Himalayan Marmot (Marmota himalayana) Redistribution to High Latitudes under Climate Change
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
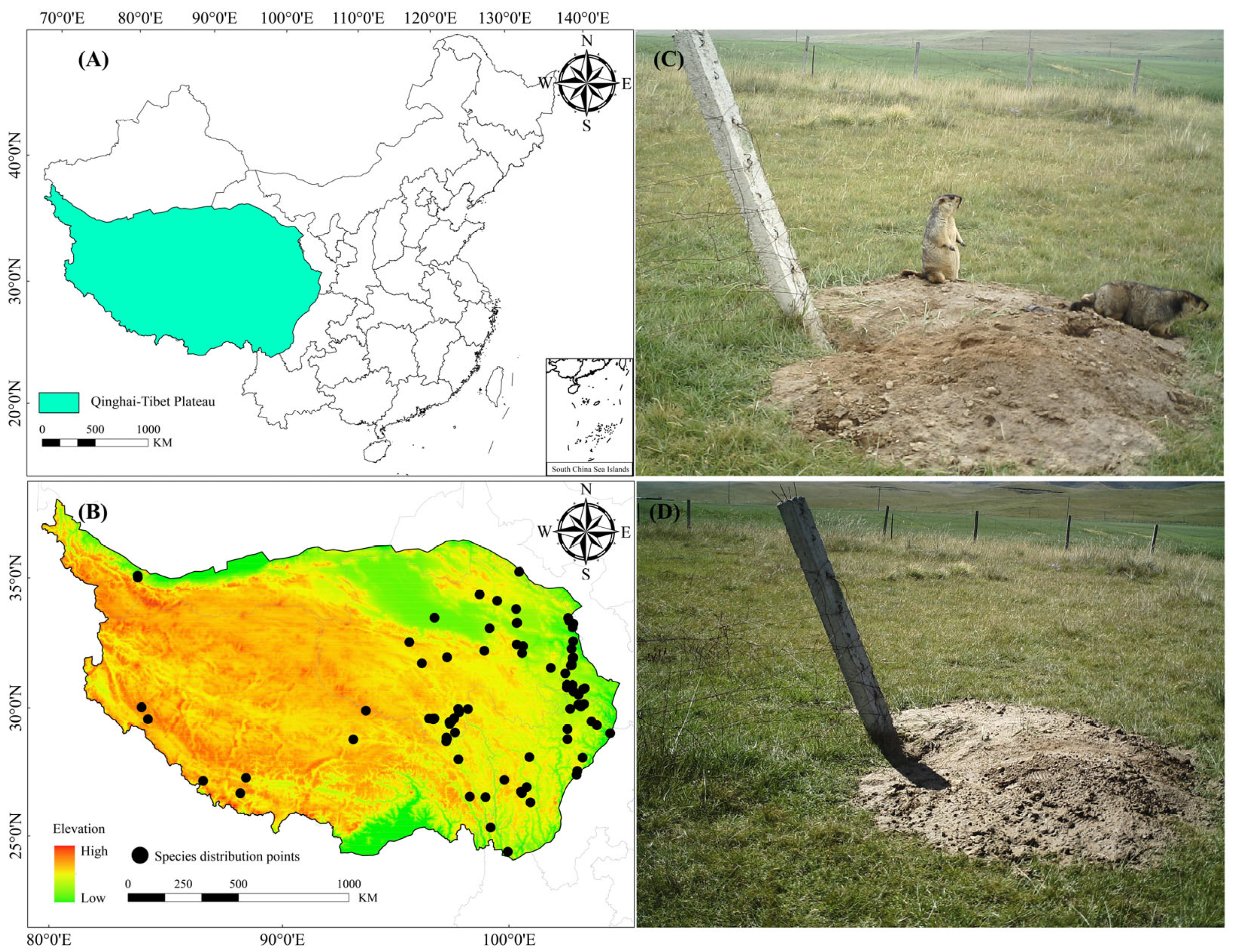
2.1. Study Area
2.2. Occurrence Data Collection
2.3. Environmental Variables
2.4. Suitable Areas Distribution Modeling
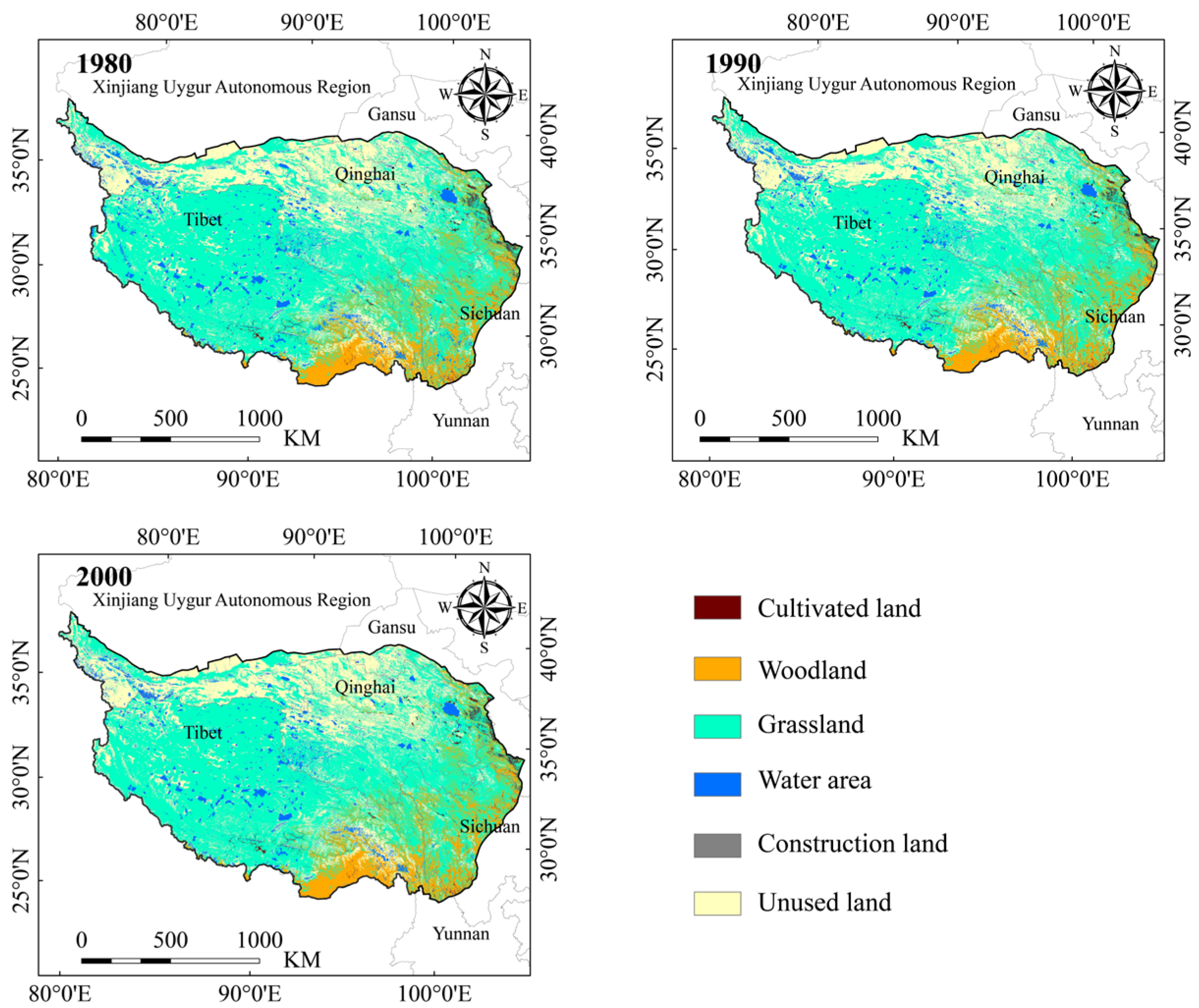
2.5. Superposition Analysis of High Suitability Area for Himalayan Marmots and Land-Use Types
2.6. Migration Trend Analysis of Himalayan Marmot
3. Results
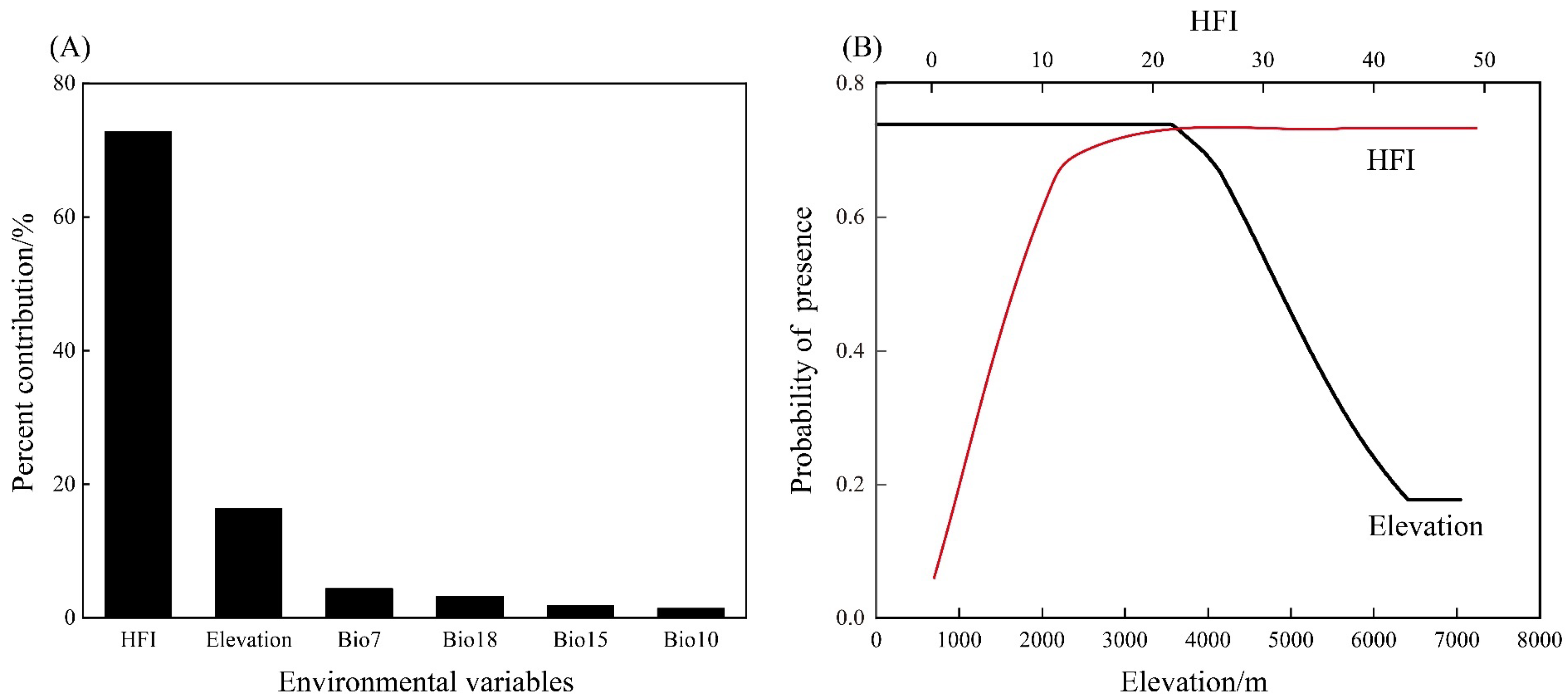
3.1. Analyses of Environmental Variables
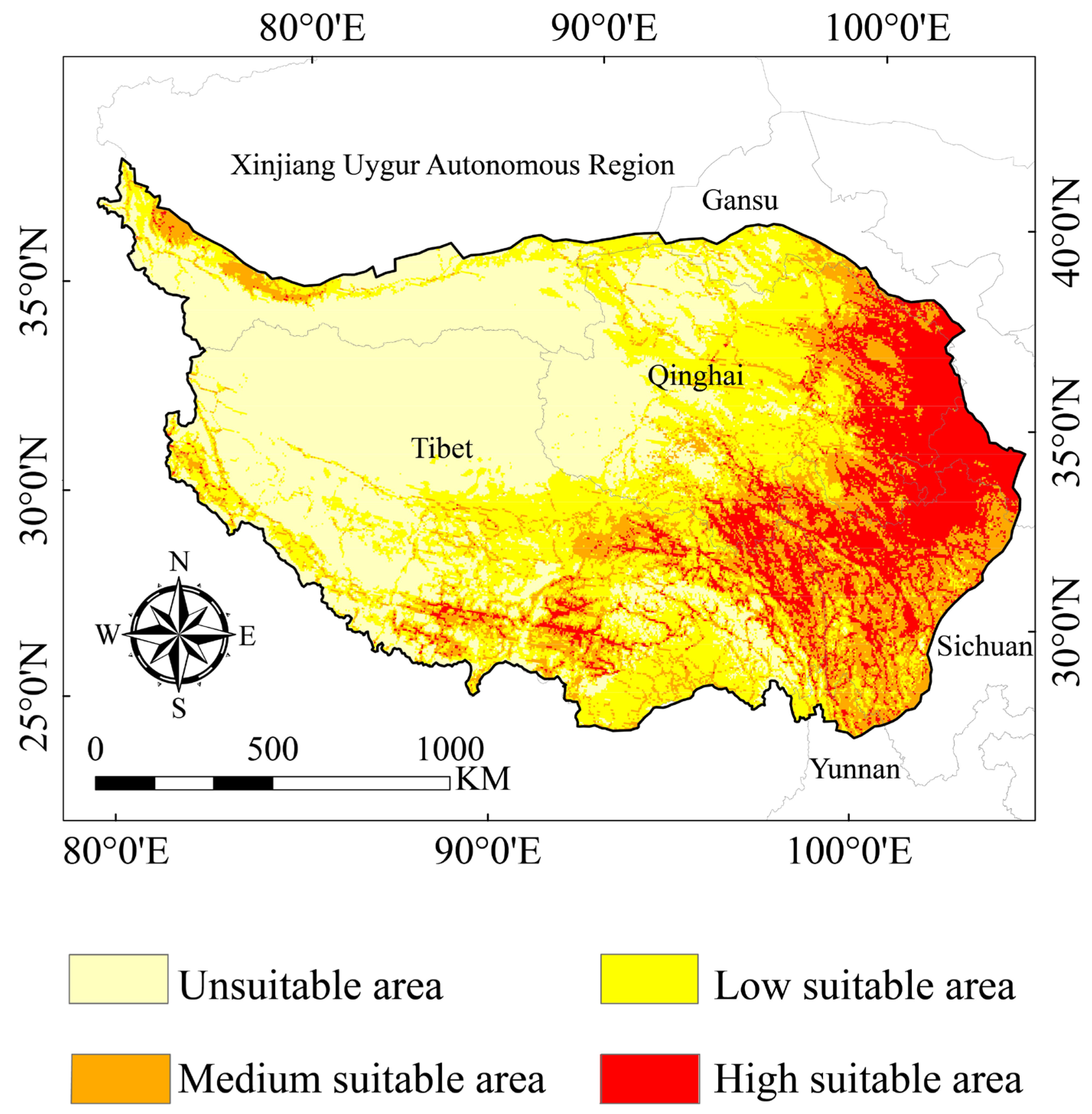
3.2. Suitable Areas for the Himalayan Marmot
3.3. Superposition Analysis of Land-Use Types and High Suitable Area
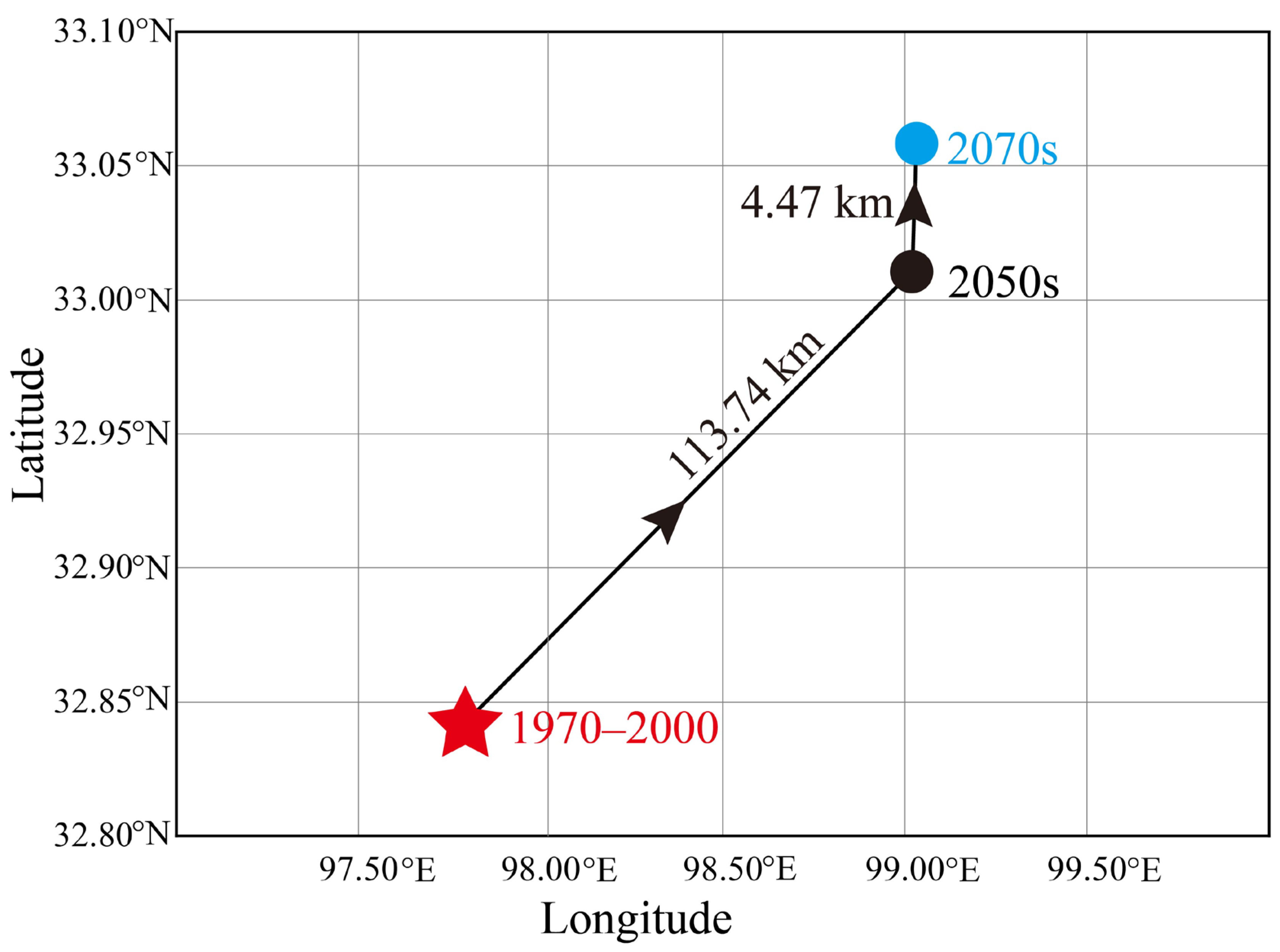
3.4. Suitable Habitat Area and Centroid Changes under Future Climate Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arneth, A.; Shin, Y.J.; Leadley, P.; Rondinini, C.; Bukvareva, E.; Kolb, M.; Midgley, G.F.; Oberdorff, T.; Palomo, I.; Saito, O. Post-biodiversity targets need to embrace climate change. Proc. Natl. Acad. Sci. USA 2020, 117, 30882–30891. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, 6089–6096. [Google Scholar] [CrossRef]
- Antão, L.H.; Weigel, B.; Strona, G.; Hällfors, M.; Kaarlejärvi, E.; Dallas, T.; Opedal, H.Ø.; Heliölä, J.; Henttonen, H.; Huitu, O. Climate change reshuffles northern species within their niches. Nat. Clim. Chang. 2022, 12, 587–592. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1297–1313. [Google Scholar] [CrossRef]
