Migration Route of Sthenoteuthis oualaniensis in the South China Sea Based on Statolith Trace Element Information
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
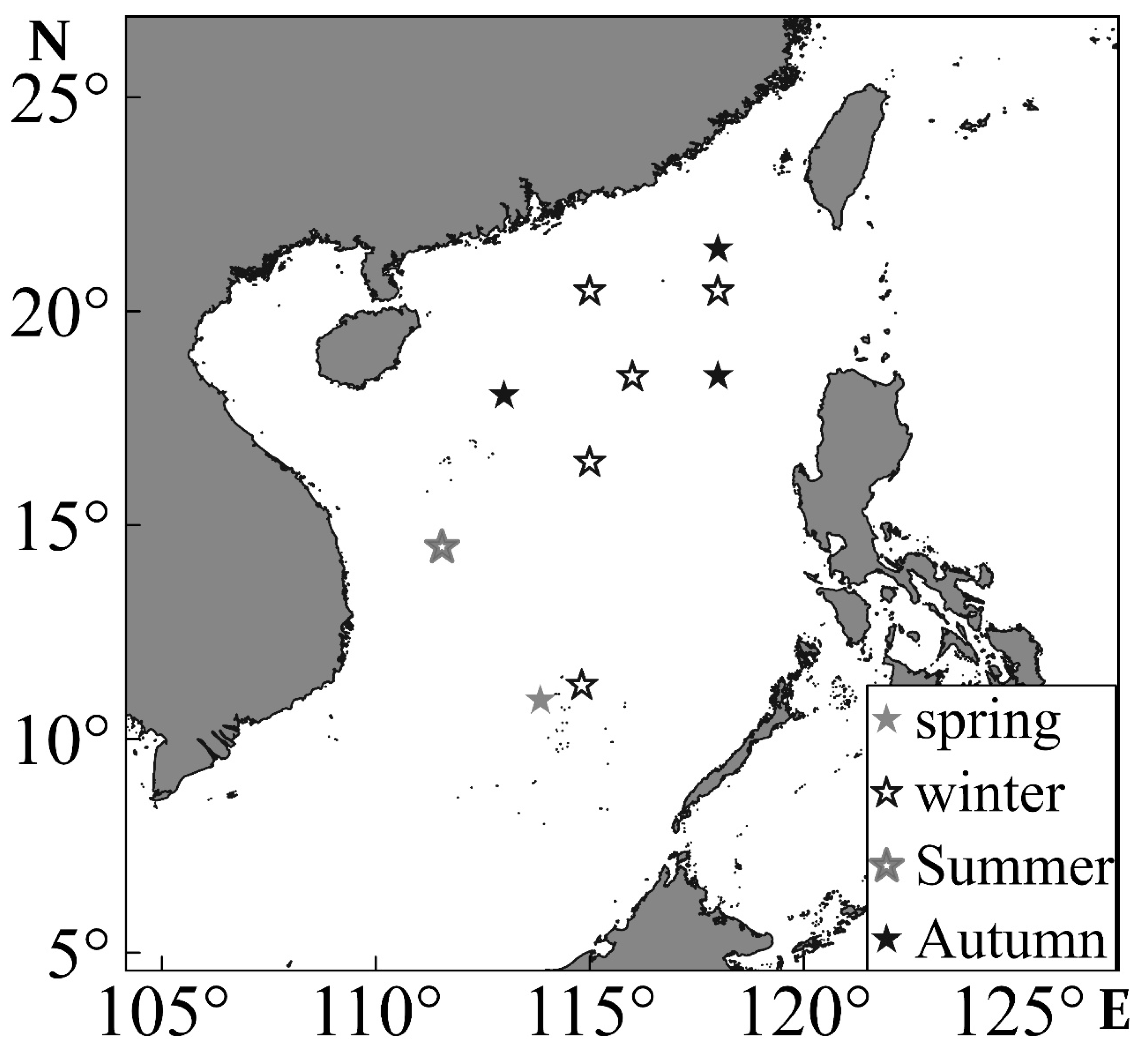
2.1. Survey Time and Sea Area
2.2. Environmental Data
2.3. Methods
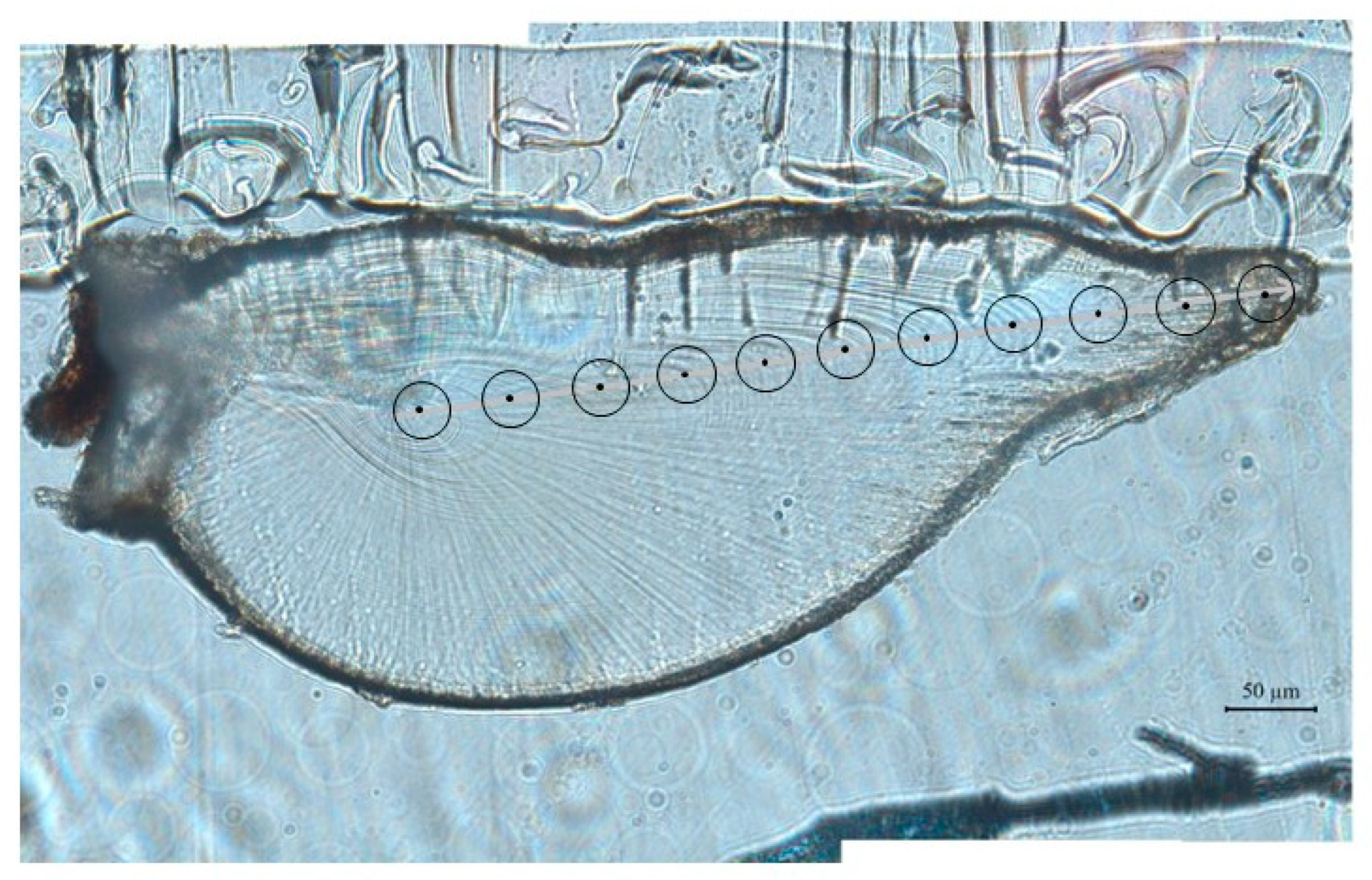
2.3.1. Statolith Measurements
2.3.2. Determination of Statolith Trace Elements
2.4. Statistical Analysis
2.5. Migratory Hypothesis and Remodeling
- (1)
- (2)
- (3)
- The calculation of the migration center of gravity [34].
3. Results
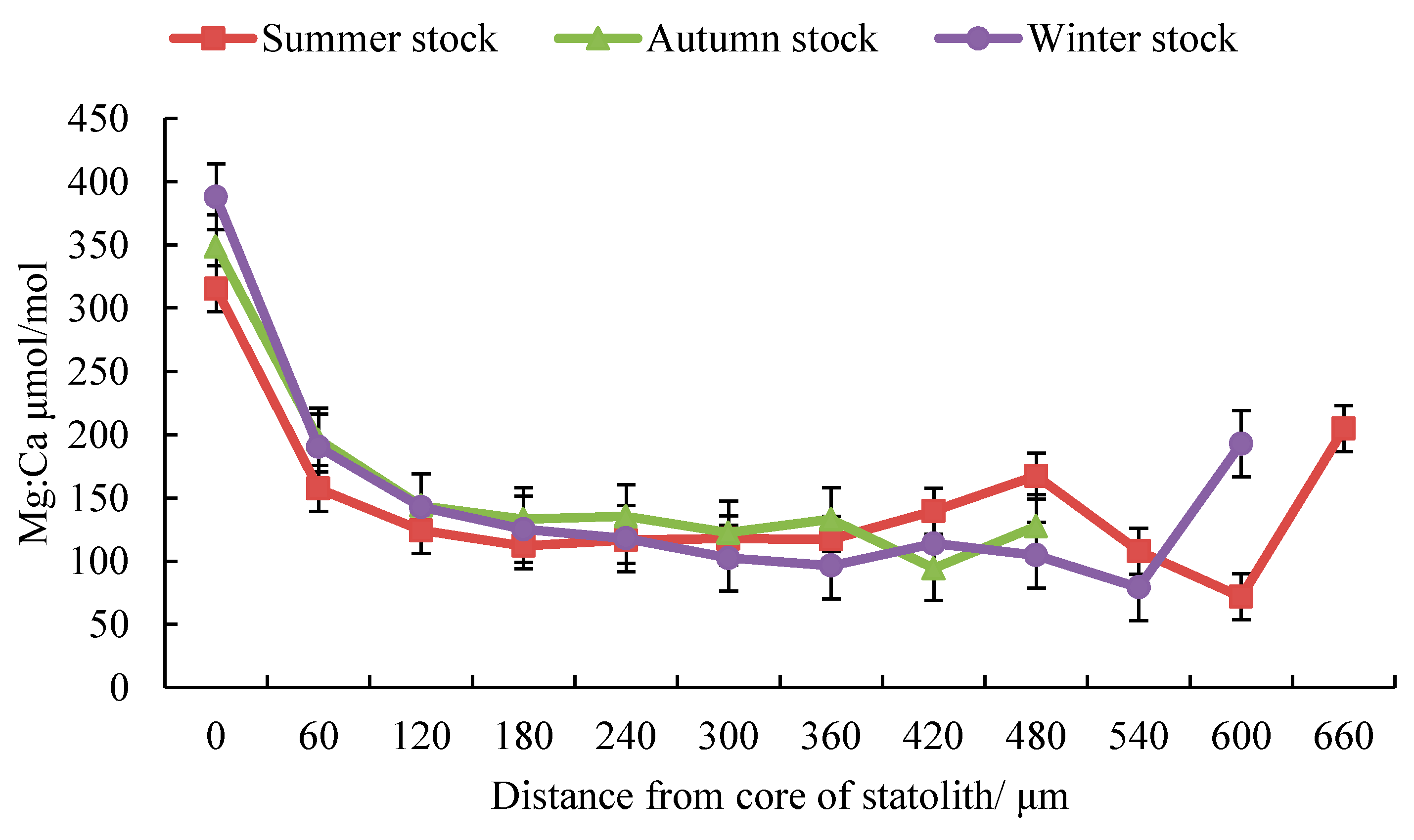
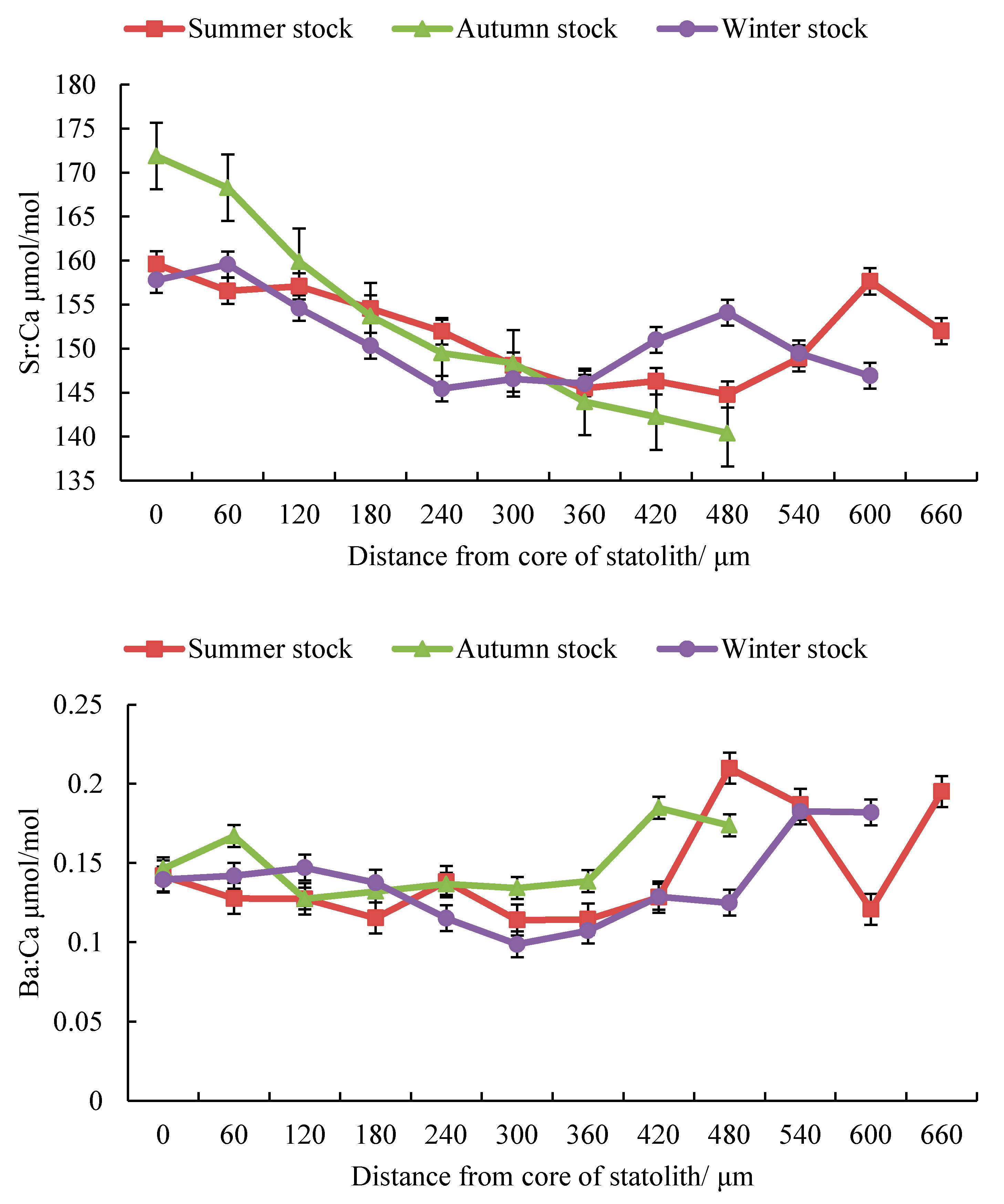
3.1. Composition of Trace Elements
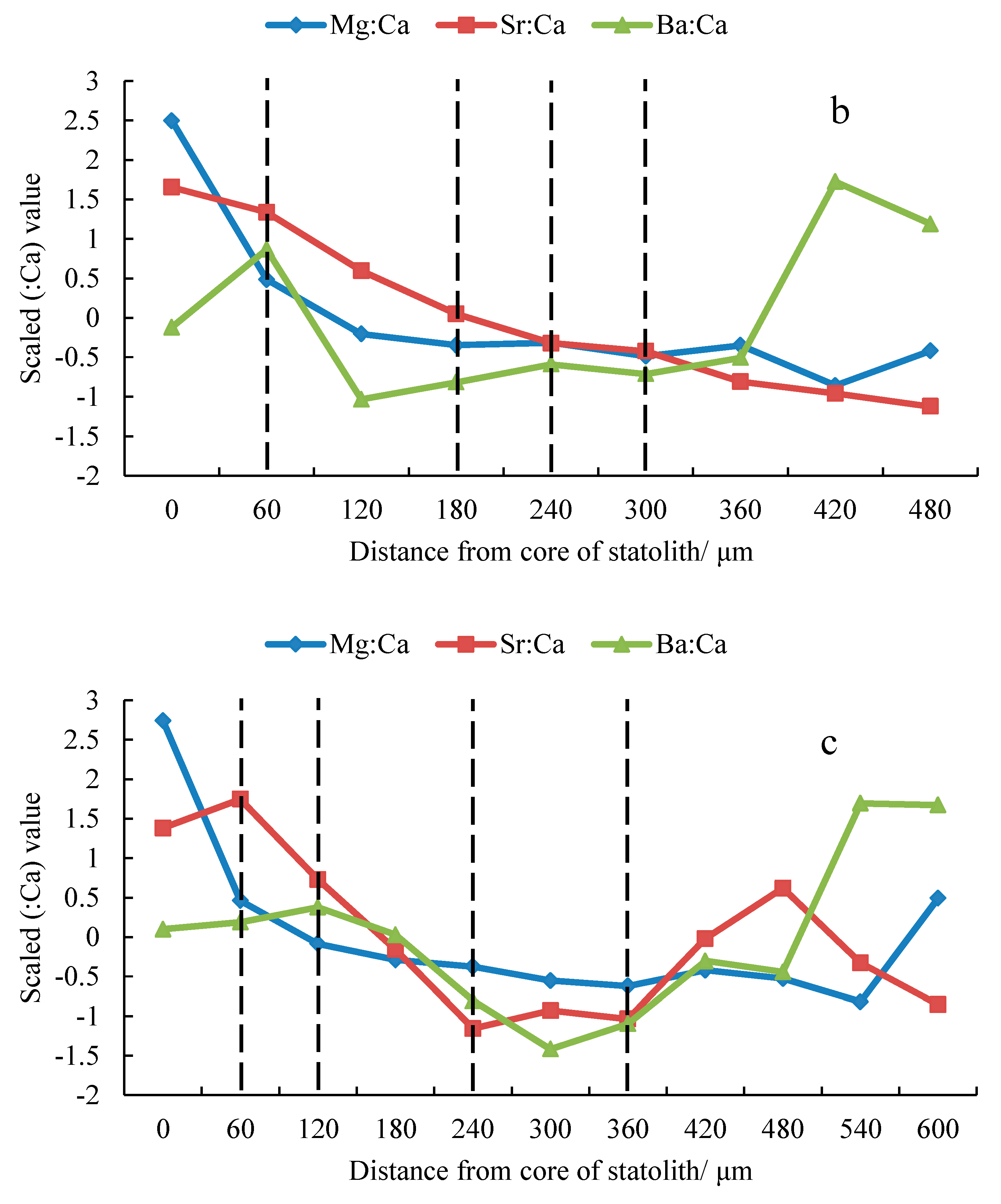
3.2. Trace Element Ratios
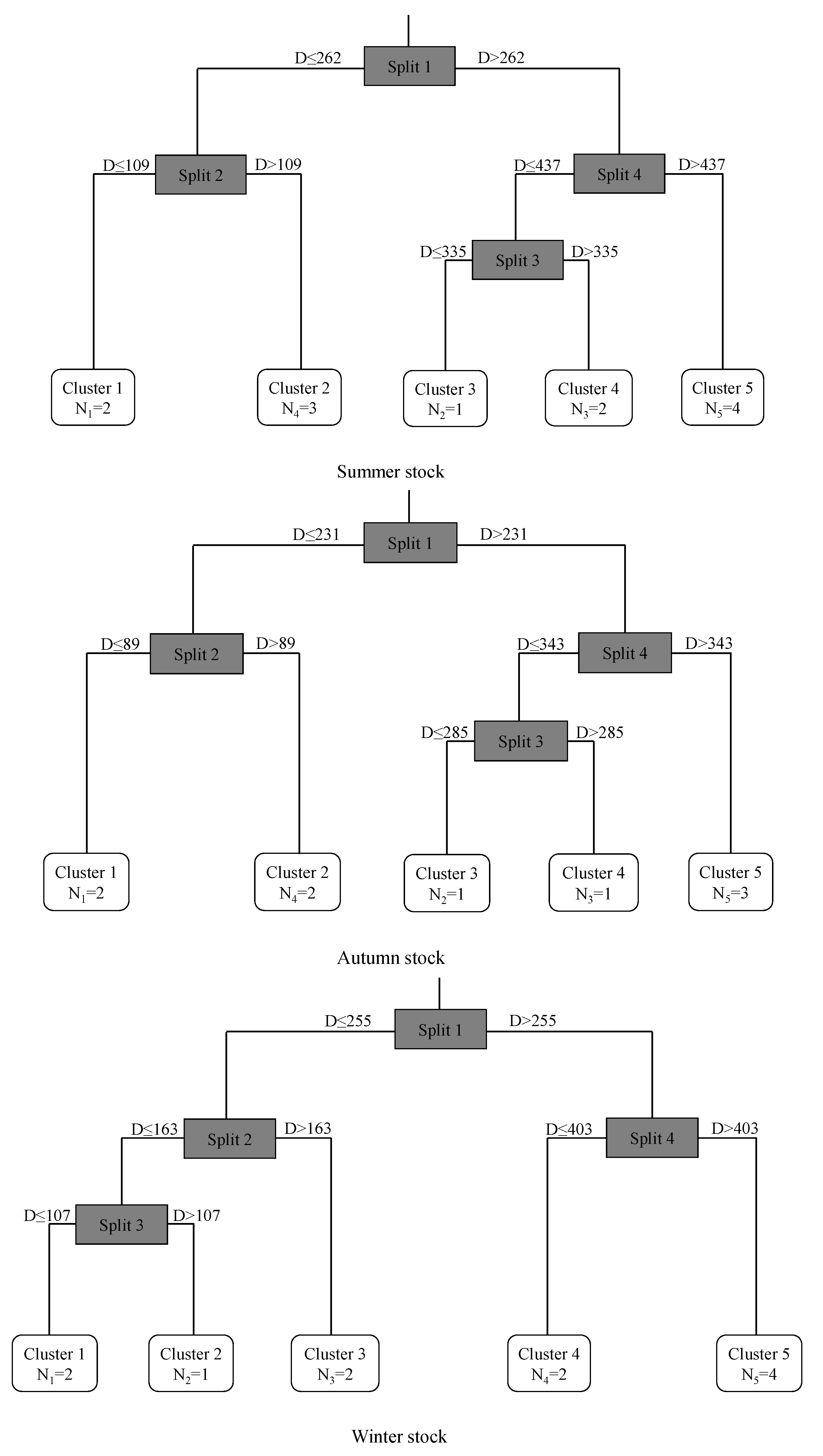
3.3. Multivariate Time Series Clustering
3.4. Relationship between Element Ratios and Temperature Variables
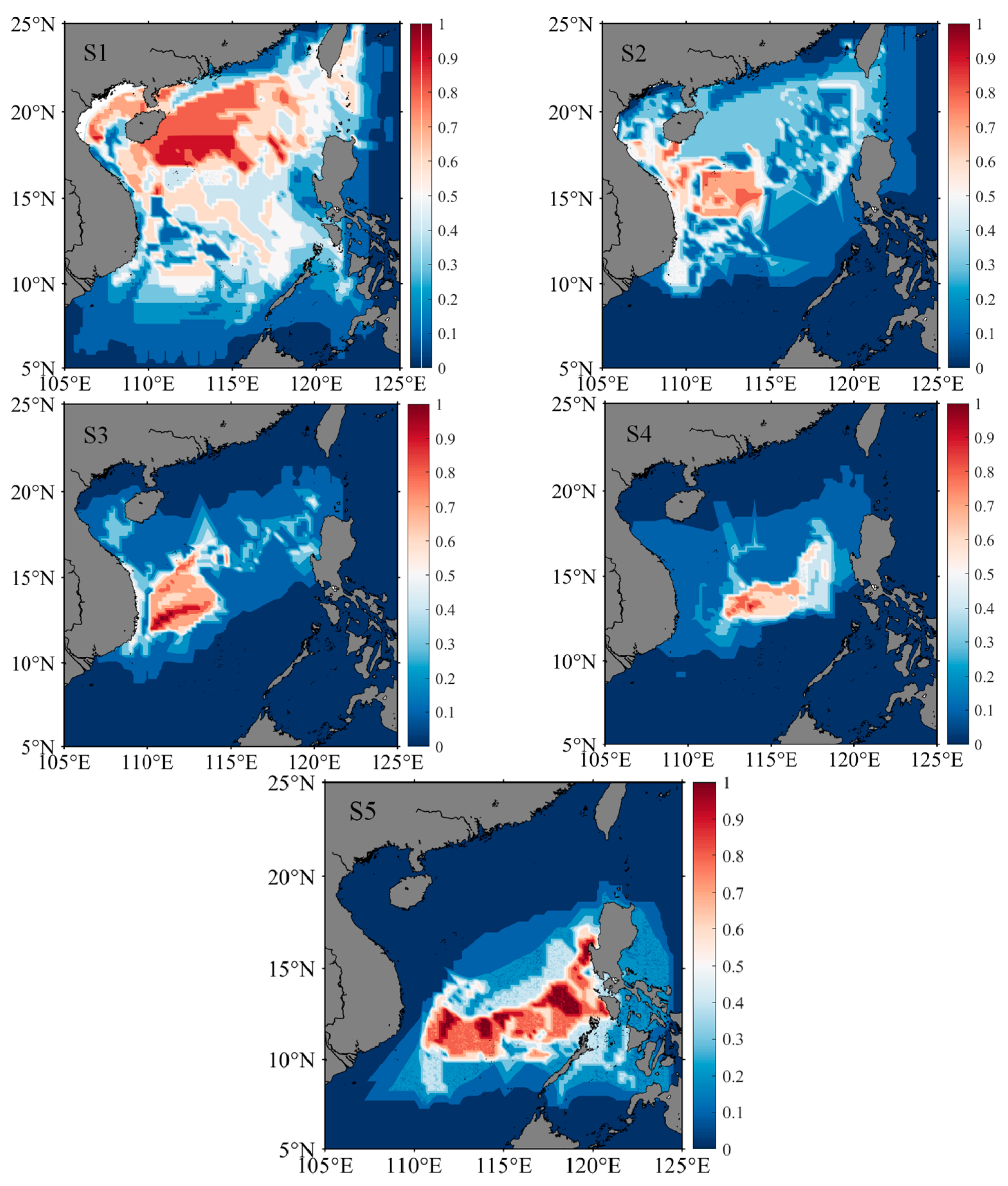
3.5. Distribution of Migratory Habitats
4. Discussion
4.1. Analysis of Trace Elements
4.2. Analyses of Effective Trace Elements Relative to Ca
4.3. Habitat Changes across Life History Stages
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World Squid Fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Hendrickson, L.C.; Ignacio, P.; Pierce, G.J.; Roa-Ureta, R.H.; Robin, J.P.; Winter, A. Stock assessment and management of cephalopods: Advances and challenges for short-lived fishery resources. ICES J. Mar. Sci. 2021, 78, 714–730. [Google Scholar] [CrossRef]
- Fan, J.T.; Fang, Z.; Ma, S.W.; Zhang, P.; Chen, Z.Z. Age, growth, and population characteristics of Sthenoteuthis oualaniensis in the South China Sea. Reg. Stud. Mar. Sci. 2021, 55, 102517. [Google Scholar] [CrossRef]
- Han, P.W.; Fang, Z.; Li, N.; Chen, X.J. Migration route reconstruction of different cohorts of Ommastrephes bartramii in the North Pacific based on statolith microchemistry. Front. Mar. Sci. 2022, 9, 832639. [Google Scholar] [CrossRef]
- Liu, B.L.; Cao, J.; Truesdell, S.B.; Chen, Y.; Chen, X.J.; Tian, S.Q. Reconstructing cephalopod migration with statolith elemental signatures: A case study using Dosidicus gigas. Fish. Sci. 2016, 82, 425–433. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Fang, Z.; Hu, S.; Song, Q. A preliminary analysis of trace-elemental signatures in statoliths of different spawning cohorts for Dosidicus gigas off EEZ waters of Chile. J. Ocean. Univ. China 2015, 14, 1059–1067. [Google Scholar] [CrossRef]
