Transcriptome Studies Reveal the N6-Methyladenosine Differences in Testis of Yaks at Juvenile and Sexual Maturity Stages
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Library Sequencing and Bioinformatics Analysis
2.4. Verification of Differential Gene Expression
3. Results
3.1. Morphological Changes in Yak Testis before and after Sexual Maturity
3.2. Yak Testis m6A Level before and after Sexual Maturity
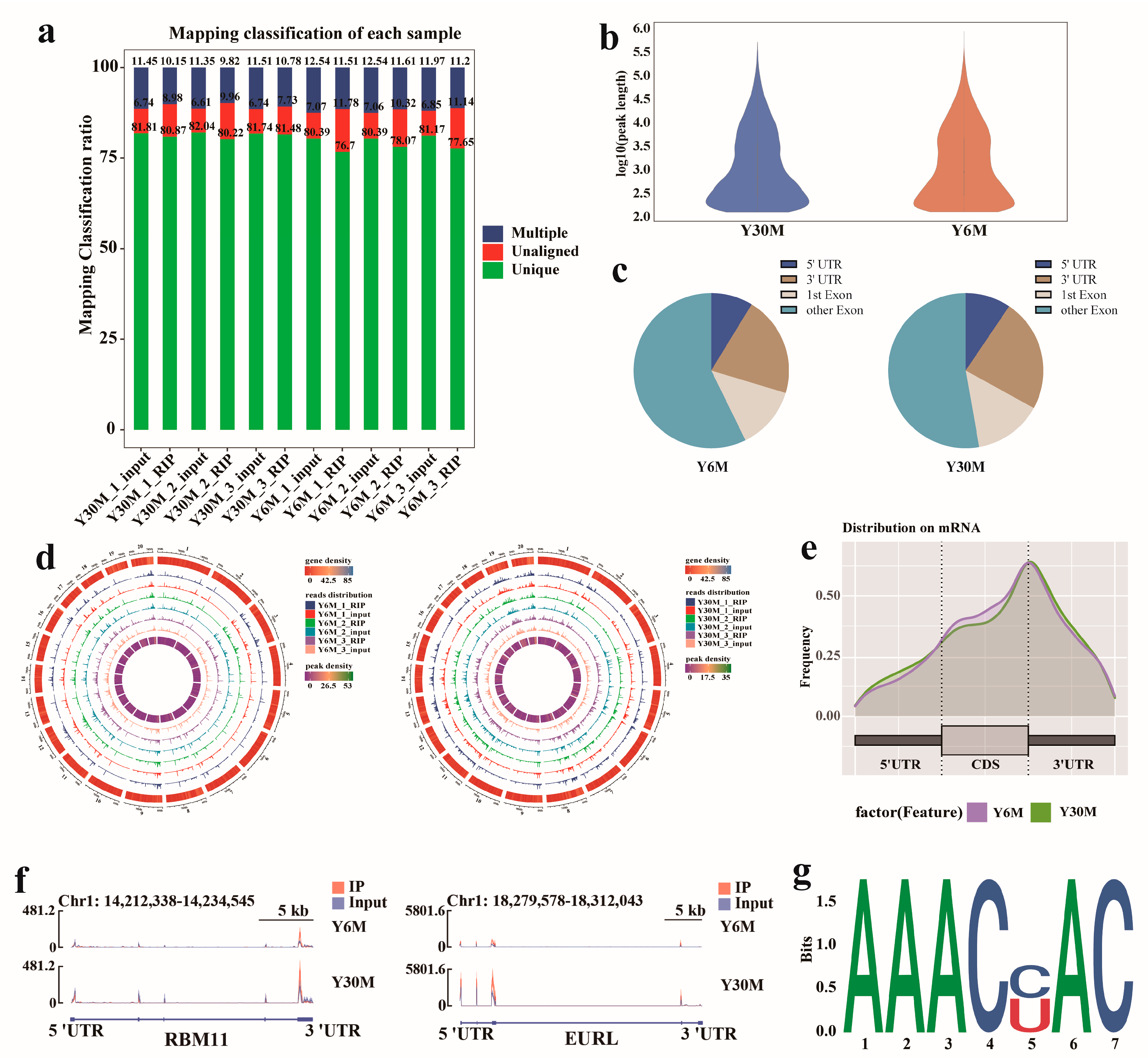
3.3. Raw Data Quality Control and Characteristics of m6A in Yak Testis
3.4. Analysis of Differentially Methylated Peaks (DMPs) of Yak Testis before and after Sexual Maturity
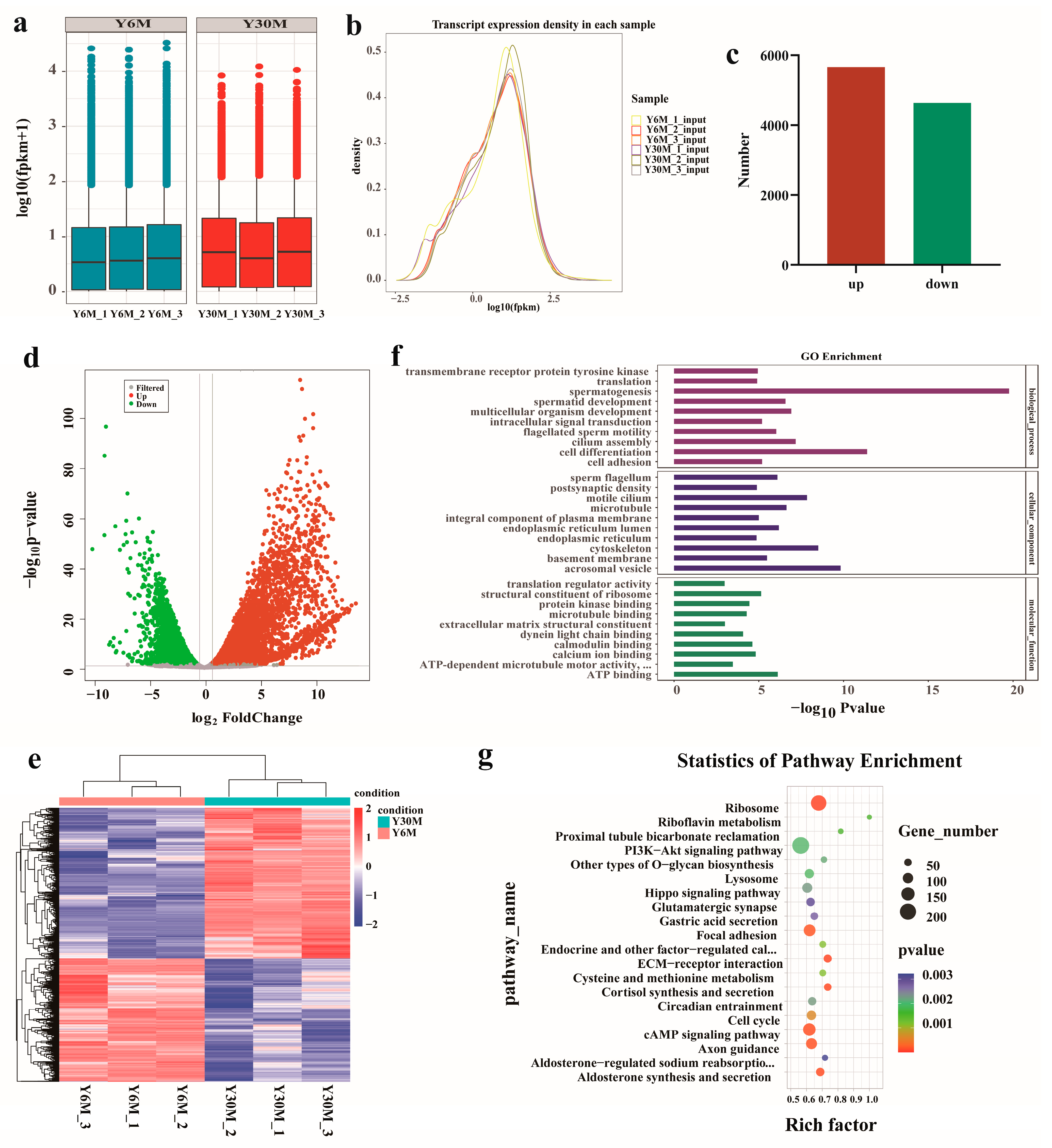
3.5. Analysis of Differentially Expressed Genes (DEGs) in Yak Testis before and after Sexual Maturity
3.6. Combined Analysis of m6A-Seq and RNA-Seq Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The yak genome and adaptation to life at high altitude. Nat. Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, Y.; Kreuzer, M.; Guo, X.; Mi, J.; Gou, Y.; Shang, Z.; Zhang, Y.; Zhou, J.; Wang, H.; et al. Seasonal variations in the fatty acid profile of milk from yaks grazing on the Qinghai-Tibetan plateau. J. Dairy Res. 2013, 80, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Xu, C.; Wu, S.; Zhao, W.; Luo, H.; Yi, C.; Liu, W.; Cai, X. Isolation and characterization of spermatogenic cells from cattle, yak and cattleyak. Anim. Reprod. Sci. 2018, 193, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Duca, Y.; La Vignera, S.; Calogero, A.E. New insights into the genetics of spermatogenic failure: A review of the literature. Hum. Genet. 2019, 138, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, J.A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis Development. Endocr. Rev. 2019, 40, 857–905. [Google Scholar] [CrossRef]
- Svingen, T.; Koopman, P. Building the mammalian testis: Origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013, 27, 2409–2426. [Google Scholar] [CrossRef]
- Ding, H.; Liu, M.; Zhou, C.; You, X.; Su, T.; Yang, Y.; Xu, D. Integrated analysis of miRNA and mRNA expression profiles in testes of Duroc and Meishan boars. BMC Genom. 2020, 21, 686. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- Fu, Y.; Dominissini, D.; Rechavi, G.; He, C. Gene expression regulation mediated through reversible m⁶A RNA methylation. Nat. Reviews. Genet. 2014, 15, 293–306. [Google Scholar] [CrossRef]
- Schwartz, S.; Mumbach, M.R.; Jovanovic, M.; Wang, T.; Maciag, K.; Bushkin, G.G.; Mertins, P.; Ter-Ovanesyan, D.; Habib, N.; Cacchiarelli, D.; et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 2014, 8, 284–296. [Google Scholar] [CrossRef]