- Ye, X.L.; Wu, X.R.; Zhao, X.M. Incorporating interspecific relationships into species distribution models can better assess the response of species to climate change, a case study of two Chinese primates. Ecol. Indic. 2022, 144, 109255. [Google Scholar] [CrossRef]
- Grinder, R.M.; Wiens, J.J. Niche width predicts extinction from climate change and vulnerability of tropical species. Glob. Chang. Biol. 2023, 29, 618–630. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate change 2021: The physical science basis. In Future Global Climate: Scenario-42 Based Projections and Near-Term Information; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Lee, H.N. Estimating Extinction Risk from Climate Change. In Climate Change Biology, 3rd ed.; Academic Press: New York, NY, USA, 2022. [Google Scholar]
- Zarnetske, P.L.; Skelly, D.K.; Urban, M.C. Ecology. Biotic multipliers of climate change. Science 2012, 336, 1516–1518. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T.; et al. Global imprint of climate change on marine life. Nature Clim. Chang. 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Marca, E.L.; Lips, K.R.; Lötters, S.; Puschendorf, R.; Ibanez, R.; Rueda-Almonacid, J.V.; Schulte, R.; Marty, C.; Castro-Herrera, F.; Manzanilla-Puppo, J.; et al. Catastrophic population declines and extinctions in neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 2005, 37, 190–201. [Google Scholar] [CrossRef]
