Chemical Composition and In Vitro Ruminal Fermentation Characteristics of Native Grasses from the Floodplain Lowlands Ecosystem in the Colombian Orinoquia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
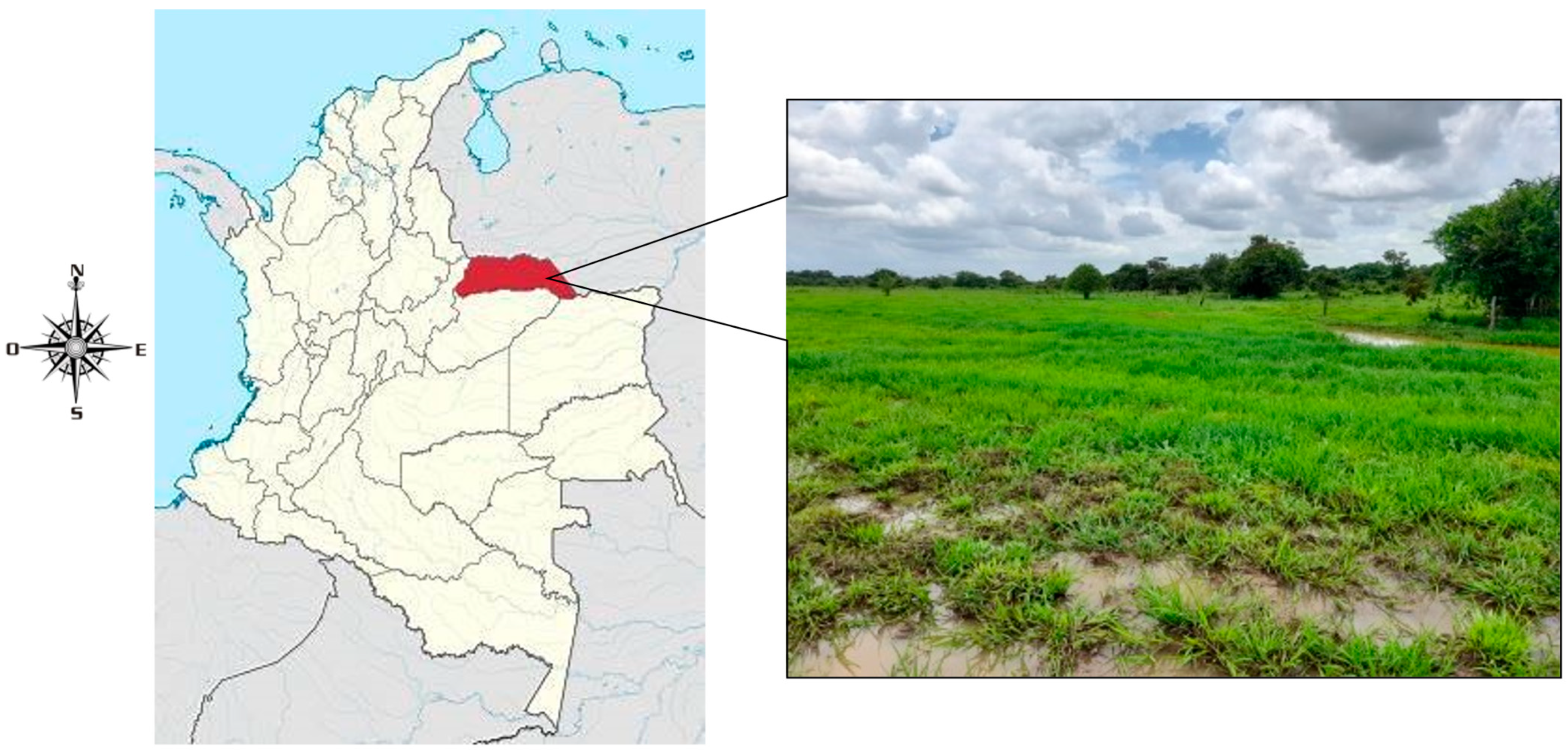
2.1. Study Site
2.2. Evaluated Species
2.3. Experimental Design
2.4. In Vitro Ruminal Fermentation Analysis
2.5. Statistical Analysis
3. Results
3.1. Nutritional Composition
3.2. Grasses Fermentation Characteristics
4. Discussion
4.1. Grasses Chemical Composition
4.2. In Vitro Fermentation Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peñuela, L.; Fernández, A.P.; Castro, F.; Ocampo, A. Uso y Manejo de Forrajes Nativos en la Sabana Inundable de la Orinoquia; Convenio de Cooperación Interinstitucional; Universidad de los Llanos, The Nature Conservancy y la Fundación Horizonte Verde con el apoyo de la Fundación Biodiversidad de España, la Corporación Autónoma Regional de la Orinoquia: Villavicencio, Colombia, 2011. [Google Scholar]
- Vélez-Terranova, M. Estrategias tecnológicas para la intensificación de la productividad ganadera en condiciones de sabanas inundables en la Orinoquía colombiana. Trop. Subtrop. Agroecosyst. 2019, 22, 257–266. [Google Scholar] [CrossRef]
- Peñuela, L.; Fernández, A. La ganadería ligada a procesos de conservación en la sabana inundable de la Orinoquia. Orinoquia 2010, 14 (Suppl. 1), 5–17. [Google Scholar]
- Salamanca-Carreño, A.; Vélez-Terranova, M.; Vargas-Corzo, O.M.; Pérez-López, O.; Castillo-Pérez, A.F.; Parés-Casanova, P.M. Relationship of Physiographic Position to Physicochemical Characteristics of Soils of the Flooded-Savannah Agroecosystem, Colombia. Agriculture 2023, 13, 220. [Google Scholar] [CrossRef]
- Pérez Bona, R.A.; Vargas Corzo, O.M. Características de la Sabana Nativa y su Potencial de Producción Bovina en la Llanura Inundable de Arauca; Boleín Técnico N° 25; Programa Regional de Investigación Pecuaria, Corpoica: Arauca, Colombia, 2001. [Google Scholar]
- Amiri, F.; Mohamed-Sharif, A.R. Comparison of nutritive values of grasses and legume species using forage quality index. Songklanakarin J. Sci. Technol. 2012, 34, 577–586. [Google Scholar]
- Rinehart, L. Ruminant Nutrition for Graziers. ATTRA—National Sustainable Agriculture Information Service. 2008. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-10/Ruminant%20Nutrition%20for%20Graziers.pdf (accessed on 15 August 2023).
- Ariza-Nieto, C.M.; Mojica, B.; Parra, D.; Afanador-Tellez, G. Use of LOCAL algorithm with near infrared spectroscopy in forage resources for grazing systems in Colombia. J. Near Infrared Spectrosc. 2018, 26, 44–52. [Google Scholar] [CrossRef]
- Salamanca-Carreño, A.; Vélez-Terranova, M.; Vargas-Corzo, O.M.; Parés-Casanova, P.M.; Bentez-Molano, J. Productive and Nutritional Characteristics of Native Grasses from the Floodplain Banks Ecosystem in the Colombian Orinoquia. Sustainability 2022, 14, 15151. [Google Scholar] [CrossRef]
