Assessment of Ruminating, Eating, and Locomotion Behavior during Heat Stress in Dairy Cattle by Using Advanced Technological Monitoring
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing, Animals, and Experimental Design
2.1.1. Housing Conditions and Feeding
2.1.2. Animals, and Experimental Design
2.2. Measurements
2.2.1. Data of Measurements
2.2.2. Duration of Measurements
2.3. Data Analysis and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yousaf, A.; Sarfaraz, I.; Zafar, M.; Abbas, R.; Hussain, A.; Manzoor, D. Effect of Treatment with Tri-Sodium Citrate Alone and in Combination with Levamisole HCl on Total Milk Bacterial Count in Dairy Buffalo Suffering from Sub-Clinical Mastitis. Rev. Vet. 2010, 21, 187–189. [Google Scholar]
- Chapter 2 Canada’s Climate Change Report. Available online: https://changingclimate.ca/CCCR2019/chapter/2-0/ (accessed on 10 August 2023).
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of Climate Change on the Livestock Food Supply Chain; a Review of the Evidence. Glob. Food Secur. 2021, 28, 100488. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.; Seneviratne, S.I. Hot Days Induced by Precipitation Deficits at the Global Scale. Proc. Natl. Acad. Sci. USA 2012, 109, 12398–12403. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Hammer, G.L.; McLean, G.; Messina, C.; Roberts, M.J.; Schlenker, W. The Critical Role of Extreme Heat for Maize Production in the United States. Nat. Clim. Chang. 2013, 3, 497–501. [Google Scholar] [CrossRef]
- Tao, S.; Orellana Rivas, R.M.; Marins, T.N.; Chen, Y.-C.; Gao, J.; Bernard, J.K. Impact of Heat Stress on Lactational Performance of Dairy Cows. Theriogenology 2020, 150, 437–444. [Google Scholar] [CrossRef]
- Rensis, F.D.; Scaramuzzi, R.J. Heat Stress and Seasonal Effects on Reproduction in the Dairy Cow—A Review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The Impact of Heat Stress on the Immune System in Dairy Cattle: A Review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Polsky, L.; von Keyserlingk, M.A.G. Invited Review: Effects of Heat Stress on Dairy Cattle Welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.P.; Zhai, P.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Scheel Monteiro, P.M. IPCC, 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Wilson, D.J.; González, R.N.; Hertl, J.; Schulte, H.F.; Bennett, G.J.; Schukken, Y.H.; Gröhn, Y.T. Effect of Clinical Mastitis on the Lactation Curve: A Mixed Model Estimation Using Daily Milk Weights. J. Dairy Sci. 2004, 87, 2073–2084. [Google Scholar] [CrossRef]
- Sejian, V.; Shashank, C.G.; Silpa, M.V.; Madhusoodan, A.P.; Devaraj, C.; Koenig, S. Non-Invasive Methods of Quantifying Heat Stress Response in Farm Animals with Special Reference to Dairy Cattle. Atmosphere 2022, 13, 1642. [Google Scholar] [CrossRef]
- Dikmen, S.; Hansen, P.J. Is the Temperature-Humidity Index the Best Indicator of Heat Stress in Lactating Dairy Cows in a Subtropical Environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Thom, E.C. The Discomfort Index. Weatherwise 1959, 12, 57–61. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.; Joy, A.; Dunshea, F.R.; Chauhan, S.S. The Impact of Heat Stress on Immune Status of Dairy Cattle and Strategies to Ameliorate the Negative Effects. Animals 2023, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Brown-Brandl, T.M. Understanding Heat Stress in Beef Cattle. Rev. Bras. Zootec. 2018, 47, e20160414. [Google Scholar] [CrossRef]