- Thomas, C.D.; Franco, A.M.; Hill, J.K. Range retractions and extinction in the face of climate warming. Trends Ecol. Evol. 2006, 21, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Lambers, J.H. Ecology. Extinction risks from climate change. Science 2015, 348, 501–502. [Google Scholar] [CrossRef]
- Sohiba, H.; Olimjon Qizi, S. Population dynamics of small mammals as a bio indicator of ecosystem status. Int. J. Sci. Res. 2020, 9, 1351–1352. [Google Scholar]
- Smith, A.; Palden, B.; Wilson, M.; Hogan, B. Functional-trait ecology of the Himalayan marmot Ochotona curzoniae (Hodgson, 1858) in the Qinghai-Tibetan Plateau ecosystem. Integr. Zool. 2018, 14, 87–103. [Google Scholar] [CrossRef]
- Krebs, C.; Boonstra, R.; Boutin, S.; Sinclair, A.; Smith, J.; Scott, B.; Martin, K.; Donoghue, M.; Turkington, R. Trophic dynamics of the boreal forests of the Kluane region. Arctic 2014, 67, 71–81. [Google Scholar] [CrossRef]
- McGill, B. Biodiversity: Land use matters. Nature 2015, 520, 38–39. [Google Scholar] [CrossRef]
- Di Marco, M.; Boitani, L.; Mallon, D.; Hoffmann, M.; Iacucci, A.; Meijaard, E.; Visconti, P.; Schipper, J.; Rondinini, C. A retrospective evaluation of the global decline of carnivores and ungulates. Conserv. Biol. 2014, 28, 1109–1118. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef]