- Getachew, G.; Blümmel, M.; Makkar, H.P.; Becker, K. In vitro measuring techniques for assessment of nutritional quality of feeds: A review. Anim. Feed Sci. Technol. 1998, 72, 261–281. [Google Scholar] [CrossRef]
- Macheboeuf, D.; Coudert, l.; Bergeault, R.; Lalière, G.; Niderkorn, V. Screening of plants from diversified natural grasslands for their potential to combine high digestibility, and low methane and ammonia production. Animal 2014, 8, 1797–1806. [Google Scholar] [CrossRef]
- Gemeda, B.S.; Hassen, A. In vitro fermentation, digestibility and methane production of tropical perennial grass especies. Crop Pasture Sci. 2014, 65, 479–488. [Google Scholar] [CrossRef]
- Getachew, G.; Robinson, P.H.; DePeters, E.J.; Taylor, S.J. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Bekele, W.; Guinguina, A.; Zegeye, A.; Simachew, A.; Ramin, M. Contemporary Methods of Measuring and Estimating Methane Emission from Ruminants. Methane 2022, 1, 82–95. [Google Scholar] [CrossRef]
- Flachowsky, G.; Lebzien, P. Effects of phytogenic substances on rumen fermentation and methane emissions: A proposal for a research process. Anim. Feed Sci. Technol. 2012, 176, 70–77. [Google Scholar] [CrossRef]
- Reihardt, M.S.; Foote, A.P.; Lambert, B.D.; Muir, J.P. Effects of protein or energy supplementation on in situ disappearance of low- and high-quality Coastal Bermudagrass hay in goats. Texas J. Agric. Nat. Res. 2011, 24, 97–105. [Google Scholar]
- Aparicio, R.; González-Ronquillo, M.; Torres, R.; Astudillo, L.; Cordova, L.; Carrasquel, J. Degradabilidad de los pastos lambedora (Leersia hexandra) y paja de agua (Hymenachne amplexicaulis) en cuatro épocas del año de una sabana inundable del estado Apure, Venezuela. Zootec. Trop. 2007, 25, 225–228. [Google Scholar]
- González-Ronquillo, M.; Aparicio, R.; Torres, R.; Domínguez-Vara, I.A. Producción de biomasa, composición química y producción de gas in vitro de la vegetación de una sabana estacional modulada. Zootec. Trop. 2009, 2, 407–417. [Google Scholar]
- Holdridge, L. Ecología Basada en Zonas de Vida; IICA: San Jose, Costa Rica, 1987; p. 216. [Google Scholar]
- Cerdas, R. Programa de fertilización de forrajes. Desarrollo de un módulo práctico para técnicos y estudiantes de ganadería de Guanacaste, Costa Rica. InterSedes 2011, 12, 109–128. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage fiber analysis (apparatus, regents, procedures and some applications). Agric. Handb. 1970, 379, 1–20. [Google Scholar]
- UN. Universidad Nacional de Colombia. Laboratorio de Biotecnología Ruminal (BIORUM)). 2023. Available online: https://direcciondelaboratorios.medellin.unal.edu.co/index.php/nuestros-laboratorios/facultad-de-ciencias-agrarias/66 (accessed on 23 June 2023).
