First Isolation and Identification of Aeromonas veronii in a Captive Giant Panda (Ailuropoda melanoleuca)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Organ Sampling and Bacterial Isolation
2.2. Histopathology of Giant Panda
2.3. Analysis of Physiological and Biochemical Features
2.4. Sequence Analysis of 16S rRNA and gyrB Genes
2.5. Molecular Identification of Virulence Genes
2.6. Antimicrobial Susceptibility Testing
2.7. Molecular Identification of Antibiotic Resistance Genes (ARGs)
2.8. The Pathogenicity Testing in Kunming Mice
3. Results
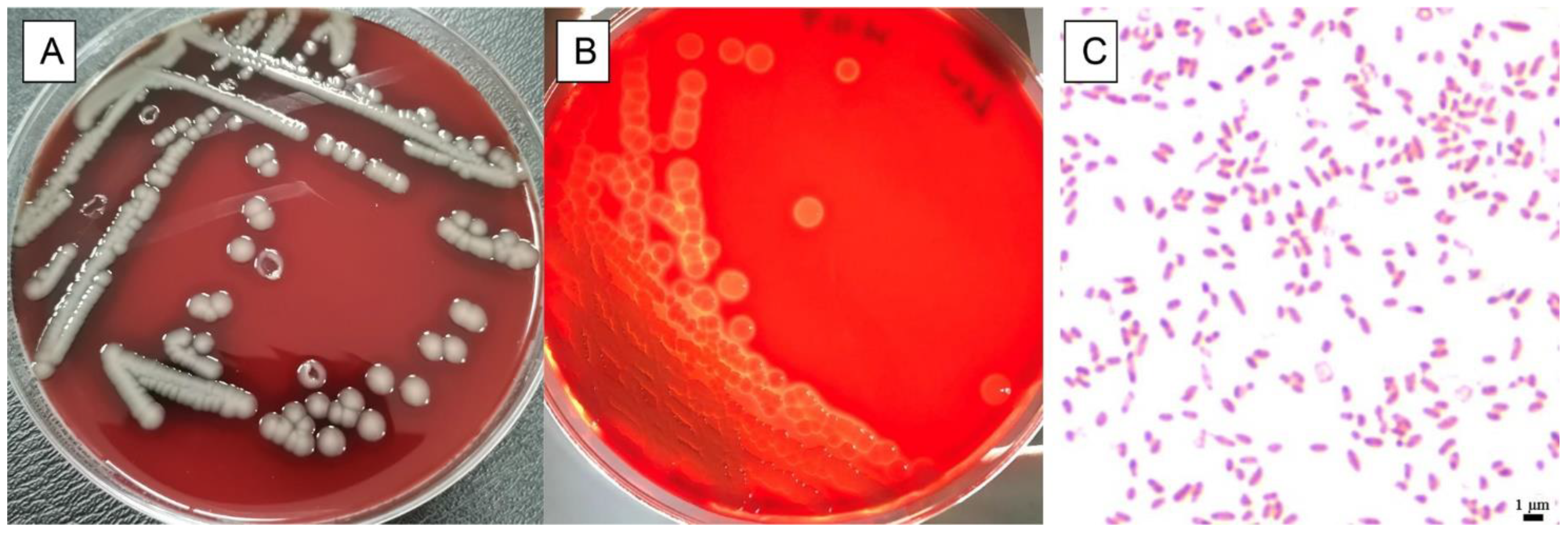
3.1. Physiological and Biochemical Characteristics of Isolate
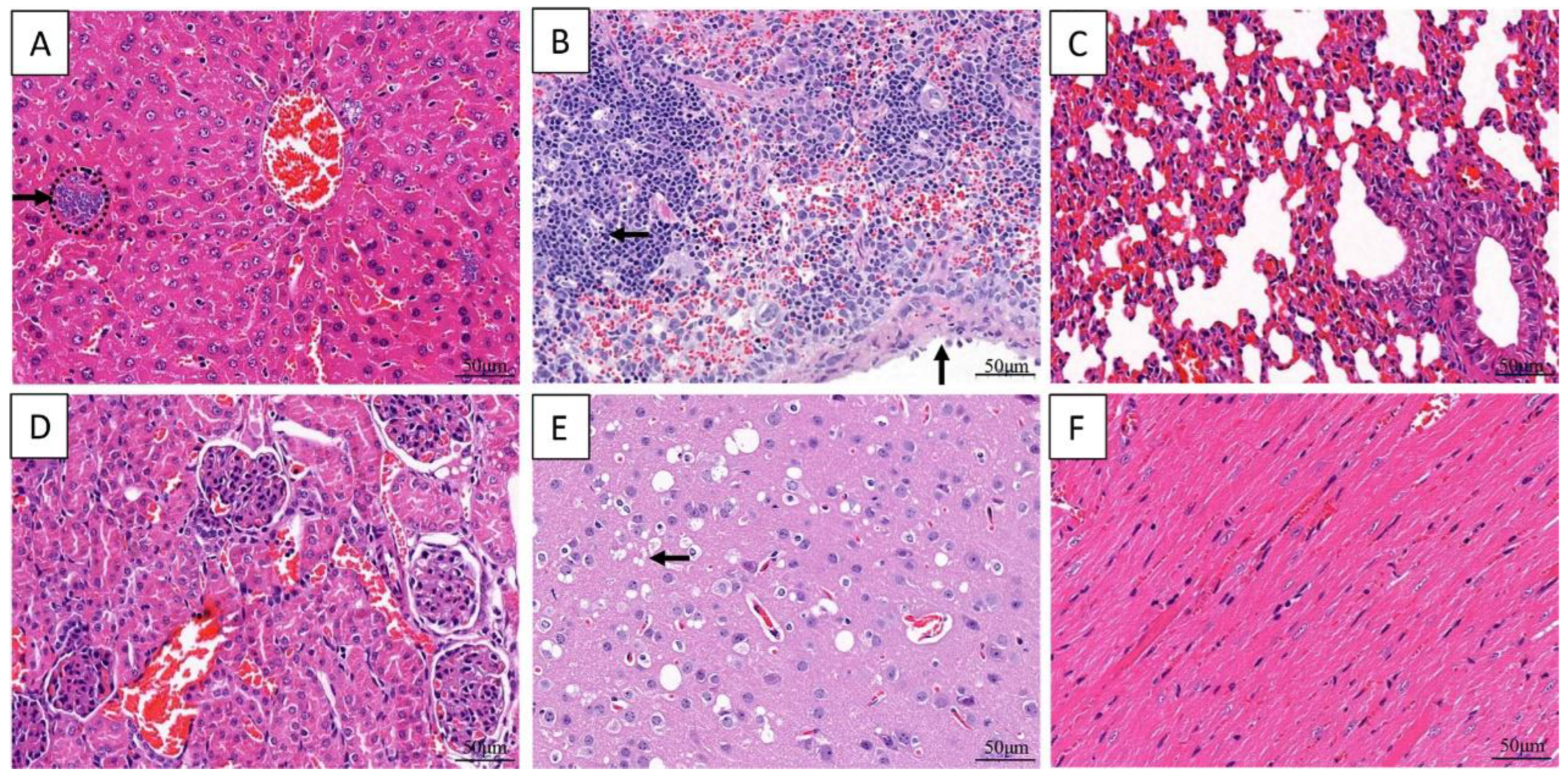
3.2. Histopathological Finding of Giant Panda
3.3. Phylogenetic Analyses of the 16S rRNA and gyrB Genes of Isolated Bacterial
3.4. The Pathogenicity Test in Mice
3.5. Histopathology of the Inoculated Mice
3.6. Virulence Gene Analyses of the Strain VGP
3.7. Antibiotic Resistance of the Strain VGP
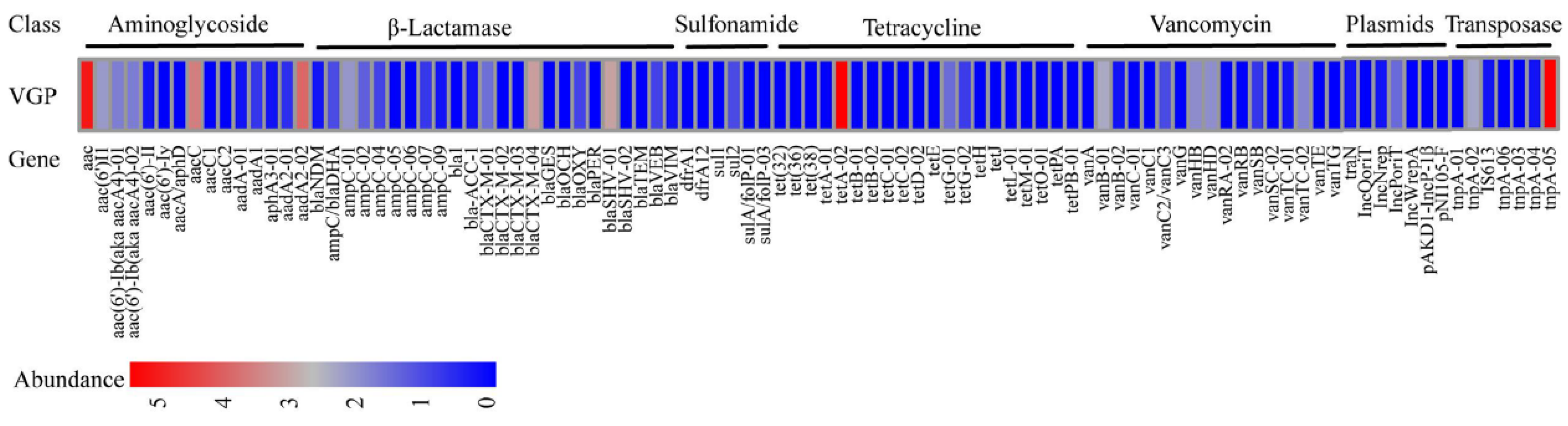
3.8. ARGs Analyses of the Strain VGP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tekedar, H.C.; Kumru, S.; Blom, J.; Perkins, A.D.; Griffin, M.J.; Abdelhamed, H.; Karsi, A.; Lawrence, M.L. Comparative genomics of Aeromonas veronii: Identification of a pathotype impacting aquaculture globally. PLoS ONE 2019, 14, e0221018. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Raza, S.H.A.; Yang, B.; Sun, Y.; Wang, G.; Sun, W.; Qian, A.; Wang, C.; Kang, Y.; Shan, X. Aeromonas veronii Infection in Commercial Freshwater Fish: A Potential Threat to Public Health. Animals 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mao, C.; Feng, J.; Li, Y.; Hu, J.; Jiang, B.; Gu, Q.; Su, Y. A First Report of Aeromonas veronii Infection of the Sea Bass, Lateolabrax maculatus in China. Front. Veter- Sci. 2021, 7, 600587. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Sun, J.; Han, Z.; Yang, X.; Xian, J.A.; Lv, A.; Hu, X.; Shi, H. Isolation, Identification and Characteristics of Aeromonas veronii From Diseased Crucian Carp (Carassius auratus gibelio). Front. Microbiol. 