- Tucker, M.A.; Böhning-Gaese, K.; Fagan, W.F.; Fryxell, J.M.; Van Moorter, B.; Alberts, S.C.; Ali, A.H.; Allen, A.M.; Attias, N.; Avgar, T.; et al. Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 2018, 359, 466–469. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Fox, A.D.; Fuller, R.; Griffin, L.; Mitchell, C.; Zhao, Y.; Moon, O.K.; Cabot, D.; Xu, Z.; et al. Stochastic simulations reveal few green wave surfing populations among spring migrating herbivorous waterfowl. Nat. Commun. 2019, 10, 2187. [Google Scholar] [CrossRef] [PubMed]
- Nienke, C.U.; Esiere, R.K.; Nnokam, B.A.; Nwalozie, R. Plague: Symptoms, transmission, diagnosis, prevention and treatment. S. Asian J. Res. Microbiol. 2023, 16, 10–16. [Google Scholar] [CrossRef]
- Eisen, R.J.; Enscore, R.E.; Biggerstaff, B.J.; Reynolds, P.J.; Ettestad, P.; Brown, T.; Pape, J.; Tanda, D.; Levy, C.E.; Engelthaler, D.M.; et al. Human plague in the southwestern United States, 1957–2004: Spatial models of elevated risk of human exposure to Yersinia pestis. J. Med. Entomol. 2007, 44, 530–537. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Atshabar, B.B.; Begon, M.; Belmain, S.R.; Bertherat, E.; Carniel, E.; Gage, K.L.; Leirs, H.; Rahalison, L. Plague: Past, present, and future. PLoS Med. 2008, 5, e3. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.B.; Zhang, C.H. The distribution of plague natural foci in the world and the prevalence of human plague. Chin. J. Endemiol. 2009, 28, 357–360. [Google Scholar]