- Littell, R.; Milliken, G.; Stroup, W.; Wolfinger, R.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2007. [Google Scholar]
- InfoStat. Software Estadistico. Versión 30/04/2020; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. [Google Scholar]
- Cruz-Hernández, A.; Hernández-Garay, A.; Aranda-Ibañez, E.; Chay-Canul, A.; Márquez-Quiroz, C.; Rojas-Garcia, A.L.; Gómez-Vázquez, A. Nutritive value of Mulato grass under dierent grazing strategies. Esosist. Recur. Agropec. 2017, 4, 65–72. [Google Scholar] [CrossRef]
- Garay, J.R.; Joaquin-Cancino, S.; Zárate-Fortuna, P.; Ibarra-Hinojosa, M.A.; Martínez-González, J.C.; González-Dávila, R.P.; Cienfuegos-Rivas, E.G. Dry matter accumulation and crude protein concentration in Brachiaria spp. cultivars in the humid tropics of Ecuador. Trop. Grasslands-Forrajes Trop. 2017, 5, 66–76. [Google Scholar] [CrossRef]
- Luce, M.S.; Gouveia, G.G.; Eudoxie, G.D. Comparative effects of food processing liquid slurry and inorganic fertilizers on tanner grass (Brachiaria arrecta) pasture: Grass yield, crude protein and P levels and residual soil N and P. Grass Forage Sci. 2016, 72, 401–413. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J. Urochloa arrecta (African signalgrass); CABI Compendium: Wallingford, UK, 2023. [Google Scholar]
- Jiménez, J.C.; Cardoso, J.A.; Leiva, L.F.; Gil, J.; Forero, M.G.; Worthington, M.L.; Miles, J.W.; Rao, I.M. Non-destructive Phenotyping to Identify Brachiaria Hybrids Tolerant to Waterlogging Stress under Field Conditions. Front. Plant Sci. 2017, 8, 167. [Google Scholar] [CrossRef]
- Dias-Filho, M.B.; Dos Santos-Lopes, M.J. Screening for tolerance to waterlogging in forage plants. In Proceedings of the III International Symposium on Forage Breeding, Bonito, MS, Brazil, 7–11 November 2011. [Google Scholar]
- Reyes-Pérez, J.J.; Méndez-Martínez, Y.; Verdecia, D.; Luna-Murillo, R.A.; Hernández Montiel, L.G.; Herrera, R. Components of the yield and bromatological composition of three Brachiaria varieties in El Empalme area, Ecuador. Cuban J. Agric. Sci. 2018, 52, 35–445. [Google Scholar]
- Cevallos, J.H.A.; Guerrero, F.C.; Zamora, G.Q.; Murillo, R.L.; Valdez, O.D.M.; Guerra, I.E.; Montes, S.Z.; Garaicoa, D.R.; Ruiz, J.V.; Mendoza, E.P. Comportamiento agronómico y composición química de tres variedades de Brachiaria en diferentes edades de cosecha. Cienc. Tecnol. 2008, 1, 87–94. [Google Scholar] [CrossRef]
- Fonseca, P.G.; Emerenciano, N.J.; Dos Santos, D.G.; Cortes, A.C.; De Oliveira, L.P.; Da Silva Santos, R. Production and quality of tropical grasses at different regrowth intervals in the Brazilian semiarid. Acta Sci. 2021, 43, e52842. [Google Scholar] [CrossRef]