2019, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Dong, N.; Chen, S.; Shu, L.; Sun, Q.; Zhou, H.; Wang, H.; Chan, E.W.; Gu, D. Detection and genetic characterization of the colistin resistance gene mcr-3.3 in an Aeromonas veronii strain isolated from alligator faeces. J. Glob. Antimicrob. Resist. 2020, 22, 860–861. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zheng, A.F.; Chen, M.M.; Lian, Y.X.; Zhang, X.K.; Zhang, S.Z.; Yu, D.; Li, J.K. Isolation and identification of pathogenic Aeromonas veronii from a dead Yangtze finless porpoise. Dis. Aquat. Org. 2018, 132, 13–22. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, Z.; Li, B.; Xue, M.; Jiang, N.; Fan, Y.; Chen, P.; Qi, F.; Kong, X.; Zhou, Y. Isolation, Identification, and Characterization of Aeromonas veronii from Chinese Soft-Shelled Turtle (Trionyx sinensis). Microorganisms 2023, 11, 1304. [Google Scholar] [CrossRef]
- Su, X.; Zhong, Y.; Wang, X.; Qin, T.; Chen, S.; Peng, D. Isolation an identification of Aeromonas veronii from duck and preliminary study on its pathogenicity. Chin. J. Prev. Vet. Med. 2020, 42, 191–194. (In Chinese) [Google Scholar]
- Li, W.; Zhao, Y.; Liu, Y.; Qi, X.; Kang, K.; Chen, M. Isolation and identification of pathogenic Aeromonas veronii from fox. Chin. J. Prev. Vet. Med. 2012, 34, 289–292. (In Chinese) [Google Scholar]
- Yi, Z.; Qiu, Z.; Ze, M.; Tang, C.; Yue, H. Isolation and identification of Aeromonas veronii from yak. J. Grass Forage Sci. (In Chinese). 2021, 3, 59. [Google Scholar]
- Rodríguez-Calleja, J.; García-López, I.; García-López, M.; Santos, J.; Otero, A. Rabbit meat as a source of bacterial foodborne pathogens. J. Food Prot. 2006, 69, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Najera, F.; Grande-Gomez, R.; Pena, J.; Vazquez, A.; Palacios, M.J.; Rueda, C.; Corona-Bravo, A.I.; Zorrilla, I.; Revuelta, L.; Gil-Molino, M.; et al. Disease Surveillance during the Reintroduction of the Iberian Lynx (Lynx pardinus) in Southwestern Spain. Animals 2021, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Borges, A.; Saavedra, M.J.; Simoes, M. Biofilm formation and multidrug-resistant Aeromonas spp. from wild animals. J. Glob. Antimicrob. Resist. 