- Tang, X.Y.; Wang, M.; Chen, H.J.; Li, H.Y.; Cai, J.Z.; He, D.L.; Cui, B.Z. Epidemiological characteristics of plague in Qinghai province based on geographic information system. Chin. J. Vector Biol. Control. 2018, 29, 60–64. [Google Scholar]
- Lu, L.; Ren, Z.; Yue, Y.; Yu, X.; Lu, S.; Li, G.; Li, H.; Wei, J.; Liu, J.; Mu, Y.; et al. Niche modeling predictions of the potential distribution of Marmota himalayana, the host animal of plague in Yushu County of Qinghai. BMC Public Health 2016, 16, 183. [Google Scholar] [CrossRef]
- Yang, L.S.; Chen, R.G.; Wang, W.Y.; Tan, J.A. The temporal and spatial distribution of the plague foci since 1840 in China. Geogr. Res. 2000, 19, 243–248. [Google Scholar]
- Wen, X.; Zhao, G.; Cheng, X.; Chang, G.; Dong, X.; Lin, X. Prediction of the potential distribution pattern of the great gerbil (Rhombomys opimus) under climate change based on ensemble modelling. Pest Manag. Sci. 2022, 78, 3128–3134. [Google Scholar] [CrossRef]
- Wu, H.L.; Wu, H.S.; Wang, Y.S.; Li, H.Y.; Tian, F.Z.; Zhou, K.Z.; Qi, Z.Z.; Zhang, Y.Q.; Zhang, Q.W.; Zhang, X.F. Analysis of Himalayan marmot distribution and plague risk in Qinghai province of China using the “3S” technology. Sci. Rep. 2023, 13, 1924. [Google Scholar] [CrossRef]
- Shrestha, T. Marmota himalayana (Errata Version Published in 2017). The IUCN Red List of Threatened Species. 2016. Available online: https://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T12826A22258911.en (accessed on 25 June 2023).