- Gaughan, J.; Mader, T.; Holt, S.; Hahn, G.; Young, B. Review of Current Assessment of Cattle and Microclimate during Periods of High Heat Load. Anim. Prod Aust 2002, 24, 77–80. [Google Scholar]
- Jo, J.-H.; Nejad, J.G.; Lee, J.-S.; Lee, H.-G. Evaluation of Heat Stress Effects in Different Geographical Areas on Milk and Rumen Characteristics in Holstein Dairy Cows Using Robot Milking and Rumen Sensors: A Survey in South Korea. Animals 2022, 12, 2398. [Google Scholar] [CrossRef] [PubMed]
- Hut, P.R.; Scheurwater, J.; Nielen, M.; van den Broek, J.; Hostens, M.M. Heat Stress in a Temperate Climate Leads to Adapted Sensor-Based Behavioral Patterns of Dairy Cows. J. Dairy Sci. 2022, 105, 6909–6922. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Weary, D.M.; Huzzey, J.M.; von Keyserlingk, M.A.G. BOARD-INVITED REVIEW: Using Behavior to Predict and Identify Ill Health in Animals1. J. Anim. Sci. 2009, 87, 770–777. [Google Scholar] [CrossRef]
- Bar, D.; Solomon, R. Rumination Collars: What Can They Tell Us. In Proceedings of the First North American Conference on Precision Dairy Management, Toronto, ON, Canada, 2–5 March 2010. [Google Scholar]
- Moretti, R.; Biffani, S.; Chessa, S.; Bozzi, R. Heat Stress Effects on Holstein Dairy Cows’ Rumination. Animal 2017, 11, 2320–2325. [Google Scholar] [CrossRef]
- Stone, A.E.; Jones, B.W.; Becker, C.A.; Bewley, J.M. Influence of Breed, Milk Yield, and Temperature-Humidity Index on Dairy Cow Lying Time, Neck Activity, Reticulorumen Temperature, and Rumination Behavior. J. Dairy Sci. 2017, 100, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Berckmans, D. General Introduction to Precision Livestock Farming. Anim. Front. 2017, 7, 6–11. [Google Scholar] [CrossRef]
- Moallem, U.; Altmark, G.; Lehrer, H.; Arieli, A. Performance of High-Yielding Dairy Cows Supplemented with Fat or Concentrate under Hot and Humid Climates. J. Dairy Sci. 2010, 93, 3192–3202. [Google Scholar] [CrossRef]
- Antanaitis, R.; Džermeikaitė, K.; Šimkutė, A.; Girdauskaitė, A.; Ribelytė, I.; Anskienė, L. Use of Innovative Tools for the Detection of the Impact of Heat Stress on Reticulorumen Parameters and Cow Walking Activity Levels. Animals 2023, 13, 1852. [Google Scholar] [CrossRef]
- Rutten, C.J.; Velthuis, A.G.J.; Steeneveld, W.; Hogeveen, H. Invited Review: Sensors to Support Health Management on Dairy Farms. J. Dairy Sci. 2013, 96, 1928–1952. [Google Scholar] [CrossRef] [PubMed]
- Lovarelli, D.; Bacenetti, J.; Guarino, M. A Review on Dairy Cattle Farming: Is Precision Livestock Farming the Compromise for an Environmental, Economic and Social Sustainable Production? J. Clean. Prod. 2020, 262, 121409. [Google Scholar] [CrossRef]
- García, R.; Aguilar, J.; Toro, M.; Pinto, A.; Rodríguez, P. A Systematic Literature Review on the Use of Machine Learning in Precision Livestock Farming. Comput. Electron. Agric. 2020, 179, 105826. [Google Scholar] [CrossRef]