2018, 12, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, D.S.; Silva, A.L.; Monteiro, F.O.; Guedes, G.M.; Sales, J.A.; Oliveira, J.S.; Maia Junior, J.E.; Miranda, S.A.; Sidrim, J.J.; Alencar, L.P.; et al. Aeromonas and Plesiomonas species from scarlet ibis (Eudocimus ruber) and their environment: Monitoring antimicrobial susceptibility and virulence. Antonie Van Leeuwenhoek 2017, 110, 33–43. [Google Scholar] [CrossRef]
- Fernandez-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Laviad-Shitrit, S.; Izhaki, I.; Arakawa, E.; Halpern, M. Wild waterfowl as potential vectors of Vibrio cholerae and Aeromonas species. Trop. Med. Int. Health 2018, 23, 758–764. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Carr, M.; Booher, C.R.; Pointer, A.M.; Mitchell, B.M.; Smith, N.; Calnan, K.; Montgomery, G.M.; Ogada, M.; Kramer, D.B. Characteristics that make trophy hunting of giant pandas inconceivable. Conserv. Biol. 2020, 34, 915–924. [Google Scholar] [CrossRef]
- Chen, D.; Zou, W.; Xie, S.; Kong, L.; Chen, Y.; Zhang, X.; Li, J.; Wang, H.; Cheng, G.; Qin, Y.; et al. Serotype and Antimicrobial Resistance of Escherichia Coli Isolated from Feces of Wild Giant Pandas (Ailuropoda Melanoleuca) in Sichuan Province, China. J. Wildl. Dis. 2018, 54, 691–699. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, L.; Jia, M.; Wang, T.; Liang, B.; Ji, X.; Sun, Y.; Liu, J.; Guo, X. Detection of Multi-Drug-Resistant Escherichia coli in a Giant Panda (Ailuropoda melanoleuca) with Extraintestinal Polyinfection. J. Wildl. Dis. 2018, 54, 626–630. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Su, X.; Gen, Y.; Huang, W.; Chen, X.; Bai, M.; Chen, Z. A Case of Klebsiella pneumoniae and Proteus mirabilis Infection in Giant Panda(Ailuropoda melanoleuca). Chin. J. Wildl. 2020, 41, 1013–1019. (In Chinese) [Google Scholar]
- Wang, C.; Li, D.; Tang, C.; Deng, L.; Huang, Z.; Han, H.; Zhang, Y. A case of giant panda reproductive tract infection with Proteus mirabilis. Sichuan J. Zool. 2007, 26, 167. (In Chinese) [Google Scholar]
- Zeng, X.; Chi, X.; Xiu, Y.; Xu, S.; Huang, Y.; Li, C. Isolation, identification and pathogenicity analysis of a strain of Proteus vulgaris from giant panda. Chin. Vet. Sci. 2020, 50, 1379–1388. (In Chinese) [Google Scholar]
- Li, C.; Wang, C.; Tang, C.; Wu, H.; Deng, L.; Zeng, C.; Zhang, Y.; Li, D. A case of Enterobacter cloacae respiratory tract infection in giant panda. Anim. Husb. Vet. Med. 2013, 45, 64–66. (In Chinese) [Google Scholar]
- Luo, L.; Wang, C.; Yang, Z.; Lan, J.; Yu, J.; Huang, X. A Subadult Giant Panda Case of Staphylococcus aureus Resparatory Tract Infection. Sichuan J. Zool. 2005, 24, 593. (In Chinese) [Google Scholar]
- Soler, L.; Yanez, M.A.; Chacon, M.R.; Aguilera-Arreola, M.G.; Catalan, V.; Figueras, M.J.; Martinez-Murcia, A.J. Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 5, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Koksal, F.; Oguzkurt, N.; Samastı, M.; Altas, K. Prevalence and antimicrobial resistance patterns of Aeromonas strains isolated from drinking water samples in istanbul, Turkey. Chemotherapy 2007, 53, 30–35. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards For Antimicrobial Susceptibility Testing: Twenty—Fourth Informational Supplement CLSI Document M100-S24; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Yan, X.; Su, X.; Ren, Z.; Fan, X.; Li, Y.; Yue, C.; Yang, M.; Deng, H.; Deng, Y.; Xu, Z.; et al. High Prevalence of Antimicrobial Resistance and Integron Gene Cassettes in Multi-Drug-Resistant Klebsiella pneumoniae Isolates From Captive Giant Pandas (Ailuropoda melanoleuca). Front. Microbiol. 2021, 12, 801292. [Google Scholar] [CrossRef]
- Busse, H.J.; Denner, E.; Lubitz, W. Classification and identification of bacteria: Current approaches to an old problem. Overview of methods used in bacterial systematics. J. Biotechnol. 1996, 47, 3–38. [Google Scholar] [CrossRef]
- Ran, C.; Qin, C.; Xie, M.; Zhang, J.; Li, J.; Xie, Y.; Wang, Y.; Li, S.; Liu, L.; Fu, X.; et al. Aeromonas veronii and aerolysin are important for the pathogenesis of motile aeromonad septicemia in cyprinid fish. Environ. Microbiol. 2018, 20, 3442–3456. [Google Scholar] [CrossRef]
- Jonson, A.B.; Normark, S.; Rhen, M. Fimbriae, pili, flagella and bacterial virulence. Contrib. Microbiol. 2005, 12, 67–89. [Google Scholar]
- Beaz-Hidalgo, R.; Figueras, M.J. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. J. Fish Dis. 2013, 36, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.M.; Dacanay, A.; Knickle, L.C.; Touhami, A.; Brown, L.L.; Jericho, M.H.; Johnson, S.C.; Reith, M. Contribution of type IV pili to the virulence of Aeromonas salmonicida subsp. salmonicida in Atlantic salmon (Salmo salar L.). Infect. Immun. 2008, 76, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Reith, M.E.; Singh, R.K.; Curtis, B.; Boyd, J.M.; Bouevitch, A.; Kimball, J.; Munholland, J.; Murphy, C.; Sarty, D.; Williams, J.; et al. The genome of Aeromonas salmonicida subsp. salmonicida A449, insights into the evolution of a fish pathogen. BMC Genom. 2008, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Vipond, R.; Bricknell, I.R.; Durant, E.; Bowden, T.J.; Ellis, A.E.; Smith, M.; MacIntyre, S. Defined deletion mutants demonstrate that the major secreted toxins are not essential for the virulence of Aeromonas salmonicida. Infect. Immun. 1998, 66, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Gao, X.; Jiang, Q.; Wen, Y.; Lin, L. Characterization of Virulence Properties of Aeromonas veronii Isolated from Diseased Gibel Carp (Carassius gibelio). Int. J. Mol. Sci. 2016, 17, 496. [Google Scholar] [CrossRef]
- Wu, J.; Su, Y.; Deng, Y.; Guo, Z.; Mao, C.; Liu, G.; Xu, L.; Cheng, C.; Bei, L.; Feng, J. Prevalence and distribution of antibiotic resistance in marine fish farming areas in Hainan, China. Sci. Total Environ. 2019, 653, 605–611. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, M.; Wang, K.Y.; Wang, J.; He, Y.; Wang, E.L.; Liu, T.; Chen, D.F.; Lai, W. Multidrug-Resistant Aeromonas veronii Recovered from Channel Catfish (Ictalurus punctatus) in China: Prevalence and Mechanisms of Fluoroquinolone Resistance. Microb. Drug Resist. 2017, 23, 473–479. [Google Scholar] [CrossRef]
- Chen, Q.; An, X.; Li, H.; Su, J.; Ma, Y.; Zhu, Y.G. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ. Int. 2016, 92–93, 1–10. [Google Scholar] [CrossRef]
- Ji, X.; Liu, J.; Liang, B.; Sun, S.; Zhu, L.; Zhou, W.; Guo, X.; Sun, Y. Molecular Characteristics of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Strains Isolated from Diseased Captive Giant Pandas (Ailuropoda melanoleuca) in China. Microb. Drug Resist. 2022, 28, 750–757. [Google Scholar] [CrossRef]
- Su, X.; Yan, X.; Li, Y.; Zhang, D.; Li, L.; Geng, Y.; Su, F.; Yue, C.; Hou, R.; Liu, S. Identification of extended-spectrum beta-lactamase (CTX-M)-producing Klebsiella pneumoniae belonging to ST37, ST290, and ST2640 in captive giant pandas. BMC Vet. Res. 2022, 18, 186. [Google Scholar] [CrossRef]




| Target Gene | Primer Sequence (5′-3′) | Product Size (bp) | Tm (°C) |
|---|---|---|---|
| aer | F: CCTATGGCCTGAGCGAGAAG | 431 | 56 |
| R: CCAGTTCCAGTCCCACCACT | |||
| act | F: GAGAAGGTGACCACCAAGAACA | 232 | 60 |
| R: AACTGACATCGGCCTTGAACTC | |||
| ser | F: CTCCTACTCCAGCGTCGGC | 128 | 64 |
| R: GATCGTCGGTGCGGTTGT | |||
| aha | F: GGCTATTGCTATCCCGGCTCTGTT | 1082 | 60 |
| R: CGGTCCACTCGTCGTCCATCTTG | |||
| lip | F: CACCTGGTKCCGCTCAAG | 247 | 56 |
| R: GTACCGAACCAGTCGGAGAA | |||
| exu | F: AGACATGCACAACCTCTTCC | 323 | 56 |
| R: GATTGGTATTGCCYTGCAA | |||
| luxs | F: GATCCTCTCCGAGGCGTGG | 369 | 58 |
| R: AGGCTTTTCAGCTTCTCTTCC | |||
| tapA | F: ATGACCTCTAGCCCCAATA | 550 | 52 |
| R: ACCCGATTGATTTCTGCC | |||
| gcaT | F: CTCCTGGAATCCCAAGTATCAG | 237 | 55 |
| R: GGCAGGTTGAACAGCAGTATCT |
| Antibiotics | Content (/disc) | Inhibition Zone (mm) | Sensitivity |
|---|---|---|---|
| PEN | 10 µg | 0.00 | R |
| PIP | 100 µg | 24.96 | S |
| AMP | 10 µg | 0.00 | R |
| OX | 1 µg | 0.00 | R |
| AMX | 20 µg | 0.00 | R |
| MOX | 30 µg | 36.05 | S |
| CAZ | 30 µg | 30.02 | S |
| CFM | 5 µg | 40.22 | S |
| CMZ | 30 µg | 35.76 | S |
| FEP | 30 µg | 34.96 | S |
| CTX | 30 µg | 39.06 | S |
| CA | 30 µg | 29.20 | S |
| CZ | 30 µg | 19.66 | S |
| CTR | 30 µg | 42.32 | S |
| FOX | 30 µg | 31.98 | S |
| TZD | 100/10 µg | 28.44 | S |
| CXM | 30 µg | 32.22 | S |
| CEC | 30 µg | 29.60 | S |
| AMS | 10/10 µg | 12.26 | I |
| CFP | 75 µg | 33.92 | S |
| ZOX | 30 µg | 35.76 | S |
| AT | 30 µg | 36.68 | S |
| MEM | 10 µg | 29.84 | S |
| IPM | 10 µg | 19.40 | R |
| K | 30 µg | 22.44 | S |
| GM | 10 µg | 22.46 | S |
| S | 10 µg | 18.49 | S |
| ENX | 10 µg | 17.54 | I |
| OFX | 5 µg | 29.54 | S |
| NOR | 10 µg | 22.45 | S |
| LOM | 10 µg | 25.59 | S |
| FOX | 5 µg | 21.02 | S |
| LVX | 5 µg | 27.48 | S |
| CIP | 5 µg | 26.86 | I |
| GAT | 5 µg | 30.24 | S |
| C | 30 µg | 32.88 | S |
| VA | 30 µg | 0.00 | R |
| AZM | 15 µg | 28.11 | S |
| DX | 30 µg | 12.83 | I |
| MI | 30 µg | 18.95 | S |
| SXT | 25 µg | 20.91 | S |
| TMP | 5 µg | 19.88 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Yang, M.; Li, Y.; Yan, X.; Hou, R.; Ayala, J.E.; Li, L.; Yue, C.; Zhang, D.; Liu, S. First Isolation and Identification of Aeromonas veronii in a Captive Giant Panda (Ailuropoda melanoleuca). Animals 2023, 13, 2779. https://doi.org/10.3390/ani13172779
Su X, Yang M, Li Y, Yan X, Hou R, Ayala JE, Li L, Yue C, Zhang D, Liu S. First Isolation and Identification of Aeromonas veronii in a Captive Giant Panda (Ailuropoda melanoleuca). Animals. 2023; 13(17):2779. https://doi.org/10.3390/ani13172779
Chicago/Turabian StyleSu, Xiaoyan, Mei Yang, Yunli Li, Xia Yan, Rong Hou, James Edward Ayala, Lin Li, Chanjuan Yue, Dongsheng Zhang, and Songrui Liu. 2023. "First Isolation and Identification of Aeromonas veronii in a Captive Giant Panda (Ailuropoda melanoleuca)" Animals 13, no. 17: 2779. https://doi.org/10.3390/ani13172779