- Yang, L. Analysis on the present situation and countermeasures of plague control in Qinghai province. Chin. J. Endemiol. 2007, 26, 464. [Google Scholar]
- Yu, D.Z. Gurrent status and development trend of plague surveillance in China. Dis. Surveill. 2007, 22, 433–435. [Google Scholar]
- Wang, S.L.; Hou, F.J. Burrow characteristics and ecological significance of Marmota himalayana in the northeastern Qinghai-Tibetan Plateau. Ecol. Evol. 2021, 11, 9100–9109. [Google Scholar] [CrossRef] [PubMed]
- Ballová, Z.; Pekarik, L.; Píš, V.; Šibík, J. How much do ecosystem engineers contribute to landscape evolution? A case study on Tatra marmots. Catena 2019, 182, 104121. [Google Scholar] [CrossRef]
- Li, L.L.; Zhang, Y.L.; Wu, J.S.; Li, S.C.; Zhang, B.H.; Zu, J.X.; Zhang, H.M.; Ding, M.J.; Paudel, B. Increasing sensitivity of alpine grasslands to climate variability along an elevational gradient on the Qinghai-Tibet Plateau. Sci. Total. Environ. 2019, 678, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D. The system of physico-geographical regions of the Qinghai-Xizang (Tibet) Plateau. Sci. China Earth Sci. 1996, 39, 410–417. [Google Scholar]
- Feng, W.; Lu, H.W.; Yao, T.C.; Yu, Q. Drought characteristics and its elevation dependence in the Qinghai-Tibet plateau during the last half-century. Sci. Rep. 2020, 10, 14323. [Google Scholar] [CrossRef]
- Ni, J. A simulation of biomes on the Tibetan Plateau and their responses to global climate change. Mt. Res. Dev. 2000, 20, 80–89. [Google Scholar] [CrossRef]
- Ni, J.; Herzschuh, U. Simulating biome distribution on the Tibetan Plateau using a modified global vegetation model. Arct. Antarct. Alp. Res. 2011, 43, 429–441. [Google Scholar] [CrossRef]
- Favre, A.; Päckert, M.; Pauls, S.U.; Jähnig, S.C.; Uhl, D.; Michalak, I.; Muellner-Riehl, A.N. The role of the uplift of the Qinghai-Tibetan plateau for the evolution of Tibetan biotas. Biol. Rev. 2015, 90, 236–253. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Wang, Z.; Shrestha, N.; Liu, J. Species divergence with gene flow and hybrid speciation on the Qinghai-Tibet Plateau. New Phytol. 2022, 234, 392–404. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, J.; Gao, H.; Cai, Z.; Zhou, X.; Li, S.; Zhang, T. Musk deer (Moschus spp.) face redistribution to higher elevations and latitudes under climate change in China. Sci. Total. Environ. 2020, 704, 135335. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Hamilton, H.H.; Kueppers, L.M. Back to the future: Using historical climate variation to project near-term shifts in habitat suitable for coast redwood. Glob. Chang. Biol. 2015, 21, 4141–4152. [Google Scholar] [CrossRef]
- Warren, D.; Glor, R.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jiang, F.; Li, G.Y.; Qin, W.; Wu, T.; Xu, F.; Hou, Y.S.; Song, P.F.; Cai, Z.Y.; Zhang, T.Z. The four antelope species on the Qinghai-Tibet plateau face habitat loss and redistribution to higher latitudes under climate change. Ecol. Indic. 2021, 123, 107337. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.G.; Li, R.M.; Wang, X.L.; Cheng, J.H.; Yang, Q.L.; Kong, H. AHP-GIS and MaxEnt for delineation of potential distribution of Arabica coffee plantation under future climate in Yunnan, China. Ecol. Indic. 2021, 132, 108339. [Google Scholar] [CrossRef]
- Liu, J.Q.; Liu, Y.P.; Zhang, J.Y.; Wen, Y.X.J.; Chu, G.M. Analysis of the potential distribution and suitability of five Kalidium species. Pratacultural Sci. 2022, 39, 33–148. [Google Scholar]
- Peng, S.J.; Zhang, J.; Zhang, X.L.; Li, Y.Q.; Liu, Y.P.; Wang, Z.H. Conservation of woody species in China under future climate and land-cover changes. J. Appl. Ecol. 2022, 59, 141–152. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Global terrestrial Human Footprint maps for 1993 and 2009. Sci. Data 2016, 3, 160067. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papes, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Ruan, L.Z.; Xu, W.; Han, Y.Q.; Zhu, C.Y.; Guan, B.C.; Xu, C.X.; Gao, B.; Zhao, D.Q. Gene flow from multiple sources maintains high genetic diversity and stable population history of Common Moorhen Gallinula chloropus in China. Ibis 2018, 160, 855–869. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.; Elith, J.; Graham, C.; Phillips, S.J.; Peterson, A. What matters for predicting the occurrences of trees: Techniques, data, or species’ characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2015, 5, 1198–1205. [Google Scholar] [CrossRef]