- Gilly, W.F.; Markaida, U.; Baxter, C.H.; Block, B.A.; Boustany, A.; Zeidberg, L.; Reisenbichler, K.; Robison, B.; Bazzino, G.; Salinas, C. Vertical and horizontal migrations by the squid Dosidicus gigas revealed by electronic tagging. Mar. Ecol. Prog. Ser. 2006, 324, 1–17. [Google Scholar] [CrossRef]
- Sakai, M.; Tsuchiya, K.; Mariategui, L.; Wakabayashi, T.; Yamashiro, C. Vertical Migratory Behavior of Jumbo Flying Squid (Dosidicus gigas) off Peru: Records of Acoustic and Pop-up Tags. Jpn. Agric. Res. Q. 2017, 51, 171–179. [Google Scholar] [CrossRef]
- Semmens, J.M.; Pecl, G.T.; Gillanders, B.M.; Waluda, C.M.; Shea, E.K.; Jouffre, D.; Shaw, P.W. Approaches to resolving cephalopod movement and migration patterns. Rev. Fish Biol. Fish. 2007, 17, 401. [Google Scholar] [CrossRef]
- Stewart, J.S.; Hazen, E.L.; Foley, D.G.; Bograd, S.J.; Gilly, W.F. Marine predator migration during range expansion: Humboldt squid Dosidicus gigas in the northern California Current System. Mar. Ecol. Prog. Ser. 2012, 471, 135–150. [Google Scholar] [CrossRef][Green Version]
- Arkhipkin, A.I.; Campana, S.E.; FitzGerald, J.; Thorrold, S.R. Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid (Loligo gahi). Can. J. Fish. Aquat. Sci. 2004, 61, 1212–1224. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Chen, Y.; Qian, W.G. Trace elements in the wtatoliths of jumbo flying squid off the Exclusive Economic Zones of Chile and Peru. Mar. Ecol. Prog. Ser. 2011, 429, 93–101. [Google Scholar] [CrossRef][Green Version]
- Radtke, R. Chemical and structural characteristics of statoliths from the shortfinned squid Illex illecebrosus. Mar. Biol. 1983, 76, 47–54. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Lu, H.J.; Ma, J. Cephalopod Statolith; Ocean Press: Beijing, China, 2011. [Google Scholar]
- Zumholz, K.; Klugel, A.; Hansteen, T.; Piatkowski, U. Statolith microchemistry traces the environmental history of the boreoatlantic armhook squid Gonatus fabricii. Mar. Ecol. Prog. 2007, 333, 195–204. [Google Scholar] [CrossRef]