- Wu, R.; Jiang, D.; Wang, Y.; Wang, X. N (6)-Methyladenosine (m(6)A) Methylation in mRNA with A Dynamic and Reversible Epigenetic Modification. Mol. Biotechnol. 2016, 58, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Boulias, K.; Greer, E.L. Biological roles of adenine methylation in RNA. Nat. Reviews. Genet. 2023, 24, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, X.; Weng, Y.L.; Lu, Z.; Liu, Y.; Lu, Z.; Li, J.; Hao, P.; Zhang, Y.; Zhang, F.; et al. m(6)A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 2018, 563, 249–253. [Google Scholar] [CrossRef]
- Cai, Z.; Niu, Y.; Li, H. RNA N6-methyladenosine modification, spermatogenesis, and human male infertility. Mol. Hum. Reprod. 2021, 27, gaab020. [Google Scholar] [CrossRef]
- Liu, S.; Lao, Y.; Wang, Y.; Li, R.; Fang, X.; Wang, Y.; Gao, X.; Dong, Z. Role of RNA N6-Methyladenosine Modification in Male Infertility and Genital System Tumors. Front. Cell Dev. Biol. 2021, 9, 676364. [Google Scholar] [CrossRef]
- Huang, T.; Guo, J.; Lv, Y.; Zheng, Y.; Feng, T.; Gao, Q.; Zeng, W. Meclofenamic acid represses spermatogonial proliferation through modulating m(6)A RNA modification. J. Anim. Sci. Biotechnol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Chang, G.; Chen, Y.; An, G.; Yan, L.; Gao, S.; Xu, Y.; Cui, Y.; Dong, J.; et al. Single-Cell RNA Sequencing Analysis Reveals Sequential Cell Fate Transition during Human Spermatogenesis. Cell Stem. Cell 2018, 23, 599–614.e594. [Google Scholar] [CrossRef]
- Tang, C.; Klukovich, R.; Peng, H.; Wang, Z.; Yu, T.; Zhang, Y.; Zheng, H.; Klungland, A.; Yan, W. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3′-UTR mRNAs in male germ cells. Proc. Natl. Acad. Sci. USA 2018, 115, E325–E333. [Google Scholar] [CrossRef]
- Tang, C.; Xie, Y.; Yu, T.; Liu, N.; Wang, Z.; Woolsey, R.J.; Tang, Y.; Zhang, X.; Qin, W.; Zhang, Y.; et al. m(6)A-dependent biogenesis of circular RNAs in male germ cells. Cell Res. 2020, 30, 211–228. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Xu, D.; Xiang, Z.; Ding, J.; Yang, X.; Li, D.; Han, X. m(6)A mRNA methylation regulates testosterone synthesis through modulating autophagy in Leydig cells. Autophagy 2021, 17, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hsu, P.J.; Xing, X.; Fang, J.; Lu, Z.; Zou, Q.; Zhang, K.J.; Zhang, X.; Zhou, Y.; Zhang, T.; et al. Mettl3-/Mettl14-mediated mRNA N(6)-methyladenosine modulates murine spermatogenesis. Cell Res. 2017, 27, 1216–1230. [Google Scholar] [CrossRef]
- Wojtas, M.N.; Pandey, R.R.; Mendel, M.; Homolka, D.; Sachidanandam, R.; Pillai, R.S. Regulation of m(6)A Transcripts by the 3’→5’ RNA Helicase YTHDC2 Is Essential for a Successful Meiotic Program in the Mammalian Germline. Mol. Cell 2017, 68, 374–387.e312. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.J.; Zhu, Y.; Ma, H.; Guo, Y.; Shi, X.; Liu, Y.; Qi, M.; Lu, Z.; Shi, H.; Wang, J.; et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017, 27, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Kasowitz, S.D.; Ma, J.; Anderson, S.J.; Leu, N.A.; Xu, Y.; Gregory, B.D.; Schultz, R.M.; Wang, P.J. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018, 14, e1007412. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Zhang, P.; Li, F.; Zhang, L.; Li, X.; Huang, T.; Zheng, Y.; Yu, T.; Zhang, T.; et al. Transcriptome-wide Dynamics of m(6)A mRNA Methylation During Porcine Spermatogenesis. Genom. Proteom. Bioinform. 2021, in press. [Google Scholar] [CrossRef]
- Wang, X.; Pei, J.; Guo, S.; Cao, M.; Kang, Y.; Xiong, L.; La, Y.; Bao, P.; Liang, C.; Yan, P.; et al. Characterization of N(6)-methyladenosine in cattle-yak testis tissue. Front. Vet. Sci. 2022, 9, 971515. [Google Scholar] [CrossRef]
- Liu, S.H.; Ma, X.Y.; Yue, T.T.; Wang, Z.C.; Qi, K.L.; Li, J.C.; Lin, F.; Rushdi, H.E.; Gao, Y.Y.; Fu, T.; et al. Transcriptome-Wide m6A Analysis Provides Novel Insights Into Testicular Development and Spermatogenesis in Xia-Nan Cattle. Front. Cell Dev. Biol. 2021, 9, 791221. [Google Scholar] [CrossRef]
- Guo, S.; Wang, X.; Cao, M.; Wu, X.; Xiong, L.; Bao, P.; Chu, M.; Liang, C.; Yan, P.; Pei, J.; et al. The transcriptome-wide N6-methyladenosine (m(6)A) map profiling reveals the regulatory role of m(6)A in the yak ovary. BMC Genom. 2022, 23, 358. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, L.; Meng, J.; Rao, M.K.; Chen, Y.; Huang, Y. MeTDiff: A Novel Differential RNA Methylation Analysis for MeRIP-Seq Data. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 15, 526–534. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; He, Q.Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 2015, 31, 2382–2383. [Google Scholar] [CrossRef]
- Wang, Y.N.; Jin, H.Z. Transcriptome-Wide m6A Methylation in Skin Lesions From Patients With Psoriasis Vulgaris. Front. Cell Dev. Biol. 2020, 8, 591629. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic. Acids. Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic. Acids. Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Gaudet, P.; Logie, C.; Lovering, R.C.; Kuiper, M.; Lægreid, A.; Thomas, P.D. Gene Ontology representation for transcription factor functions. Biochim. Biophys. Acta. Gene Regul. Mech. 2021, 1864, 194752. [Google Scholar] [CrossRef]
- Xu, Z.; Miyata, H.; Kaneda, Y.; Castaneda, J.M.; Lu, Y.; Morohoshi, A.; Yu, Z.; Matzuk, M.M.; Ikawa, M. CIB4 is essential for the haploid phase of spermatogenesis in mice. Biol. Reprod. 2020, 103, 235–243. [Google Scholar] [CrossRef]
- Mohammadabadi, M.R.; Jafari, A.H.D.; Bordbar, F. Molecular analysis of CIB4 gene and protein in Kermani sheep. Braz. J. Med. Biol. Res. = Rev. Bras. Pesqui. Medicas E Biol. 2017, 50, e6177. [Google Scholar] [CrossRef]