- Canchila, E.R.; Soca, M.; Ojeda, F.; Machado, R. Evaluación de la composición bromatológica de 24 accesiones de Brachiaria spp. Pastos Forrajes 2009, 32, 1–9. [Google Scholar]
- Liu, J.; Duan, C.; Zhang, X.; Zhu, Y.; Lu, X. Potential of Leersia hexandra Swartz for phytoextraction of Cr from soil. J. Hazard. Mater. 2011, 188, 85–91. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, N.; Rivera-Cruz, M.C.; Trujillo-Narcía, A.; Almaráz-Suárez, J.J.; Salgado-García, S. Spatial Distribution of Oil and Biostimulation Through the Rhizosphere of Leersia hexandra in Degraded Soil. Water Air Soil Pollut. 2016, 227, 319. [Google Scholar] [CrossRef]
- Muñoz-González, J.C.; Huerta-Bravo, M.; Lara, B.A.; Rangel, S.R.; De la Rosa, A.J. Production and nutritional quality of forages in conditions Humid Tropics of Mexico. Rev. Mex. Cienc. Agríc. Pub. Esp. 2016, 16, 3315–3327. [Google Scholar]
- Harper, K.J.; McNeill, D.M. The Role iNDF in the Regulation of Feed Intake and the Importance of Its Assessment in Subtropical Ruminant Systems (the Role of iNDF in the Regulation of Forage Intake). Agriculture 2015, 5, 778–790. [Google Scholar] [CrossRef]
- Carrillo-Díaz, M.I.; Miranda-Romero, L.A.; Chávez-Aguilar, G.; Zepeda-Batista, J.L.; González-Reyes, M.; García-Casillas, A.C.; Tirado-González, D.N.; Tirado-Estrada, G. Improvement of Ruminal Neutral Detergent Fiber Degradability by Obtaining and Using Exogenous Fibrolytic Enzymes from White-Rot Fungi. Animals 2022, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Mwendia, S.W.; Ohmstedt, U.; Nyakundi, F.; Notenbaert, A.; Peters, P. Does harvesting Urochloa and Megathyrsus forages at short intervals confer an advantage on cumulative dry matter yields and quality? J. Sci. Food Agric. 2022, 102, 750–756. [Google Scholar] [CrossRef]
- Fonseca-Pereira, G.; Emerenciano-Neto, J.V.; dos Santos-Difante, G.; Cortes-Assis, L.C.; de Oliveira-Lima, P. Morphogenic and structural characteristics of tropical forage grasses managed under different regrowth periods in the Brazilian semi-arid region. Semin. Ciências Agrárias 2019, 40, 283–292. [Google Scholar] [CrossRef]
- Bhatta, R.; Tajima, K.; Kurihara, M. Influence of temperature and pH on fermentation pattern and methane production in the rumen simulating fermenter (RUSITEC). Asian-Aust. J. Anim. Sci. 2006, 19, 376–380. [Google Scholar] [CrossRef]
- Gaviria-Uribe, X.; Bolívar-Vergara, D.M.; Chirinda, N.; Molina-Botero, I.C.; Mazabel, J.; Barahona-Rosales, R.; Arango, J. In vitro methane production and ruminal fermentation parameters of tropical grasses and grass-legume associations commonly used for cattle feeding in the tropics. Livest. Res. Rural Dev. 2022, 34, 1–17. [Google Scholar]