- Jin, Y.; Li, N.; Yu, J.; Fang, Z.; Chen, X.J. Preliminary Study on the migration characteristic of two Loligo species in the northern south China Sea based on otolith microchemistry. Ocean. Et Lim. Sin. 2021, 52, 1540–1548. [Google Scholar] [CrossRef]
- Izzo, C.; Doubleday, Z.A.; Schultz, A.G.; Woodcock, S.H.; Gillanders, B.M. Contribution of water chemistry and fish condition to otolith chemistry: Comparisons across salinity environments. J. Fish Biol. 2015, 86, 1680–1698. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.O.; Bockmon, E.E.; Frieder, C.A.; Gonzalez, J.P.; Levin, L.A. Environmental pH, O-2 and capsular effects on the geochemical composition of statoliths of embryonic squid Doryteuthis opalescens. Water 2014, 6, 2233–2254. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawakami, Y.; Matsuyama, M. Analysis of the hatching site and migratory behaviour of the swordtip squid (Uroteuthis edulis) caught in the Japan Sea and Tsushima Strait in autumn estimated by statolith analysis. Mar. Biol. Res. 2018, 14, 105–112. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wu, X.C.; Lu, H.J.; Liu, K.; Ren, P.; Ning, X.; Chen, X.J. Beak microstructure and growth characteristics of Sthenoteuthis oualaniensis in the Xisha islands waters of the south China sea. Oceanol. Limnol. Sin. 2021, 52, 1293–1302. [Google Scholar] [CrossRef]
- Jereb, P.; Roper, C.F.E. Cephalopods of the World. An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date. Vol. 2. Myopsid and Oegopsid Squids; FAO Species Catalogue for Fishery Purposes; FAO: Rome, Italy, 2010. [Google Scholar]
- Liu, Y.; Wang, X.H.; Du, F.Y.; Liu, B.L.; Zhang, P.; Liu, M.N.; Qiu, Y.S. Age and growth of Sthenoteuthis oualaniensis based on statolith microstructure in the South China sea. J. Trop. Oceanogr. 2019, 38, 62–73. [Google Scholar]
- Arkhipkin, A.I. Squid as nutrient vectors linking Southwest Atlantic marine ecosystems. Deep. Sea. Res. II Top. Stud. Oceanogr. 2013, 95, 7–20. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, B.L.; Tian, S.Q.; Qian, W.G.; Zhao, X.H. Fishery biology of purpleback squid, Sthenoteuthis oualaniensis, in the Northwest Indian Ocean. Fish. Res. 2007, 83, 98–104. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Zhong, J.S. Age, growth and population composition of squid Sthenoteuthis oualaniensis in northwest Indian Ocean by statolith microstructure. J. Dalian Ocean. Univ. 2009, 24, 2016–2212. [Google Scholar] [CrossRef]