- Cao, W.; Haig-Ladewig, L.; Gerton, G.L.; Moss, S.B. Adenylate kinases 1 and 2 are part of the accessory structures in the mouse sperm flagellum. Biol. Reprod. 2006, 75, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, G.; Zhang, H.; Chen, F.; Chen, Y.; Zhuang, Y.; Huang, Z.; Zou, F.; Liu, M.; An, G.; et al. Adenylate kinase 1 deficiency disrupts mouse sperm motility under conditions of energy stress. Biol. Reprod. 2020, 103, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.R.; Wu, Q.Q.; Wu, Y.J.; Hu, Y.Q.; Jiang, Z.X.; Lv, H.; Qian, W.Z.; Cai, C.; Wu, J.W. Germline FOXJ2 overexpression causes male infertility via aberrant autophagy activation by LAMP2A upregulation. Cell Death Dis. 2022, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Granadino, B.; Arias-de-la-Fuente, C.; Pérez-Sánchez, C.; Párraga, M.; López-Fernández, L.A.; del Mazo, J.; Rey-Campos, J. Fhx (Foxj2) expression is activated during spermatogenesis and very early in embryonic development. Mech. Dev. 2000, 97, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Vicens, A.; Gómez Montoto, L.; Couso-Ferrer, F.; Sutton, K.A.; Roldan, E.R. Sexual selection and the adaptive evolution of PKDREJ protein in primates and rodents. Mol. Hum. Reprod. 2015, 21, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chiang, H.S.; Cheng, C.Y.; Wu, Y.N.; Lin, Y.C.; Liu, H.C.; Tsai, W.K.; Chen, Y.L.; Lin, Y.H. SLC9A3 Protein Is Critical for Acrosomal Formation in Postmeiotic Male Germ Cells. Int. J. Mol. Sci. 2017, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Luangpraseuth-Prosper, A.; Lesueur, E.; Jouneau, L.; Pailhoux, E.; Cotinot, C.; Mandon-Pépin, B. TOPAZ1, a germ cell specific factor, is essential for male meiotic progression. Dev. Biol. 2015, 406, 158–171. [Google Scholar] [CrossRef]
- Kojima, S.; Hatano, M.; Okada, S.; Fukuda, T.; Toyama, Y.; Yuasa, S.; Ito, H.; Tokuhisa, T. Testicular germ cell apoptosis in Bcl6-deficient mice. Development 2001, 128, 57–65. [Google Scholar] [CrossRef]
- Singh, S.P.; Kharche, S.D.; Pathak, M.; Soni, Y.K.; Pawaiya, R.V.S.; Quadri, S.A.; Singh, M.K.; Chauhan, M.S. Establishment of effective and safe recipient preparation for germ-cell transplantation with intra-testicular busulfan treatment in pre-pubertal Barbari goats. Theriogenology 2022, 189, 270–279. [Google Scholar] [CrossRef]
- Mutoji, K.; Singh, A.; Nguyen, T.; Gildersleeve, H.; Kaucher, A.V.; Oatley, M.J.; Oatley, J.M.; Velte, E.K.; Geyer, C.B.; Cheng, K.; et al. TSPAN8 Expression Distinguishes Spermatogonial Stem Cells in the Prepubertal Mouse Testis. Biol. Reprod. 2016, 95, 117. [Google Scholar] [CrossRef]
- Morimoto, H.; Kanatsu-Shinohara, M.; Orwig, K.E.; Shinohara, T. Expression and functional analyses of ephrin type-A receptor 2 in mouse spermatogonial stem cells. Biol. Reprod. 2020, 102, 220–232. [Google Scholar] [CrossRef]