- Wilson-García, C.Y.; Sánchez-Santillán, P.; López-Zerón, N.E.; Domínguez-Rodríguez, I.E.; Ayala-Monter, M.A.; Torres-Salado, N.; Valenzuela Lagarda, J.L. In vitro fermentative characteristics and chemical quality of Guinea grass with organic and chemical fertilization Agro Productividad. Agro Product. 2022, 15, 79–86. [Google Scholar] [CrossRef]
- Kamalak, A.; Canbolat, O.; Gurbuz, Y.; Ozay, O. Comparison of in vitro gas production technique with in situ nylon bag technique to estimate dry matter degradation. Czech J. Anim. Sci. 2005, 50, 60–67. [Google Scholar] [CrossRef]
- Makkar, H.P. In vitro gas methods for evaluation of feeds containing phytochemicals. Anim. Feed Sci. Technol. 2005, 123–124, 291–302. [Google Scholar] [CrossRef]
- Vélez, O.M.; Campos, R.; Sánchez, H. Uso de metabolitos secundarios de las Plantas para reducir la metanogénesis ruminal. Trop. Subtrop. Agroecos. 2014, 17, 489–499. [Google Scholar]
- Miguel, M.A.; Lee, S.S.; Mamuad, L.L.; Choi, Y.J.; Jeong, C.D.; Son, A.; Cho, K.K.; Kim, E.T.; Kim, S.B.; Lee, S.S. Enhancing Butyrate Production, Ruminal Fermentation and Microbial Population through Supplementation with Clostridium saccharobutylicum. J. Microbiol. Biotechnol. 2019, 29, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.K.; Durmic, Z.; Erskine, W.; Ghamkhar, K.; Revell, C. In vitro ruminal fermentation characteristics and methane production differ in selected key pasture species in Australia. Crop Pasture Sci. 2013, 64, 935–942. [Google Scholar] [CrossRef]
- Giraldo-Parra, P.A. Efecto de Varios Aditivos y Suplementos Nutricionales en las Emisiones de Metano y los Parámetros de la Fermentación Ruminal In Vitro; Tesis Maestría en Ciencias Agrarias, Universidad Nacional de Colombia Medellín: Medellín, Colombia, 2013. [Google Scholar]
- Bryant, M.P.; Robinson, I.M. Some nutritional characteristics of predominant culturable ruminal bacteria. J. Bacteriol. 1962, 84, 605–614. [Google Scholar] [CrossRef]
- Leng, R.A. Factors affecting the utilization of “poor-quality” forages by ruminants particularly under tropical conditions. Nutr. Res. Rev. 1990, 3, 277–303. [Google Scholar] [CrossRef]
- Marín, A.; Giraldo, L.A.; Correa, G. Parámetros de fermentación ruminal in vitro del pasto Kikuyo (Pennisetum clandestinum). Livest. Res. Rural Dev. 2014, 26, 1–6. [Google Scholar]
- Ley de Coss, A.; Guerra-Medina, C.; Montañez-Valdez, O.; Guevara, F.; Pinto, R.; Reyes-Gutiérrez, J. In vitro production of gas methane by tropical grasses. Rev. MVZ Córdoba 2018, 23, 6788–6798. [Google Scholar] [CrossRef]
- Vélez-Terranova, M.; Campos-Gaona, R.; Sánchez-Guerrero, H.; Giraldo, L.A. Fermentation dynamics and methane production of diets based on Brachiaria humidicola with high inclusion Levels of Enterolobium schomburgkii and Senna occidentalis in a Rusitec system. Trop. Subtrop. Agroecos. 2018, 21, 163–175. [Google Scholar]