- Zhao, C.X.; Shen, C.Y.; Andrew, B.; Yan, Y.R.; Kang, B. Purpleback flying squid Sthenoteuthis oualaniensis in the South China sea: Growth, resources and association with the environment. Water 2021, 13, 65. [Google Scholar] [CrossRef]
- Bower, J.R.; Ichii, T. The red flying squid (Ommastrephes bartramii): A review of recent research and the fishery in Japan. Fish. Res. 2005, 76, 39–55. [Google Scholar] [CrossRef]
- Jiang, Y.E.; Fang, Z.Q.; Lin, Z.J.; Zhang, P.; Chen, Z.Z. Trace elements in statoliths of Sthenoteuthis oualaniensis in the South China Sea. South China Fish. Sci. 2016, 12, 71–79. [Google Scholar] [CrossRef]
- Fan, J.T.; Yu, W.; Ma, S.W.; Chen, Z.Z. Spatio-temporal variability of habitat distribution of Sthenoteuthis oualaniensis in South China Sea and its interannual variation. South China Fish. Sci. 2022, 18, 1–9. [Google Scholar] [CrossRef]
- Hu, Z.C.; Gao, S.; Liu, Y.S.; Hu, S.; Chen, H.; Yuan, H. Signal enhancement in laser ablation ICP-MS by addition of nitrogen in the central channel gas. J. Anal. At. Spectrom. 2008, 23, 1093–1101. [Google Scholar] [CrossRef]
- Jones, J.B.; Arkhipkin, A.I.; Marriott, A.L.; Pierce, G.J. Reprint of using statolith elemental signatures to confirm ontogenetic migrations of the squid Doryteuthis gahi around the Falkland Islands (Southwest Atlantic). Chem. Geol. 2019, 526, 165–174. [Google Scholar] [CrossRef]
- Pan, X.D.; Zhang, C.; Ye, Z.J.; Xu, B.D.; Li, J.C.; Liu, Y. Trace elements in the otoliths of the Japanese Spanish mackerel (Scomberomorus niphonius) in the southern Yellow Sea. J. Fish. China 2019, 43, 907–916. [Google Scholar] [CrossRef]
- Li, N.; Fang, Z.; Chen, X.J.; Feng, Z.P. Preliminary study on the migration characteristics of swordtip squid (Uroteuthis edulis) based on the trace elements of statolith in the East China Sea. Reg. Stud. Mar. Sci. 2021, 46, 101879. [Google Scholar] [CrossRef]
- Li, N.; Han, P.W.; Wang, C.; Chen, X.J.; Fang, Z. Migration routes of the swordtip squid Uroteuthis edulis in the East China Sea determined based on the statolith trace element information. Hydrobiologia 2023, 850, 861–880. [Google Scholar] [CrossRef]
