860–2872. [Google Scholar] [CrossRef]
| Sample | Raw_Reads | Clean_Reads | Clean_Bases | Error_Rate | Q20 | Q30 | GC_Pct (%) |
|---|---|---|---|---|---|---|---|
| 1 | 47,274,858 | 44,974,632 | 6.75 G | 0.03 | 96.49 | 90.94 | 48.85 |
| 2 | 46,793,042 | 43,993,002 | 6.6 G | 0.03 | 96.53 | 90.92 | 49.15 |
| 3 | 45,919,032 | 43,032,780 | 6.45 G | 0.03 | 96.9 | 91.75 | 49.65 |
| 4 | 47,007,730 | 44,086,276 | 6.61 G | 0.03 | 96.37 | 90.58 | 48.31 |
| 5 | 43,530,104 | 41,775,200 | 6.27 G | 0.03 | 96.81 | 91.54 | 48.85 |
| 6 | 46,781,622 | 43,964,854 | 6.59 G | 0.03 | 96.61 | 91.16 | 49.68 |
| 7 | 47,396,944 | 44,495,152 | 6.67 G | 0.03 | 96.79 | 91.46 | 49.65 |
| 8 | 45,488,812 | 42,321,398 | 6.35 G | 0.03 | 96.85 | 91.6 | 49.67 |
| 9 | 50,102,200 | 46,354,446 | 6.95 G | 0.03 | 96.95 | 91.87 | 49.44 |
| 10 | 45,492,008 | 43,214,822 | 6.48 G | 0.03 | 96.64 | 91.22 | 49.56 |
| 11 | 51,245,632 | 46,862,522 | 7.03 G | 0.03 | 96.93 | 91.8 | 49.66 |
| 12 | 47,303,766 | 44,999,926 | 6.75 G | 0.03 | 96.67 | 91.23 | 49.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yang, Y.; Wang, T.; Zhang, W.; Hua, S.; Ruan, Q.; Wang, X.; Zhu, C.; Meng, Z. Liver Transcriptome Shows Differences between Acute Hypoxia-Tolerant and Intolerant Individuals of Greater Amberjack (Seriola dumerili). Animals 2023, 13, 2717. https://doi.org/10.3390/ani13172717
Li D, Yang Y, Wang T, Zhang W, Hua S, Ruan Q, Wang X, Zhu C, Meng Z. Liver Transcriptome Shows Differences between Acute Hypoxia-Tolerant and Intolerant Individuals of Greater Amberjack (Seriola dumerili). Animals. 2023; 13(17):2717. https://doi.org/10.3390/ani13172717
Chicago/Turabian StyleLi, Duo, Yang Yang, Tong Wang, Weiwei Zhang, Sijie Hua, Qingxin Ruan, Xi Wang, Chunhua Zhu, and Zining Meng. 2023. "Liver Transcriptome Shows Differences between Acute Hypoxia-Tolerant and Intolerant Individuals of Greater Amberjack (Seriola dumerili)" Animals 13, no. 17: 2717. https://doi.org/10.3390/ani13172717