- Kulivand, M.; Kafilzadeh, F. Correlation between chemical composition, kinetics of fermentation and methane production of eight pasture grasses. Acta Sci. Anim. Sci. Mar. 2015, 37, 9–14. [Google Scholar] [CrossRef]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef]
- Meale, S.J.; Chaves, A.V.; Baah, J.; McAllister, T.A. Methane Production of Different Forages in In vitro Ruminal Fermentation. Asian-Aust. J. Anim. Sci. 2012, 25, 86–91. [Google Scholar] [CrossRef]
- Montalvão Lima, D.; Abdalla Filho, A.L.; Tavares Lima, P.M.; Zanuto Sakita, G.; Dias e Silva, T.P.; McManus, C.; Abdalla, A.L.; Louvandini, H. Morphological characteristics, nutritive quality, and methane production of tropical grasses in Brazil. Pesq. Agropec. Bras. 2018, 53, 323–331. [Google Scholar] [CrossRef]
- Kondo, M.; Yoshida, M.; Loresco, M.; Lapitan, R.M.; Herrera, J.R.; Del Barrio, A.N.; Uyeno, Y.; Matsui, H.; Fujihara, T. Nutrient Contents and In vitro Ruminal Fermentation of Tropical Grasses Harvested in Wet Season in the Philippines. Adv. Anim. Vet. Sci. 2015, 3, 694–699. [Google Scholar] [CrossRef]
- Chino Velasquez, L.B.; Molina-Botero, I.C.; Moscoso Muñoz, J.E.; Gómez Bravo, C. Relationship between Chemical Composition and In Vitro Methane Production of High Andean Grasses. Animals 2022, 12, 2348. [Google Scholar] [CrossRef] [PubMed]

| Species | Common Name | Growth Habit | Characteristic |
|---|---|---|---|
| Leersia hexandra Sw. (1788) | Lambedora grass | Rhizomatous-Stoloniferous | Native |
| Acroceras zizanioides (Kunth) Dandy (1931) | Black water straw | Stoloniferous | Native |
| Hymenachne amplexicaulis (Rudge) Nees (1829) | Water straw | Stoloniferous | Native |
| Urochloa arrecta (Hack ex T. Duran and Schinz) Stent (control) | Tanner grass | Stoloniferous | Introduced |
| Species | Age (days) | Height (cm) | GFY (Ton/ha) | DMY (Ton/ha) | DM (%) | CP (%) | NDF (%) | ADF (%) |
| A. z | 30 | 29.1 f ± 1.3 | 4.0 cd ± 0.31 | 1.1 de ± 0.1 | 27.8 c ± 1.0 | 11.6 ab ± 0.6 | 60.6 cd ± 1.1 | 38.8 ab ± 1.2 |
| Control | 47.8 de ± 2.3 | 3.9 cd ± 0.31 | 0.8 fg ± 0.1 | 19.8 f ± 1.0 | 10.1 bc ± 0.6 | 60.3 cd ± 1.1 | 29.8 d ± 1.2 | |
| H. a | 68.9 b ± 2.6 | 3.6 d ± 0.31 | 0.7 g ± 0.1 | 18.8 f ± 1.7 | 7.5 cde ± 1.1 | 66.3 ab ± 1.8 | 40.6 a ± 2 | |
| L. h | 45.0 de ± 1.9 | 4.4 cd ± 0.31 | 1.7 b ± 0.1 | 39.1 a ± 1.0 | 12.1 a ± 0.6 | 59.9 cd ± 1.1 | 37.8 abc ± 1.2 | |
| A. z | 40 | 43.1 e ± 1.3 | 5.7 a ± 0.31 | 1.4 c ± 0.1 | 25.1 cde ± 1.0 | 11.3 ab ± 0.6 | 61.6 c ± 1.1 | 34.9 c ± 1.2 |
| Control | 64.6 bc ± 1.9 | 4.6 bc ± 0.31 | 1.1 e ± 0.1 | 23.0 e ± 1.0 | 8.2 cd ± 0.6 | 66.1 b ± 1.1 | 34.7 c ± 1.2 | |
| H. a | 85.8 a ± 2.1 | 4.5 cd ± 0.31 | 1.0 ef ± 0.1 | 22.5 ef ± 1.0 | 7.2 de ± 0.6 | 67.7 ab ± 1.1 | 37.1 abc 1.2 | |
| L. h | 59.9 c ± 1.5 | 5.7 a ± 0.31 | 1.9 ab ± 0.1 | 33.5 b ± 1.0 | 8.1 cd ± 0.6 | 57.5 de ± 1.1 | 35.4 bc ± 1.2 | |