- Zhang, K.; Li, M.; Liu, L.; Liu, Y.; Xiong, W.; Hu, S.H. Correlation between microchemical characteristics of fish otolithes and water environment. Chem. Bioeng. 2017, 34, 28–35. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, X.J.; Chen, Y.; Tian, S.Q. Geographic variation in statolith trace elements of the Humboldt squid, Dosidicus gigas, in high seas of Eastern Pacific Ocean. Mar. Biol. 2013, 160, 2853–2862. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Wilkinson, L.M.; Munro, A.R.; Vries, M.C. Statolith chemistry of two life history stages of cuttlefish: Effects of temperature and seawater trace element concentration. Geochim. Cosmochim. Acta 2013, 101, 12–33. [Google Scholar] [CrossRef]
- Lacoue-Labarthe, T.; Reveillac, E.; Oberhansli, F.; Teyssie, J.L.; Jeffree, R.; Gattuso, J.P. Effects of ocean acidification on trace element accumulation in the early-life stages of squid Loligo vulgaris. Aquat. Toxical. 2011, 105, 166–176. [Google Scholar] [CrossRef]
- Lu, H.J.; Ning, X.; Liu, W.; Zhang, Y.X.; Chen, Z.Y.; Chen, X.J. Comparison in fishery biology of Sthenoteuthis oualaniensis in different climate events in the South China Sea. Oceanol. Limnol. Sin. 2021, 52, 1029–1038. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawakami, Y.; Matsuyama, M. Migratory routes of the swordtip squid Uroteuthis edulis inferred from statolith analysis. Aquat. Biol. 2015, 24, 53–60. [Google Scholar] [CrossRef]
- Bettencourt, V.; Guerra, A. Growth increments and biomineralization process in cephalopod statoliths. J. Exp. Mar. Biol. Ecol. 2000, 248, 191–205. [Google Scholar] [CrossRef]
- Morris, C.C. Statocyst fluid composition and its effects on calcium carbonate precipitation in the squid Alloteuthis subulata (Lamarck, 1798): Towards a model for biomineralization. Bull. Mar. Sci. 1991, 49, 379–388. [Google Scholar]
- Ichii, T.; Mahapatra, K.; Sakai, M.; Okada, Y. Life history of the neon flying squid: Effect of the oceanographic regime in the North Pacific Ocean. Mar. Ecol. Prog. Ser. 2009, 378, 1–11. [Google Scholar] [CrossRef]