- Hope, A.G.; Malaney, J.L.; Bell, K.C.; Salazar-Miralles, F.; Chavez, A.S.; Barber, B.R.; Cook, J.A. Revision of widespread red squirrels (genus: Tamiasciurus) highlights the complexity of speciation within North American forests. Mol. Phylogenet. Evol. 2016, 100, 170–182. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Lay, G.L.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Sahlean, T.C.; Gherghel, I.; Papeş, M.; Strugariu, A.; Zamfirescu, Ş.R. Refining climate change projections for organisms with low dispersal abilities: A case study of the Caspian whip snake. PLoS ONE 2014, 9, e91994. [Google Scholar] [CrossRef]
- Johnson, T.L.; Bjork, J.K.; Neitzel, D.F.; Dorr, F.M.; Schiffman, E.K.; Eisen, R.J. Habitat Suitability Model for the Distribution of Ixodes scapularis (Acari: Ixodidae) in Minnesota. J. Med. Entomol. 2016, 53, 598–606. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Xu, X.L.; Liu, J.Y.; Zhang, S.W.; Li, R.D.; Yan, C.Z.; Wu, S.X. China’s Multi-Period Land Use Land Cover Remote Sensing Monitoring Dataset (CNLUCC). In Data Registration and Publishing System of the Resource and Environmental Science Data Center of the Chinese Academy of Sciences; Resources and Environment Science Data Registration and Publishing System: Beijing, China, 2018. [Google Scholar]
- Cong, M.; Xu, Y.; Tang, L.; Yang, W.; Jian, M. Predicting the dynamic distribution of Sphagnum bogs in China under climate change since the last interglacial period. PLoS ONE 2020, 15, e0230969. [Google Scholar] [CrossRef]
- Hu, X.G.; Jin, Y.; Wang, X.R.; Mao, J.F.; Li, Y. Predicting impacts of future climate change on the distribution of the widespread conifer Platycladus orientalis. PLoS ONE 2015, 10, e0132326. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Wang, Z.C.; Yao, B.H.; An, K.; Pu, Q.S.; Zhang, C.J.; Zhang, Z.M.; Hou, Q.Q.; Zhang, D.G.; Su, J.H. Environmental and climatic drivers of phenotypic evolution and distribution changes in a widely distributed subfamily of subterranean mammals. Sci. Total Environ. 2023, 878, 163177. [Google Scholar] [CrossRef] [PubMed]
- Armitage, K.B. The evolution, ecology, and systematics of marmots. Oecologia Mont. 2000, 9, 1–18. [Google Scholar]
- Nikol’skii, A.; Ulak, A. Key factors determining the ecological niche of the Himalayan marmot, Marmota himalayana Hodgson (1841). Russ. J. Ecol. 2006, 37, 46–52. [Google Scholar] [CrossRef]
- Gao, M.X.; Li, X.W.; Cao, C.X.; Zhang, H.; Li, Q.; Zhou, H.; He, Q.S.; Xu, M.; Zhao, J.; Zheng, S.; et al. Spatial prediction and analysis of Himalayan marmot plague natural epidemic foci in China based on HJ-1 satellite data. Sci. China Earth Sci. 2010, 53, 8–15. [Google Scholar] [CrossRef]
- Qian, Q.; Zhao, J.; Fang, L.Q.; Zhou, H.; Zhang, W.Y.; Wei, L.; Yang, H.; Yin, W.W.; Cao, W.C.; Li, Q. Mapping risk of plague in Qinghai-Tibetan Plateau, China. BMC Infect. Dis. 2014, 1, 382. [Google Scholar] [CrossRef]
- Bai, L.; Liu, B.N.; Ji, C.M.; Zhao, S.H.; Liu, S.Y.; Wang, R.; Wang, W.R.; Yao, P.; Li, X.M.; Fu, X.J. Hypoxic and cold adaptation insights from the himalayan marmot genome. iScience 2019, 11, 505–507. [Google Scholar] [CrossRef]
- Bao, Z.; Li, C.; Guo, C.; Xiang, Z. Convergent Evolution of Himalayan Marmot with Some High-Altitude Animals through ND3 Protein. Animals 2021, 11, 251. [Google Scholar] [CrossRef]
- Yan, X.B.; Guo, Y.X.; Zhou, H.; Wang, K. Effect of geographical factors on genetic variation of Elymus nutans indigenous in the Qinghai-Tibetan Plateau. Acta Bot. Boreal. Occident. Sin. 2007, 27, 328–333. [Google Scholar]
- Richard, T.T.; Alexander, E. Roads and their major ecological effects. Annu. Rev. Ecol. Evol. S. 1998, 29, 207–231. [Google Scholar]