- Metcalf, C.E.; Wassarman, D.A. Nucleolar colocalization of TAF1 and testis-specific TAFs during Drosophila spermatogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2007, 236, 2836–2843. [Google Scholar] [CrossRef]
- Hiller, M.; Chen, X.; Pringle, M.J.; Suchorolski, M.; Sancak, Y.; Viswanathan, S.; Bolival, B.; Lin, T.Y.; Marino, S.; Fuller, M.T. Testis-specific TAF homologs collaborate to control a tissue-specific transcription program. Development 2004, 131, 5297–5308. [Google Scholar] [CrossRef] [PubMed]
- Cressman, V.L.; Backlund, D.C.; Avrutskaya, A.V.; Leadon, S.A.; Godfrey, V.; Koller, B.H. Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice. Mol. Cell. Biol. 1999, 19, 7061–7075. [Google Scholar] [CrossRef] [PubMed]
- Carré, G.A.; Siggers, P.; Xipolita, M.; Brindle, P.; Lutz, B.; Wells, S.; Greenfield, A. Loss of p300 and CBP disrupts histone acetylation at the mouse Sry promoter and causes XY gonadal sex reversal. Hum. Mol. Genet. 2018, 27, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Imura-Kishi, K.; Uchida, A.; Hiramatsu, R.; Kurohmaru, M.; Kanai, Y. Regionally distinct patterns of STAT3 phosphorylation in the seminiferous epithelia of mouse testes. Mol. Reprod. Dev. 2018, 85, 262–270. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wen, X.X.; Zhao, L.; He, J.P. Immunolocalization of Smad4 protein in the testis of domestic fowl (Gallus domesticus) during postnatal development. Acta Histochem. 2012, 114, 429–433. [Google Scholar] [CrossRef]
- Cheng, L.F.; Liu, M.; Zhu, M.Y.; Chen, D.Y. Seasonal changes in spermatogenesis in silver fox. Carcinog. Teratog. Mutagen. 2016, 28, 393–397. [Google Scholar]
- Prakash, B.S.; Sarkar, M.; Mondal, M. An update on reproduction in yak and mithun. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 217–223. [Google Scholar] [CrossRef]
- Cao, M.; Wang, X.; Guo, S.; Kang, Y.; Pei, J.; Guo, X. F1 Male Sterility in Cattle-Yak Examined through Changes in Testis Tissue and Transcriptome Profiles. Animals 2022, 12, 2711. [Google Scholar] [CrossRef]
- Yu, S.J. The challenges and progress in the management of reproduction in yaks. Soc. Reprod. Fertil. Suppl. 2007, 64, 283–296. [Google Scholar] [CrossRef]
- Patra, S.K. Epigenetics of reproductive infertility. Front. Biosci. 2017, 9, 509–535. [Google Scholar] [CrossRef]
- Xia, H.; Zhong, C.; Wu, X.; Chen, J.; Tao, B.; Xia, X.; Shi, M.; Zhu, Z.; Trudeau, V.L.; Hu, W. Mettl3 Mutation Disrupts Gamete Maturation and Reduces Fertility in Zebrafish. Genetics 2018, 208, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Sai, L.; Li, Y.; Zhang, Y.; Zhang, J.; Qu, B.; Guo, Q.; Han, M.; Jia, Q.; Yu, G.; Li, K.; et al. Distinct m(6)A methylome profiles in poly(A) RNA from Xenopus laevis testis and that treated with atrazine. Chemosphere 2020, 245, 125631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.X.; Wang, J.K.; Shen, L.J.; Long, C.L.; Liu, B.; Wei, Y.; Han, L.D.; Wei, Y.X.; Wu, S.D.; Wei, G.H. Increased m6A RNA modification is related to the inhibition of the Nrf2-mediated antioxidant response in di-(2-ethylhexyl) phthalate-induced prepubertal testicular injury. Environ. Pollut. 