| A. z | 50 | 49.1 d ± 1.3 | 5.8 a ± 0.31 | 1.4 c ± 0.1 | 24.5 de ± 1.0 | 12.2 a ± 0.6 | 61.4 c ± 1.1 | 39.8 a ± 1.2 |
| Control | 67.7 b ± 1.9 | 4.8 bc ± 0.31 | 1.2 cde ± 0.1 | 26.2 cd ± 1.0 | 6.1 e ± 0.6 | 66.9 ab ± 1.1 | 34.6 c ± 1.2 | |
| H. a | 89.0 a ± 2.1 | 5.4 ab ± 0.31 | 1.3 cd ± 0.1 | 24.5 de ± 1.0 | 6.6 de ± 0.6 | 69.6 a ± 1.1 | 39.1 a ± 1.2 | |
| L. h | 63.5 bc ± 1.5 | 5.7 a ± 0.31 | 2.0 a ± 0.1 | 35.6 b ± 1.0 | 10.6 ab ± 0.6 | 56.6 e ± 1.1 | 37.2 abc ± 1.2 | |
| Interaction (p-value) | NS | NS | NS | 0.0001 | 0.0066 | 0.0046 | 0.0199 | |
| Species | Age (days) | Cellulose (%) | Hemicelulose (%) | Lignin (%) | Ash (%) | Ca (%) | P (%) | |
| A. z | 30 | 28.89 abc ±1.14 | 21.77 cd ± 0.84 | 9.9 ab ± 0.4 | 9.8 bc ± 0.9 | 0.54 a ± 0.05 | 0.26 bc ± 0.01 | |
| Control | 23.26 e ± 1.14 | 30.52 a ± 0.84 | 6.5 e ± 0.4 | 7.8 cd ± 0.9 | 0.46 abc ± 0.05 | 0.23 cd ± 0.01 | ||
| H. a | 31.14 ab ± 1.97 | 25.65 b ± 1.46 | 9.5 abcd ± 0.8 | 8.3 bcd ± 1.2 | 0.23 c ± 0.1 | 0.28 abc ± 0.02 | ||
| L. h | 27.42 bcd ± 1.14 | 22.04 c ± 0.84 | 10.4 a ± 0.4 | 15.8 a ± 0.9 | 0.44 abc ± 0.05 | 0.17 e ± 0.01 | ||
| A. z | 40 | 25.87 cde ± 1.14 | 26.73 b ± 0.84 | 9.1 bcd ± 0.4 | 9.2 bc ± 0.9 | 0.59 a ± 0.05 | 0.28 ab ± 0.01 | |
| Control | 25.93 cde ± 1.14 | 31.43 a ± 0.84 | 8.8 bcd ± 0.4 | 7.2 cd ± 0.9 | 0.34 bc ± 0.05 | 0.23 cd ± 0.01 | ||
| H. a | 28.05 abcd ± 1.14 | 30.64 a ± 0.84 | 9.0 bcd ± 0.4 | 6.7 d ± 0.9 | 0.26 c ± 0.05 | 0.27 bc ± 0.01 | ||
| L. h | 27.35 bcd ± 1.14 | 22.15 c ± 0.84 | 8.1 d ± 0.4 | 13.8 a ± 0.9 | 0.35 bc ± 0.05 | 0.16 e ± 0.01 | ||
| A. z | 50 | 31.37 a ± 1.14 | 21.59 cd ± 0.84 | 8.5 cd ± 0.4 | 10.9 b ± 0.9 | 0.49 ab ± 0.05 | 0.32 a ± 0.01 | |
| Control | 25.37 de ± 1.14 | 32.29 a ± 0.84 | 9.2 abcd ± 0.4 | 5.8 d ± 0.9 | 0.23 c ± 0.05 | 0.21 d ± 0.01 | ||
| H. a | 29.5 ab ± 1.14 | 30.52 a ± 0.84 | 9.6 abc ± 0.4 | 6.5 d ± 0.9 | 0.28 c ± 0.05 | 0.25 bc ± 0.01 | ||
| L. h | 27.72 bcd ± 1.14 | 19.34 d ± 0.84 | 9.5 abcd ± 0.4 | 14.6 a ± 0.9 | 0.28 c ± 0.05 | 0.14 e ± 0.01 | ||
| Interaction (p-value) | NS | <0.0001 | 0.0004 | 0.0476 | NS | 0.0244 | ||
| Species | Time | Gas Production (mL/g DM) | DMD (%) | pH |
|---|---|---|---|---|
| A. zizanioides | 12 | 126.7 ± 2.75 | 25.5 f ± 1.2 | 6.7 ± 0.02 |
| U. arrecta (control) | 124.7 ± 2.75 | 31.9 e ± 1.2 | 6.8 ± 0.02 | |
| H. amplexicaulis | 120.7 ± 2.75 | 20.8 g ± 1.2 | 6.8 ± 0.02 | |
| L. hexandra | 125.3 ± 2.75 | 26.8 f ± 1.2 | 6.7 ± 0.02 | |
| A. zizanioides | 24 | 154.3 ± 2.75 | 37.1 d ± 1.2 | 6.6 ± 0.02 |
| U. arrecta (control) | 160.0 ± 2.75 | 48.8 bc ± 1.2 | 6.7 ± 0.02 | |
| H. amplexicaulis | 147.7 ± 2.75 | 35.4 de ± 1.2 | 6.6 ± 0.02 | |
| L. hexandra | 158.0 ± 2.75 | 38.9 d ± 1.2 | 6.6 ± 0.02 | |
| A. zizanioides | 48 | 197.3 ± 2.75 | 47.5 c ± 1.2 | 6.6 ± 0.02 |
| U. arrecta (control) | 201.7 ± 2.75 | 60.6 a ± 1.2 | 6.6 ± 0.02 | |