- Vijai, D.; Sakai, M.; Sakurai, Y. Embryonic and paralarval development following artificial fertilization in the neon flying squid Ommastrephes bartramii. Zoomorphology 2015, 134, 417–430. [Google Scholar] [CrossRef]
- He, Y.H.; Cai, S.Q.; Wang, S.A. Advances of the study of the North Equatorial Current bifurcation and the northern circulation of the South China Sea. J. Mar. Sci. 2009, 27, 74–84. [Google Scholar]
- Nie, Y.H.; Zhan, J.M.; Chen, Z.W. Simulation and Influence Factor Analysis of Circulation and Thermal Structure of the Surface Layer of the South China Sea. Acta Scientiarum. Nat. Univ. Sunyatseni 2011, 50, 134–138. [Google Scholar]
- Su, J.L. Overview of the South China Sea circulation and its dynamics. Acta Oceanol. Sin. 2005, 27, 1–8. [Google Scholar]
- Xu, T.; Cao, Y.G. Numerical simulation study on the seasonal variations of ocean circulation in the South China Sea. Coastal. Eng. 2017, 36, 62–71. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.Z.; Chen, G.B.; Qiu, Y.S.; Liu, S.G.; Yao, Z. Hydroacoustic detection and estimation techniques of squid Sthenoteuthis oualaniensis in the South China Sea. South China Fish. Sci. 2014, 10, 1–11. [Google Scholar] [CrossRef]



| Stocks | Sampling Seasons | Measurement Quantities | Mantle Length/mm | Age/Day | Weight/g | Gonad Maturity | Sex Ratios F/M |
|---|---|---|---|---|---|---|---|
| Winter | Autumn | 28 | 119–142 | 184–223 | 29–195 | I–IV | 0.6:1 |
| Summer | Spring | 28 | 103–152 | 172–259 | 26–1109 | I–IV | 1:1 |
| Autumn | Summer | 26 | 98–124 | 182–244 | 46–131 | I–IV | 1:1 |
| Stock | Concentrations/μmol/mol | ||||||
|---|---|---|---|---|---|---|---|
| Na | Mg | Ca | Fe | Sr | Ba | ||
| Summer stock | Min | 3270.39 | 2238.22 | 40,933,039.82 | 63.86 | 4925.29 | 2.93 |
| Max | 5479.59 | 50,109.44 | 41,209,251.08 | 1185.97 | 7711.54 | 53.64 | |
| Mean | 4266.74 | 6818.52 | 41,103,854.93 | 348.02 | 6239.47 | 6.32 | |
| Std | 532.22 | 6580.35 | 58,092.83 | 360.85 | 441.79 | 5.97 | |
| Autumn stock | Min | 3670.79 | 3133.53 | 40,942,215.57 | 61.93 | 5415.46 | 3.78 |
| Max | 5122.58 | 118,998.03 | 41,198,206.00 | 432.18 | 7842.94 | 17.32 | |
| Mean | 4385.96 | 8809.93 | 41,099,665.07 | 169.84 | 6313.49 | 6.09 | |
| Std | 321.13 | 15,931.08 | 50,312.46 | 103.90 | 506.55 | 2.04 | |