2020, 259, 113911. [Google Scholar] [CrossRef] [PubMed]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Anim. Int. J. Anim. Biosci. 2018, 12, s27–s35. [Google Scholar] [CrossRef]
- Liu, P.; Dong, Q.; Liu, S.; Degen, A.; Zhang, J.; Qiu, Q.; Jing, X.; Shang, Z.; Zheng, W.; Ding, L. Postpartum oestrous cycling resumption of yak cows following different calf weaning strategies under range conditions. Anim. Sci. J. 2018, 89, 1492–1503. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. CMLS 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Thumfart, K.M.; Mansuy, I.M. What are Sertoli cells? Historical, methodological, and functional aspects. Andrology 2023, 11, 849–859. [Google Scholar] [CrossRef]
- Wong, E.W.; Mruk, D.D.; Cheng, C.Y. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim. Biophys. Acta 2008, 1778, 692–708. [Google Scholar] [CrossRef]
- Mäkelä, J.A.; Hobbs, R.M. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction 2019, 158, R169–R187. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, T.; Han, D. Testicular immunoregulation and spermatogenesis. Semin. Cell Dev. Biol. 2016, 59, 157–165. [Google Scholar] [CrossRef]
- Young, J.C.; Kerr, G.; Micati, D.; Nielsen, J.E.; Rajpert-De Meyts, E.; Abud, H.E.; Loveland, K.L. WNT signalling in the normal human adult testis and in male germ cell neoplasms. Hum. Reprod. 2020, 35, 1991–2003. [Google Scholar] [CrossRef]
- Deng, C.Y.; Lv, M.; Luo, B.H.; Zhao, S.Z.; Mo, Z.C.; Xie, Y.J. The Role of the PI3K/AKT/mTOR Signalling Pathway in Male Reproduction. Curr. Mol. Med. 2021, 21, 539–548. [Google Scholar] [CrossRef]
- Shawki, H.H.; Ishikawa-Yamauchi, Y.; Kawashima, A.; Katoh, Y.; Matsuda, M.; Al-Soudy, A.S.; Minisy, F.M.; Kuno, A.; Gulibaikelamu, X.; Hirokawa, T.; et al. EFCAB2 is a novel calcium-binding protein in mouse testis and sperm. PLoS ONE 2019, 14, e0214687. [Google Scholar] [CrossRef]
- Liao, H.Q.; Guo, Z.Y.; Huang, L.H.; Liu, G.; Lu, J.F.; Zhang, Y.F.; Xing, X.W. WDR87 interacts with CFAP47 protein in the middle piece of spermatozoa flagella to participate in sperm tail assembly. Mol. Hum. Reprod. 2022, 29, gaac042. [Google Scholar] [CrossRef]
- Kiani, M.; Movahedin, M.; Halvaei, I.; Soleimani, M. Formation of organoid-like structures in the decellularized rat testis. Iran. J. Basic Med. Sci. 2021, 24, 1523–1528. [Google Scholar] [CrossRef]
- Tovo-Neto, A.; Martinez, E.R.M.; Melo, A.G.; Doretto, L.B.; Butzge, A.J.; Rodrigues, M.S.; Nakajima, R.T.; Habibi, H.R.; Nóbrega, R.H. Cortisol Directly Stimulates Spermatogonial Differentiation, Meiosis, and Spermiogenesis in Zebrafish (Danio rerio) Testicular Explants. Biomolecules 2020, 10, 429. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004, 95, 866–871. [Google Scholar] [CrossRef]
- Blackshear, P.E.; Goldsworthy, S.M.; Foley, J.F.; McAllister, K.A.; Bennett, L.M.; Collins, N.K.; Bunch, D.O.; Brown, P.; Wiseman, R.W.; Davis, B.J. Brca1 and Brca2 expression patterns in mitotic and meiotic cells of mice. Oncogene 1998, 16, 61–68. [Google Scholar] [CrossRef]
- Chaudhary, J.; Skinner, M.K. Role of the transcriptional coactivator CBP/p300 in linking basic helix-loop-helix and CREB responses for follicle-stimulating hormone-mediated activation of the transferrin promoter in Sertoli cells. Biol. Reprod. 2001, 65, 568–574. [Google Scholar] [CrossRef]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Jia, Y.; Ohanyan, A.; Lue, Y.H.; Swerdloff, R.S.; Liu, P.Y.; Cohen, P.; Wang, C. The effects of humanin and its analogues on male germ cell apoptosis induced by chemotherapeutic drugs. Apoptosis Int. J. Program. Cell Death 2015, 20, 551–561. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinform. (Oxf. Engl.) 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef]
- Cui, X.; Wei, Z.; Zhang, L.; Liu, H.; Sun, L.; Zhang, S.W.; Huang, Y.; Meng, J. Guitar: An R/Bioconductor Package for Gene Annotation Guided Transcriptomic Analysis of RNA-Related Genomic Features. BioMed Res. Int. 2016, 2016, 8367534. [Google Scholar] [CrossRef]
- Ramírez, F.; Dündar, F.; Diehl, S.; Grüning, B.A.; Manke, T. deepTools: A flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014, 42, W187–W191. [Google Scholar] [CrossRef]
- Schulz, M.H.; Devanny, W.E.; Gitter, A.; Zhong, S.; Ernst, J.; Bar-Joseph, Z. DREM 2.0: Improved reconstruction of dynamic regulatory networks from time-series expression data. BMC Syst. Biol. 2012, 6, 104. [Google Scholar] [CrossRef]


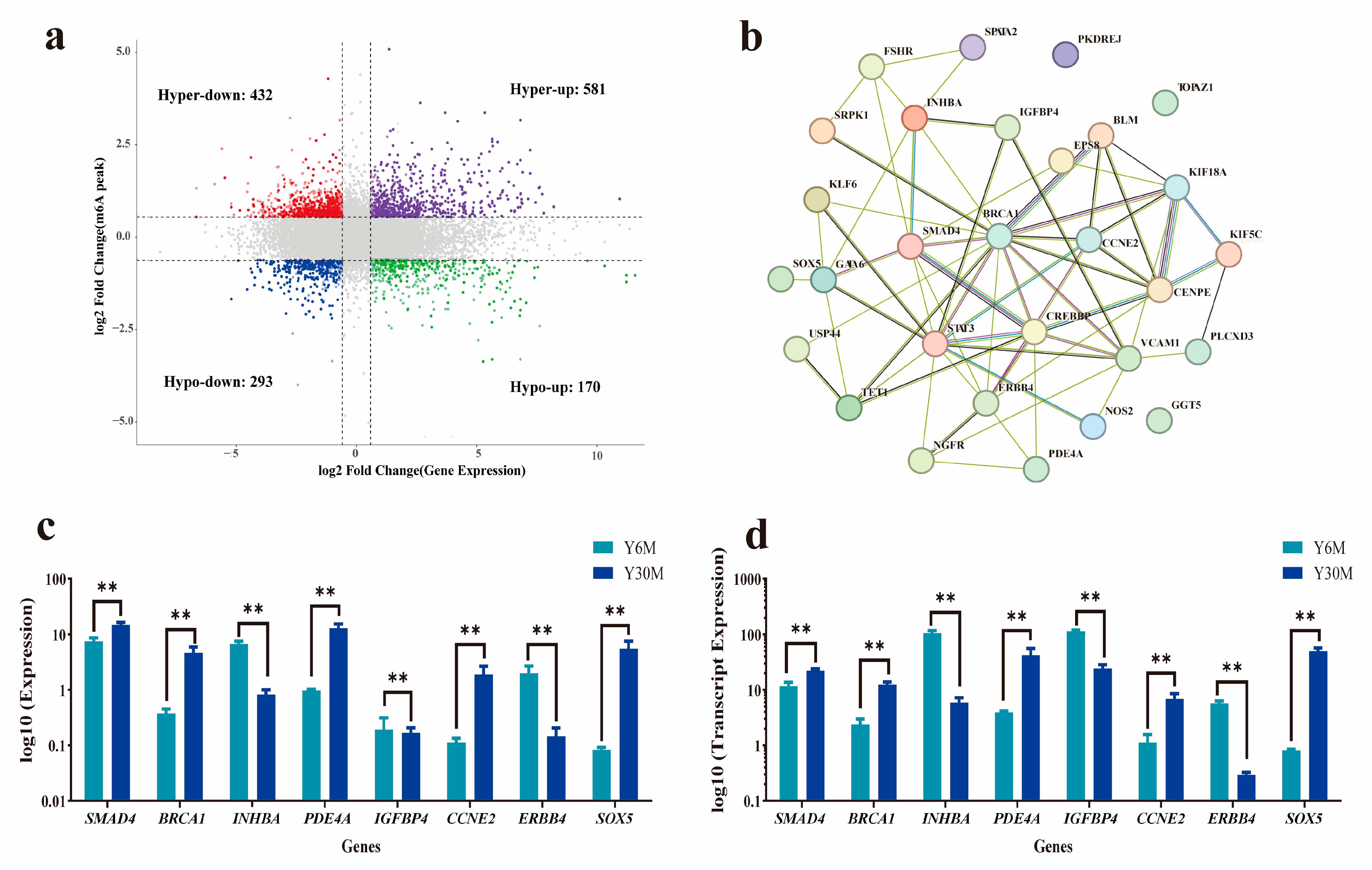
| Name | Pattern | Chr | Start | End | log2FC (m6A) | log2FC (mRNA) | p-Value |
|---|---|---|---|---|---|---|---|
| PKDREJ | hyper-up | 5 | 125,560,273 | 125,560,773 | 2.62 | 5.63 | 2.65 × 10−55 |
| SOX5 | hyper-up | 5 | 16,470,677 | 16,470,826 | 1.08 | 5.87 | 1.96 × 10−47 |
| INHBA | hyper-down | 4 | 44,549,993 | 44,550,142 | 0.91 | −4.30 | 1.08 × 10−35 |
| SRPK1 | hypo-up | 24 | 26,610,618 | 26,610,814 | −0.68 | 3.99 | 4.83 × 10−28 |
| ERBB4 | hypo-down | 2 | 41,164,674 | 41,164,923 | −0.91 | −4.36 | 1.56 × 10−27 |
| USP44 | hypo-up | 5 | 82,720,213 | 82,720,562 | −1.18 | 5.95 | 3.89 × 10−21 |
| KIF5C | hypo-up | 2 | 98,167,244 | 98,168,507 | −0.69 | 4.00 | 7.66 × 10−21 |