| H. amplexicaulis | 189.7 ± 2.75 | 49.1 bc ± 1.2 | 6.6 ± 0.02 | |
| L. hexandra | 198.0 ± 2.75 | 51.8 b ± 1.2 | 6.6 ± 0.02 | |
| p-value | Specie | 0.0260 | 0.0001 | NS |
| Time | <0.0001 | <0.0001 | <0.0001 | |
| Interaction | NS | 0.0178 | NS |
| Species | Time | Ammonia (mg/dL) | Ch4 (ml/gDMD) | TVFA (mmol/L) | Acetic (mmol/L) | Propionic (mmol/L) | Butyric (mmol/L) |
|---|---|---|---|---|---|---|---|
| A. zizanioides | 38.5 d ± 1.1 | 31.5 bc ± 1.7 | 26.1 ± 1.7 | 15.5 ± 1.0 | 8.4 ± 0.6 | 2.2 ± 0.2 | |
| U. arrecta (control) | 42.8 c ± 1.2 | 29.6 bc ± 1.7 | 24.6 ± 1.7 | 14.0 ± 1.0 | 8.7 ± 0.6 | 1.8 ± 0.2 | |
| H. amplexicaulis | 24 | 38.5 d ± 1.1 | 33.8 b ± 1.7 | 21.5 ± 1.7 | 13.0 ± 1.0 | 6.7 ± 0.6 | 1.8 ± 0.2 |
| L. hexandra | 42.2 c ± 1.1 | 27.8 c ± 1.7 | 28.7 ± 1.7 | 17.6 ± 1.0 | 8.6 ± 0.6 | 2.4 ± 0.2 | |
| A. zizanioides | 46.6 b ± 1.1 | 43.5 a ± 1.7 | 60.4 ± 1.7 | 36.3 ± 1.0 | 16.9 ± 0.6 | 7.1 ± 0.2 | |
| U. arrecta (control) | 51.6 a ± 1.2 | 39.6 a ± 1.7 | 60.8 ± 1.7 | 36.2 ± 1.0 | 17.6 ± 0.6 | 7.0 ± 0.2 | |
| H. amplexicaulis | 48 | 45.1 bc ± 1.1 | 41.8 a ± 1.9 | 57.7 ± 1.7 | 35.8 ± 1.0 | 15.8 ± 0.6 | 6.7 ± 0.2 |
| L. hexandra | 52.6 a ± 1.1 | 43.5 a ± 1.7 | 62.3 ± 1.7 | 37.8 ± 1.0 | 16.9 ± 0.6 | 7.6 ± 0.2 | |
| Species | 0.011 | NS | 0.029 | NS | NS | 0.0069 | |
| p-value | Time | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Interaction | <0.0001 | 0.0437 | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-Terranova, M.; Salamanca-Carreño, A.; Vargas-Corzo, O.M.; Parés-Casanova, P.M.; Arias-Landazábal, J.N. Chemical Composition and In Vitro Ruminal Fermentation Characteristics of Native Grasses from the Floodplain Lowlands Ecosystem in the Colombian Orinoquia. Animals 2023, 13, 2760. https://doi.org/10.3390/ani13172760
Vélez-Terranova M, Salamanca-Carreño A, Vargas-Corzo OM, Parés-Casanova PM, Arias-Landazábal JN. Chemical Composition and In Vitro Ruminal Fermentation Characteristics of Native Grasses from the Floodplain Lowlands Ecosystem in the Colombian Orinoquia. Animals. 2023; 13(17):2760. https://doi.org/10.3390/ani13172760
Chicago/Turabian StyleVélez-Terranova, Mauricio, Arcesio Salamanca-Carreño, Oscar M. Vargas-Corzo, Pere M. Parés-Casanova, and José N. Arias-Landazábal. 2023. "Chemical Composition and In Vitro Ruminal Fermentation Characteristics of Native Grasses from the Floodplain Lowlands Ecosystem in the Colombian Orinoquia" Animals 13, no. 17: 2760. https://doi.org/10.3390/ani13172760
APA StyleVélez-Terranova, M., Salamanca-Carreño, A., Vargas-Corzo, O. M., Parés-Casanova, P. M., & Arias-Landazábal, J. N. (2023). Chemical Composition and In Vitro Ruminal Fermentation Characteristics of Native Grasses from the Floodplain Lowlands Ecosystem in the Colombian Orinoquia. Animals, 13(17), 2760. https://doi.org/10.3390/ani13172760