- Zehner, N.; Umstätter, C.; Niederhauser, J.J.; Schick, M. System Specification and Validation of a Noseband Pressure Sensor for Measurement of Ruminating and Eating Behavior in Stable-Fed Cows. Comput. Electron. Agric. 2017, 136, 31–41. [Google Scholar] [CrossRef]
- Gantner, V.; Mijić, P.; Kuterovac, K.; Solić, D.; Gantner, R. Temperature-Humidity Index Values and Their Significance on the Daily Production of Dairy Cattle. Mljekarstvo Časopis Za Unaprjedjenje Proizv. Prerade Mlijeka 2011, 61, 56–63. [Google Scholar]
- Davison, C.; Michie, C.; Hamilton, A.; Tachtatzis, C.; Andonovic, I.; Gilroy, M. Detecting Heat Stress in Dairy Cattle Using Neck-Mounted Activity Collars. Agriculture 2020, 10, 210. [Google Scholar] [CrossRef]
- Ramón-Moragues, A.; Carulla, P.; Mínguez, C.; Villagrá, A.; Estellés, F. Dairy Cows Activity under Heat Stress: A Case Study in Spain. Animals 2021, 11, 2305. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- West, J.W. Effects of Heat-Stress on Production in Dairy Cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and Hormonal Acclimation to Heat Stress in Domesticated Ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Settivari, R.S.; Spain, J.N.; Ellersieck, M.R.; Byatt, J.C.; Collier, R.J.; Spiers, D.E. Relationship of Thermal Status to Productivity in Heat-Stressed Dairy Cows Given Recombinant Bovine Somatotropin1. J. Dairy Sci. 2007, 90, 1265–1280. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of Heat Stress and Plane of Nutrition on Lactating Holstein Cows: I. Production, Metabolism, and Aspects of Circulating Somatotropin1. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Shwartz, G.; Rhoads, M.L.; VanBaale, M.J.; Rhoads, R.P.; Baumgard, L.H. Effects of a Supplemental Yeast Culture on Heat-Stressed Lactating Holstein Cows1. J. Dairy Sci. 2009, 92, 935–942. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of Dairy Cows During the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Maia, G.G.; Siqueira, L.G.B.; de Paula Vasconcelos, C.O.; Tomich, T.R.; de Almeida Camargo, L.S.; Rodrigues, J.P.P.; de Menezes, R.A.; Goncalves, L.C.; Teixeira, B.F.; de Oliveira Grando, R.; et al. Effects of Heat Stress on Rumination Activity in Holstein-Gyr Dry Cows. Livest. Sci. 2020, 239, 104092. [Google Scholar] [CrossRef]
- Meneses, J.A.M.; de Sá, O.A.A.L.; Coelho, C.F.; Pereira, R.N.; Batista, E.D.; Ladeira, M.M.; Casagrande, D.R.; Gionbelli, M.P. Effect of Heat Stress on Ingestive, Digestive, Ruminal and Physiological Parameters of Nellore Cattle Feeding Low- or High-Energy Diets. Livest. Sci. 2021, 252, 104676. [Google Scholar] [CrossRef]
- Gouvêa, V.N.; Cooke, R.F.; Marques, R.S. Impacts of Stress-Induced Inflammation on Feed Intake of Beef Cattle. Front. Anim. Sci. 2022, 3, 962748. [Google Scholar] [CrossRef]
- Provolo, G.; Riva, E. One Year Study of Lying and Standing Behaviour of Dairy Cows in a Frestall Barn in Italy. J. Agric. Eng. 2009, 40, 27–34. [Google Scholar] [CrossRef]