| TET1 | hyper-down | 27 | 24,532,195 | 24,532,594 | 0.86 | −3.90 | 5.95 × 10−15 |
| NOS2 | hypo-down | 19 | 49,714,496 | 49,720,399 | −0.64 | −3.58 | 1.03 × 10−14 |
| IGFBP4 | hypo-down | 19 | 25,616,251 | 25,617,161 | −0.67 | −2.38 | 2.37 × 10−14 |
| KLF6 | hyper-down | 12 | 36,414,828 | 36,415,221 | 1.10 | −2.31 | 8.18× 10−55 |
| PLCXD3 | hypo-down | 23 | 50,340,333 | 50,417,181 | −1.06 | −4.24 | 2.38 × 10−13 |
| NGFR | hyper-down | 19 | 29,717,922 | 29,719,581 | 0.62 | −2.57 | 3.59 × 10−12 |
| GATA6 | hyper-down | 21 | 30,279,347 | 30,302,478 | 0.67 | −2.60 | 7.39 × 10−12 |
| TOPAZ1 | hypo-up | 22 | 49,897,438 | 49,897,685 | −1.74 | 3.79 | 1.22 × 10−11 |
| FSHR | hyper-down | 9 | 51,638,767 | 51,638,966 | 1.17 | −2.66 | 1.27 × 10−11 |
| PDE4A | hypo-up | 8 | 15,331,939 | 15,332,787 | −0.63 | 3.41 | 1.29 × 10−11 |
| BRCA1 | hyper-up | 19 | 22,819,897 | 22,820,196 | 1.03 | 2.27 | 1.21 × 10−9 |
| SPATA2 | hypo-up | 12 | 74,095,786 | 74,096,077 | −0.67 | 2.52 | 6.29 × 10−9 |
| STAT3 | hypo-down | 19 | 23,617,050 | 23,617,579 | −1.47 | −1.80 | 2.49 × 10−7 |
| VCAM1 | hypo-down | 3 | 28,996,951 | 28,999,908 | −2.03 | −2.94 | 4.33 × 10−7 |
| CCNE2 | hypo-up | 18 | 61,692,482 | 61,693,870 | −0.84 | 2.50 | 1.43 × 10−6 |
| GGT5 | hypo-down | 16 | 79,102,897 | 79,103,146 | −1.30 | −1.65 | 4.48 × 10−6 |
| VDAC3 | hypo-up | 28 | 16,482,220 | 16,482,368 | −0.61 | 1.78 | 1.97 × 10−5 |
| EPS8 | hypo-down | 5 | 8,127,710 | 8,130,594 | −1.72 | −1.40 | 5.12 × 10−5 |
| CREBBP | hyper-down | 26 | 44,363,116 | 44,364,442 | 0.77 | −1.16 | 0.00016 |
| BLM | hyper-up | 17 | 22,597,323 | 22,597,571 | 0.72 | 1.55 | 0.00033 |
| SMAD4 | hyper-down | 21 | 48,509,408 | 48,509,706 | 0.72 | −1.10 | 0.00047 |
| KIF18A | hyper-up | 13 | 9,570,719 | 9,570,965 | 0.65 | 2.25 | 0.0006 |
| CENPE | hyper-up | 6 | 105,142,758 | 105,146,881 | 0.82 | 1.41 | 0.0345 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Pei, J.; Wang, X.; Cao, M.; Xiong, L.; Kang, Y.; Ding, Z.; La, Y.; Chu, M.; Bao, P.; et al. Transcriptome Studies Reveal the N6-Methyladenosine Differences in Testis of Yaks at Juvenile and Sexual Maturity Stages. Animals 2023, 13, 2815. https://doi.org/10.3390/ani13182815
Guo S, Pei J, Wang X, Cao M, Xiong L, Kang Y, Ding Z, La Y, Chu M, Bao P, et al. Transcriptome Studies Reveal the N6-Methyladenosine Differences in Testis of Yaks at Juvenile and Sexual Maturity Stages. Animals. 2023; 13(18):2815. https://doi.org/10.3390/ani13182815
Chicago/Turabian StyleGuo, Shaoke, Jie Pei, Xingdong Wang, Mengli Cao, Lin Xiong, Yandong Kang, Ziqiang Ding, Yongfu La, Min Chu, Pengjia Bao, and et al. 2023. "Transcriptome Studies Reveal the N6-Methyladenosine Differences in Testis of Yaks at Juvenile and Sexual Maturity Stages" Animals 13, no. 18: 2815. https://doi.org/10.3390/ani13182815