| Winter stock | Min | 3315.17 | 2731.72 | 40,964,425.73 | 64.70 | 5519.29 | 2.75 |
| Max | 6067.07 | 31,658.01 | 41,216,403.36 | 553.18 | 7062.98 | 10.28 | |
| Mean | 4238.17 | 6535.40 | 41,120,434.76 | 210.02 | 6224.37 | 5.38 | |
| Std | 501.68 | 5517.04 | 52,934.93 | 127.05 | 387.61 | 1.48 | |
| Stock | Stage | Distance | Number Spots | Mg:Ca μmol/mol | Sr:Ca μmol/mol | Ba:Ca μmol/mol | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std | Mean | Std | Mean | Std | ||||
| Summer | Embryonic | D ≤ 109 | 2 | 236.42 | 122.58 | 158.08 | 6.49 | 0.13 | 0.02 |
| Larval | 109 < D ≤ 262 | 3 | 117.70 | 23.71 | 154.54 | 11.52 | 0.12 | 0.02 | |
| Juvenile | 262 < D ≤ 335 | 1 | 117.83 | 41.21 | 148.08 | 6.91 | 0.11 | 0.02 | |
| Sub-adult | 335 < D ≤ 437 | 2 | 128.38 | 47.05 | 145.90 | 3.88 | 0.12 | 0.02 | |
| Adult | D ≥ 437 | 4 | 139.23 | 45.02 | 145.38 | 9.71 | 0.21 | 0.11 | |
| Autumn | Embryonic | D ≤ 89 | 2 | 243.88 | 97.77 | 170.08 | 7.76 | 0.15 | 0.01 |
| Larval | 89 < D ≤ 231 | 2 | 138.36 | 18.89 | 156.78 | 5.24 | 0.12 | 0.01 | |
| Juvenile | 231 < D ≤ 285 | 1 | 135.33 | 28.78 | 149.49 | 4.58 | 0.13 | 0.01 | |
| Sub-adult | 285 < D ≤ 343 | 1 | 122.38 | 19.71 | 148.34 | 3.73 | 0.13 | 0.04 | |
| Adult | D ≥ 343 | 3 | 121.08 | 33.91 | 143.13 | 4.58 | 0.14 | 0.02 | |
| Winter | Embryonic | D ≤ 107 | 2 | 289.29 | 168.24 | 158.68 | 8.87 | 0.14 | 0.02 |
| Larval | 107 < D ≤ 163 | 1 | 142.77 | 25.20 | 154.61 | 8.31 | 0.14 | 0.01 | |
| Juvenile | 163 < D ≤ 255 | 2 | 121.60 | 23.59 | 147.88 | 5.41 | 0.12 | 0.01 | |
| Sub-adult | 255 < D ≤ 403 | 2 | 99.44 | 21.08 | 146.30 | 6.57 | 0.10 | 0.01 | |
| Adult | D ≥ 403 | 4 | 102.67 | 22.47 | 153.51 | 9.44 | 0.14 | 0.03 | |
| Stocks | Environment Variables | Elements Ratios | Equations | R2 | AIC | P | Sign |
|---|---|---|---|---|---|---|---|
| Winter | Temp_5 | Sr:Ca | T5 = 31.56 − 0.02 Sr:Ca | 0.22 | 17.85 | p > 0.05 | ns |
| Temp_25 | Sr:Ca | T25 = 33.12 − 0.03 Sr:Ca | 0.33 | 11.85 | p < 0.05 | * | |
| Temp_55 | Sr:Ca | T55 = 46.12 − 0.14 Sr:Ca | 0.10 | 43.28 | p > 0.05 | ns | |
| Temp_75 | Sr:Ca | T75 = 45.23 − 0.15 Sr:Ca | 0.10 | 44.07 | p > 0.05 | ns | |
| Temp_105 | Sr:Ca | T105 = 31.51 − 0.08 Sr:Ca | 0.13 | 32.47 | p > 0.05 | ns | |
| Summer | Temp_5 | Ba:Ca | T5 = 27.72 + 0.18 Ba:Ca | 0.34 | 27.56 | p < 0.05 | * |