- Lokhorst, C.; De Mol, R.M.; Kamphuis, C. Invited Review: Big Data in Precision Dairy Farming. Animal 2019, 13, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Lamp, O.; Derno, M.; Otten, W.; Mielenz, M.; Nürnberg, G.; Kuhla, B. Metabolic Heat Stress Adaption in Transition Cows: Differences in Macronutrient Oxidation between Late-Gestating and Early-Lactating German Holstein Dairy Cows. PLoS ONE 2015, 10, e0125264. [Google Scholar] [CrossRef]
- Ammer, S.; Lambertz, C.; Gauly, M. Comparison of Different Measuring Methods for Body Temperature in Lactating Cows under Different Climatic Conditions. J. Dairy Res. 2016, 83, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Anskienė, L.; Rapaliutė, E.; Bilskis, R.; Džermeikaitė, K.; Bačėninaitė, D.; Juškienė, V.; Juška, R.; Meškinytė, E. Relationship between Reticulorumen Parameters Measured in Real Time and Methane Emission and Heat Stress Risk in Dairy Cows. Animals 2022, 12, 3257. [Google Scholar] [CrossRef]
- González Pereyra, A.V.; Maldonado May, V.; Catracchia, C.G.; Herrero, M.A.; Flores, M.C.; Mazzini, M. Influence of Water Temperature and Heat Stress on Drinking Water Intake in Dairy Cows. Chil. J. Agric. Res. 2010, 70, 328–336. [Google Scholar] [CrossRef]
- McDowell, R.E.; Hooven, N.W.; Camoens, J.K. Effect of Climate on Performance of Holsteins in First Lactation. J. Dairy Sci. 1976, 59, 965–971. [Google Scholar] [CrossRef]
- Cook, N.B. Prevalence of Lameness among Dairy Cattle in Wisconsin as a Function of Housing Type and Stall Surface. J. Am. Vet. Med. Assoc. 2003, 223, 1324–1328. [Google Scholar] [CrossRef]
- Rulquin, H.; Caudal, J. Effects of Lying or Standing on Mammary Blood Flow and Heart Rate of Dairy Cows. Ann. Zootech 1992, 41, 101. [Google Scholar] [CrossRef]
- Igono, M.O.; Bjotvedt, G.; Sanford-Crane, H.T. Environmental Profile and Critical Temperature Effects on Milk Production of Holstein Cows in Desert Climate. Int. J. Biometeorol. 1992, 36, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Abeni, F.; Galli, A. Monitoring Cow Activity and Rumination Time for an Early Detection of Heat Stress in Dairy Cow. Int. J. Biometeorol. 2017, 61, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Tullo, E.; Mattachini, G.; Riva, E.; Finzi, A.; Provolo, G.; Guarino, M. Effects of Climatic Conditions on the Lying Behavior of a Group of Primiparous Dairy Cows. Animals 2019, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Fregonesi, J.A.; Leaver, J.D. Behaviour, Performance and Health Indicators of Welfare for Dairy Cows Housed in Strawyard or Cubicle Systems. Livest. Prod. Sci. 2001, 68, 205–216. [Google Scholar] [CrossRef]
- Müschner-Siemens, T.; Hoffmann, G.; Ammon, C.; Amon, T. Daily Rumination Time of Lactating Dairy Cows under Heat Stress Conditions. J. Therm. Biol. 2020, 88, 102484. [Google Scholar] [CrossRef]
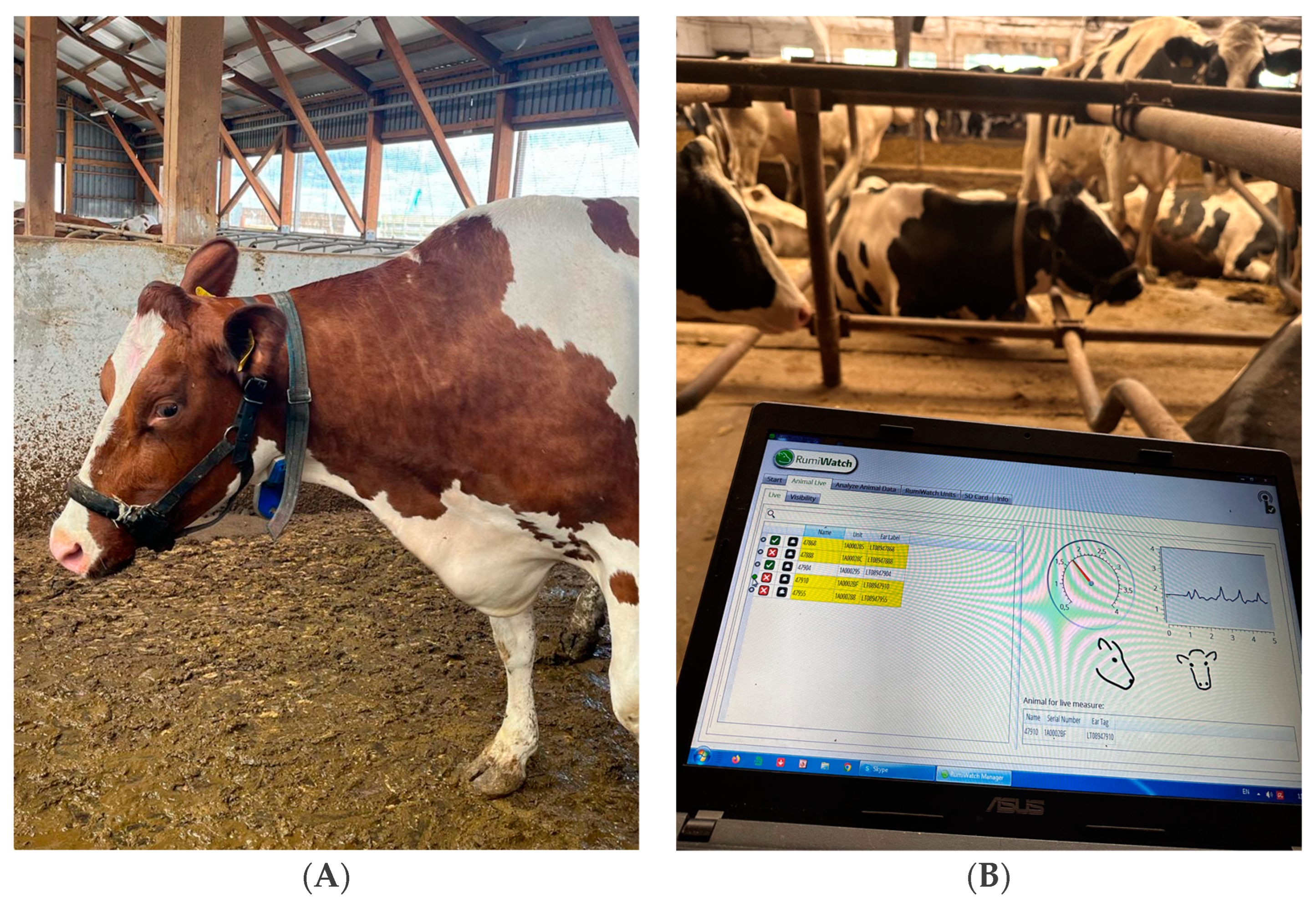
| Parameter | Abbreviation | Description |
|---|---|---|
| Rumination time | RT | Time spent chewing or ruminating, including 5 s intervals. |
| Eating time | ET | Time spent chewing or ruminating, including 5 s intervals. |
| Drinking time | DT | Drinking time, including up to 5 s breaks between gulps |
| Rumination chews | RC | During rumination, molar chews for mechanical reduction of regurgitated items into smaller masses |
| