Effect of Perineal Urethrostomy on the Length of the Urethra of the Cat: A Cadaveric Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
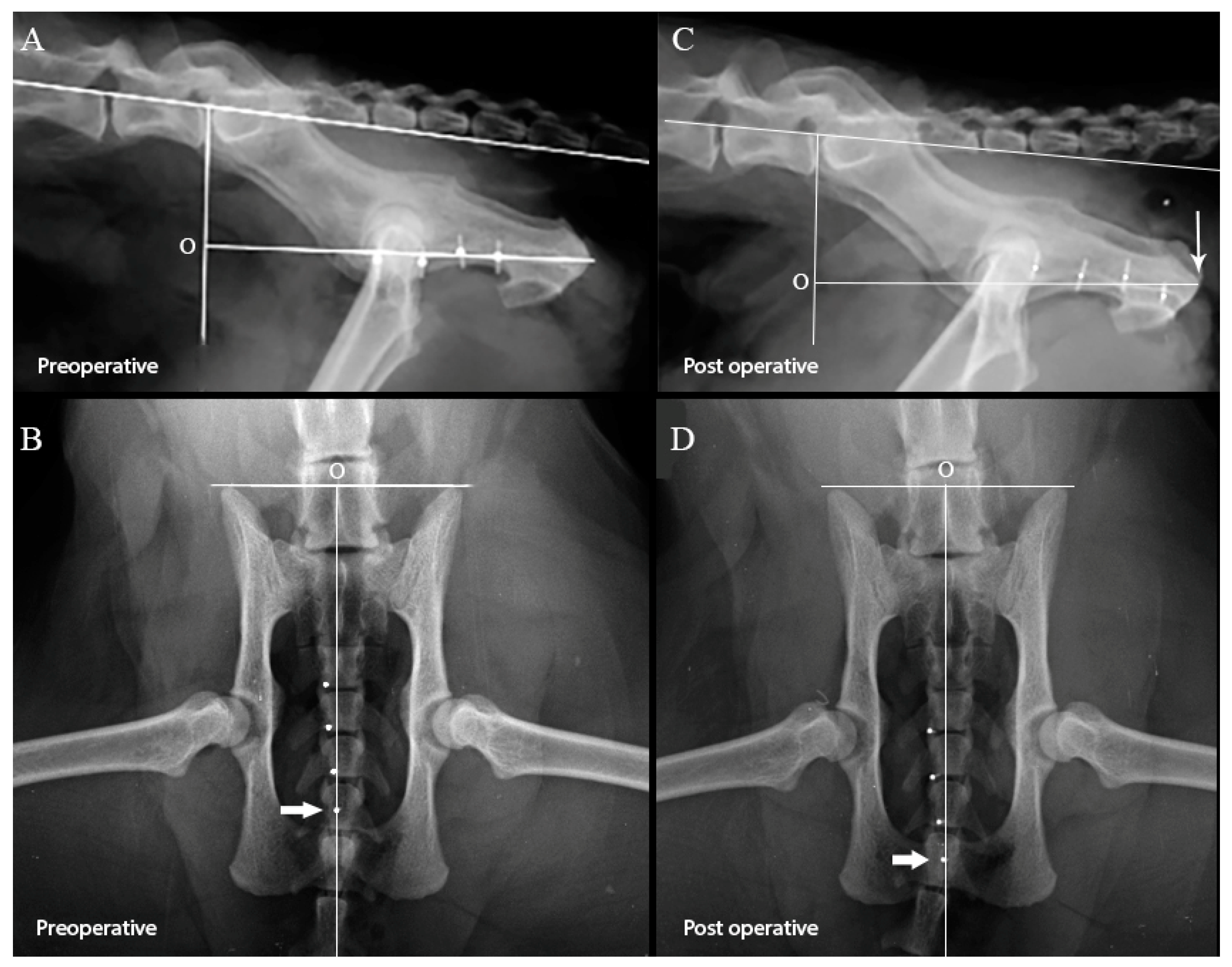
2.1. Effect of Perineal Urethrostomy on the Pelvic Urethra
2.2. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Osborne, C.A.; Kruger, J.M.; Lulich, J.P. Feline lower urinary tract disorders—Definition of terms and concepts. Vet. Clin. N. Am. -Small Anim. Pract. 1996, 26, 169–179. [Google Scholar] [CrossRef]
- Kruger, J.M.; Osborne, C.A.; Lulich, J.P. Changing Paradigms of Feline Idiopathic Cystitis. Vet. Clin. N. Am. -Small Anim. Pract. 2009, 39, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Buffington, C.A.T. Idiopathic Cystitis in Domestic Cats-Beyond the Lower Urinary Tract. J. Vet. Intern. Med. 2011, 25, 784–796. [Google Scholar] [CrossRef]
- Jones, E.; Palmieri, C.; Thompson, M.; Jackson, K.; Allavena, R. Feline Idiopathic Cystitis: Pathogenesis, Histopathology and Comparative Potential. J. Comp. Pathol. 2021, 185, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.A.; Caywood, D.D.; Johnston, G.R.; Polzin, D.J.; Lulich, J.P.; Kruger, J.M. Perineal Urethrostomy Versus Dietary-Management in Prevention of Recurrent Lower Urinary-Tract Disease. J. Small Anim. Pract. 1991, 32, 296–305. [Google Scholar] [CrossRef]
- Osborne, C.A.; Caywood, D.D.; Johnston, G.R.; Polzin, D.J.; Lulich, J.P.; Kruger, J.M.; Ulrich, L.K. Feline perineal urethrostomy—A potential cause of feline lower urinary tract disease. Vet. Clin. N. Am.-Small Anim. Pract. 1996, 26, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Cuddy, L.C.; McAlinden, A.B. Urethra. In Veterinary Surgery Small Animal, 2nd ed.; Johnston, S.A., Tobias, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2. [Google Scholar]
- Gerber, B.; Eichenberger, S.; Reusch, C.E. Guarded long-term prognosis in male cats with urethral obstruction. J. Feline Med. Surg. 2008, 10, 16–23. [Google Scholar] [CrossRef]
- Hauptman, J. Perineal Urethrostomy—Surgical Technique and Management of Complications. Vet. Clin. N. Am.-Small Anim. Pract. 1984, 14, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.P.; Harrison, W. Perineal Urethrostomy in Cats. J. Am. Vet. Med. Assoc. 1971, 159, 1789. [Google Scholar] [PubMed]
- Griffin, D.W.; Gregory, C.R. Prevalence of Bacterial Urinary-Tract Infection After Perineal Urethrostomy in Cats. J. Am. Vet. Med. Assoc. 1992, 200, 681–684. [Google Scholar]
- Addison, E.S.; Halfacree, Z.; Moore, A.H.; Demetriou, J.; Parsons, K.; Tivers, M. A retrospective analysis of urethral rupture in 63 cats. J. Feline Med. Surg. 2014, 16, 300–307. [Google Scholar] [CrossRef]
- Corgozinho, K.B.; de Souza, H.J.; Pereira, A.N.; Belchior, C.; da Silva, M.A.; Martins, M.C.L.; Damico, C.B. Catheter-induced urethral trauma in cats with urethral obstruction. J. Feline Med. Surg. 2007, 9, 481–486. [Google Scholar] [CrossRef]
- Shipov, A.; Zafrany, A.; Kahana, N.; Peery, D.; Sommer, A.; Winkler, R.; Segev, G. Successful Balloon Expandable Stent Placement in the Management of Severe Proximal Urethral Stricture in a Cat. Isr. J. Vet. Med. 2014, 69, 88–91. [Google Scholar]
- de Faria, B.G.O.; da Silva, V.M.; Silva, J.A.; Santos, S.C.A.; Sala, P.L.; Quessada, A.M.; Muramoto, C.; da Costa Neto, J.M. Autogenous Vascularized Intestinal Grafting for Urethral Reconstruction in Feline. Acta Sci. Vet. 2020, 48. [Google Scholar] [CrossRef]
- Segev, G.; Livne, H.; Ranen, E.; Lavy, E. Urethral obstruction in cats: Predisposing factors, clinical, clinicopathological characteristics and prognosis. J. Feline Med. Surg. 2011, 13, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.S.; Rozanski, E.A.; Wayne, A.S. Prazosin administration increases the rate of recurrent urethral obstruction in cats: 388 cases. J. Am. Vet. Med. Assoc. 2022, 260, S7–S11. [Google Scholar] [CrossRef]
- Cosford, K.L.; Koo, S.T. In-hospital medical management of feline urethral obstruction: A review of recent clinical research. Can. Vet. J. 2020, 61, 595–604. [Google Scholar]
- Nye, A.K.; Luther, J.K. Feline Perineal Urethrostomy: A Review of Past and Present Literature. Top. Companion Anim. Med. 2018, 33, 77–82. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, M.A. Complications of Lower Urinary Tract Surgery in Small Animals. Vet. Clin. N. Am. -Small Anim. Pract. 2011, 41, 889–913. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.; Holt, D.E. Surgical revision of the urethral stoma following perineal urethrostomy in 11 cats: (1998–2004). J. Am. Anim. Hosp. Assoc. 2006, 42, 218–222. [Google Scholar] [CrossRef]
- Bernarde, A.; Viguier, E. Transpelvic urethrostomy in 11 cats using an ischial ostectomy. Vet. Surg. 2004, 33, 246–252. [Google Scholar] [CrossRef]
- Baines, S.J.; Rennie, S.; White, R.A.S. Prepubic urethrostomy: A long-term study in 16 cats. Vet. Surg. 2001, 30, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ellison, G.W.; Lewis, D.D.; Boren, F.C. Subpubic Urethrostomy to Salvage a Failed Perineal Urethrostomy in a Cat. Compend. Contin. Educ. Pract. Vet. 1989, 11, 946–951. [Google Scholar]
- Williams, J. Surgical Management of Blocked Cats Which approach and when? J. Feline Med. Surg. 2009, 11, 14–22. [Google Scholar] [CrossRef]
- Smith, C.W. Perineal urethrostomy. Vet. Clin. N. Am.-Small Anim. Pract. 2002, 32, 917. [Google Scholar] [CrossRef]
- Smith, C.W.; Schiller, A.G. Perineal Urethrostomy in Cat—Retrospective Study of Complications. J. Am. Anim. Hosp. Assoc. 1978, 14, 225–228. [Google Scholar]
- Sousa-Filho, R.P.; Nunes-Pinheiro, D.C.; Sampaio, K.O.; da Silva, E.C.; Cavalcanti, G.A.; da Cunha, M.G.M.M. Clinical outcomes of 28 cats 12–24 months after urethrostomy. J. Feline Med. Surg. 2020, 22, 890–897. [Google Scholar] [CrossRef]
- Griffin, D.W.; Gregory, C.R.; Kitchell, R.L. Preservation of Striated-Muscle Urethral Sphincter Function with Use of a Surgical Technique for Perineal Urethrostomy in Cats. J. Am. Vet. Med. Assoc. 1989, 194, 1057–1060. [Google Scholar]
- Yoo, P.B.; Woock, J.P.; Grill, W.M. Somatic innervation of the feline lower urinary tract. Brain Res. 2008, 1246, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Dumartinet, C.; Bernard, F.; Bernarde, A. Outcomes and postoperative complications after transpelvic urethrostomy used as first-line surgery in 38 male cats with obstructive lower urinary tract disease. J. Feline Med. Surg. 2022, 24, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, M.; Stamenova, P.; Lee, K. Comparison of surgical indications and short- and long-term complications in 56 cats undergoing perineal, transpelvic or prepubic urethrostomy. J. Feline Med. Surg. 2021, 23, 477–486. [Google Scholar] [CrossRef] [PubMed]
| Markers | Distance on Lateral View [Median (Range), mm] | p Value | Distance on Ventro-Dorsal View [Median (Range), mm] | p Value | ||
|---|---|---|---|---|---|---|
| Pre PU | Post PU | Pre PU | Post PU | |||
| Markers 1 and 2 | 8.5 (5.0–11.1) | 8.3 (5.4–11.0) | 0.610 | 8.7 (6.0–10.5) | 8.5 (5.9–13.2) | 0.310 |
| Markers 2 and 3 | 9.1 (7.1–9.6) | 9.2 (7.6–11.5) | 0.230 | 9.2 (6.9–10.1) | 9.8 (6.7–11.0) | 0.052 |
| Markers 3 and 4 | 9.0 (6.4–10.6) | 9.9 (6.4–11.4) | 0.059 | 9.5 (6.1–10.4) | 9.4 (5.8–12.0) | 0.553 |
| Origin and marker 1 | 26.8 (19.2–31.9) | 34.0 (18.7–37.6) | 0.022 | 43.7 (39.3–51.8) | 51.3 (43.2–58.1) | 0.009 |
| Origin and marker 2 | 34.7 (25.4–41.8) | 41.77 (29.7–47.0) | 0.037 | 53.8 (48.3–59.1) | 61.1 (51.4–66.0) | 0.005 |
| Origin and marker 3 | 43.0 (34.6–51.6) | 50.44 (37.3–58.5) | 0.013 | 63.6 (55.9–67.9) | 69.4 (61.2–75.9) | 0.005 |
| Origin and marker 4 | 49.6 (43.7–61.3) | 58.7 (47.0–69.5) | 0.009 | 71.4 (63.1–77.5) | 77.6 (71.2–86.6) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shipov, A.; Israeli, I.; Billet, J.-P.; Adam, Y.; Milgram, J. Effect of Perineal Urethrostomy on the Length of the Urethra of the Cat: A Cadaveric Study. Animals 2023, 13, 2810. https://doi.org/10.3390/ani13182810
Shipov A, Israeli I, Billet J-P, Adam Y, Milgram J. Effect of Perineal Urethrostomy on the Length of the Urethra of the Cat: A Cadaveric Study. Animals. 2023; 13(18):2810. https://doi.org/10.3390/ani13182810
Chicago/Turabian StyleShipov, Anna, Inbar Israeli, Jean-Philippe Billet, Yoav Adam, and Joshua Milgram. 2023. "Effect of Perineal Urethrostomy on the Length of the Urethra of the Cat: A Cadaveric Study" Animals 13, no. 18: 2810. https://doi.org/10.3390/ani13182810
APA StyleShipov, A., Israeli, I., Billet, J.-P., Adam, Y., & Milgram, J. (2023). Effect of Perineal Urethrostomy on the Length of the Urethra of the Cat: A Cadaveric Study. Animals, 13(18), 2810. https://doi.org/10.3390/ani13182810