| Temp_25 | Mg:Ca Sr:Ca Ba:Ca | T25 = 27.19 + 0.0006 Mg:Ca + 0.0031 Sr:Ca − 0.2019 Ba:Ca | 0.75 | 25.03 | p < 0.01 | ** | |
| Temp_55 | Sr:Ca | T55 = 27.27 − 0.0003 Sr:Ca | 0.12 | 52.95 | p > 0.05 | ns | |
| Temp_75 | Ba:Ca | T75 = 26.04 + 1.12 Ba:Ca | 0.03 | 32.29 | p > 0.05 | ns | |
| Temp_105 | Ba:Ca | T105 = 21.54 + 1.13 Ba:Ca | 0.10 | 38.53 | p > 0.05 | ns | |
| Autumn | Temp_5 | Mg:Ca | T5 = 28.29 + 0.0054 Mg:Ca | 0.12 | 14.75 | p > 0.05 | ns |
| Temp_25 | Sr:Ca Ba:Ca | T25 = 23.80 + 0.02 Sr:Ca + 2.79 Ba:Ca | 0.40 | 5.13 | p < 0.05 | * | |
| Temp_55 | Mg:Ca | T55 = 25.35 − 0.02 Mg:Ca | 0.16 | 29.31 | p > 0.05 | ns | |
| Temp_75 | Mg:Ca | T75 = 23.64 − 0.03 Mg:Ca | 0.17 | 33.38 | p > 0.05 | ns | |
| Temp_105 | Mg:Ca | T105 = 19.79 − 0.02 Mg:Ca | 0.18 | 29.33 | p > 0.05 | ns |
| Growth Stage | Winter Stock | Summer Stock | Autumn Stock |
|---|---|---|---|
| Temperature/°C | Temperature/°C | Temperature/°C | |
| Embryonic | 28.05–28.88 | 27.70–27.92 | 27.38–27.92 |
| Larval | 28.16–28.89 | 27.65–27.81 | 27.15–27.51 |
| Juvenile | 28.45–28.94 | 27.64–27.76 | 27.01–27.27 |
| Sub–adult | 28.27–28.97 | 27.67–27.73 | 26.98–27.45 |
| Adult | 28.05–28.91 | 27.65–27.72 | 26.92–27.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Fang, Z.; Ma, S.; Zhang, P.; Feng, X.; Chen, Z. Migration Route of Sthenoteuthis oualaniensis in the South China Sea Based on Statolith Trace Element Information. Animals 2023, 13, 2811. https://doi.org/10.3390/ani13182811
Fan J, Fang Z, Ma S, Zhang P, Feng X, Chen Z. Migration Route of Sthenoteuthis oualaniensis in the South China Sea Based on Statolith Trace Element Information. Animals. 2023; 13(18):2811. https://doi.org/10.3390/ani13182811
Chicago/Turabian StyleFan, Jiangtao, Zhou Fang, Shengwei Ma, Peng Zhang, Xue Feng, and Zuozhi Chen. 2023. "Migration Route of Sthenoteuthis oualaniensis in the South China Sea Based on Statolith Trace Element Information" Animals 13, no. 18: 2811. https://doi.org/10.3390/ani13182811
APA StyleFan, J., Fang, Z., Ma, S., Zhang, P., Feng, X., & Chen, Z. (2023). Migration Route of Sthenoteuthis oualaniensis in the South China Sea Based on Statolith Trace Element Information. Animals, 13(18), 2811. https://doi.org/10.3390/ani13182811