- Bergstrom, R.; Skarpe, C. The abundance of large wild herbivores in a semi-arid savanna in relation to reasons, pans and livestock. Afr. J. Ecol. 1999, 37, 12–26. [Google Scholar] [CrossRef]
- Yang, S.M.; Wei, W.H.; Yin, B.F.; Fan, N.C.; Zhou, W.Y. The predation risks of the Himalayan marmot and plateau zokor and their survival strategies in the Alpine meadow ecosystem. Acta Ecol. Sinica 2007, 27, 4972–4978. [Google Scholar]
- Wei, W.R. The Relationship between Population Density, Vegetation community structure and predation risk of Himalayan marmot. Acta Agrestia Sin. 2019, 27, 350–355. [Google Scholar]
- Luan, S.L.; Ren, H.Y.; Shi, R.H. Research progress on identification and monitoring of the appropriate habitat of Himalayan marmot. Chin. J. Endem. 2021, 40, 1024–1030. [Google Scholar]
- Guo, C.; Gao, S.; Zhou, S.L.; Zhang, L.; Xiang, Z.F. Burrowing and anti-predator requirements determine the microhabitat selection of himalayan marmot in zoige wetland. Zoolog Sci. 2020, 37, 554–562. [Google Scholar] [CrossRef]
- Wu, J.P.; Gong, X.Y.; Yao, X.X.; Casper, D.P. Plant communities responding to grazing pressure by sheep in an Alpine meadow. Transl. Anim. Sci. 2020, 4, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.J.; Lu, L.; Wu, H.X.; Wang, J.; Liu, Q.Y. Analysis of ecological environment factors of Himalayan marmot based on global positioning system, remote sensing and geographical information system in Yushu City, Qinghai Province. Chin. J. Endemiol. 2016, 35, 329–332. [Google Scholar]
- Borgo, A. Habitat requirements of the Alpine marmot Marmota marmota in re-introduction areas of the Eastern Italian Alps. Formulation and validation of habitat suitability models. Acta Theriol. 2003, 48, 557–569. [Google Scholar] [CrossRef]
- Shi, S.M.; Liu, F.Y.; Yan, X.B. Habitat selection by Himalayana marmot in the eastern Qilian Mountains. J. Gansu Agric. Univ. 2008, 43, 125–130. [Google Scholar]
- Řičánková, V.P.; Riegert, J.; Semančíková, E.; Hais, E.; Čejková, A.; Prach, K. Habitat preferences in gray marmots (Marmota baibacina). Acta Theriol. 2014, 59, 317–324. [Google Scholar] [CrossRef]
- Jiang, D.C.; Zhao, X.M.; López-Pujol, J.; Wang, Z.Q.; Qu, Y.H.; Zhang, Y.M.; Zhang, T.Z.; Li, D.Y.; Jiang, K.; Wang, B.; et al. Effects of climate change and anthropogenic activity on ranges of vertebrate species endemic to the Qinghai-Tibet Plateau over 40 years. Conserv. Biol. 2023, 8, e14069. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Xu, T.; Xu, W.; Wu, K.; Yang, W.; Blank, D. Piosphere effect on soil seed bank in desert steppe. Chin. J. Ecol. 2015, 34, 2778–2784. [Google Scholar]
- Zhu, B.R.; Verhoeven, M.A.; Velasco, N.; Sanchez-Aguilar, L.; Zhang, Z.; Piersma, T. Current breeding distributions and predicted range shifts under climate change in two subspecies of Black-tailed Godwits in Asia. Glob. Chang. Biol. 2022, 28, 5416–5426. [Google Scholar] [CrossRef]
- Hou, Z.; Jin, P.; Liu, H.; Qiao, H.; Sket, B.; Cannizzaro, A.G.; Berg, D.J.; Li, S. Past climate cooling promoted global dispersal of amphipods from Tian Shan montane lakes to circumboreal lakes. Glob. Chang. Biol. 2022, 28, 3830–3845. [Google Scholar] [CrossRef]
- Buyandelger, S.; Tojoo, E.; Otgonbayar, B.; Bayartogtokh, B.; Reading, R.P. Ecosystem engineering effects of Mongolian marmots (Marmota sibirica) on terrestrial arthropod communities in Mongolia. Mong. J. Biol. Sci. 2021, 19, 17–30. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Kang, Y.; Wang, Y.; Tan, Y.; Yao, B.; An, K.; Su, J. Himalayan Marmot (Marmota himalayana) Redistribution to High Latitudes under Climate Change. Animals 2023, 13, 2736. https://doi.org/10.3390/ani13172736
Wang Z, Kang Y, Wang Y, Tan Y, Yao B, An K, Su J. Himalayan Marmot (Marmota himalayana) Redistribution to High Latitudes under Climate Change. Animals. 2023; 13(17):2736. https://doi.org/10.3390/ani13172736
Chicago/Turabian StyleWang, Zhicheng, Yukun Kang, Yan Wang, Yuchen Tan, Baohui Yao, Kang An, and Junhu Su. 2023. "Himalayan Marmot (Marmota himalayana) Redistribution to High Latitudes under Climate Change" Animals 13, no. 17: 2736. https://doi.org/10.3390/ani13172736




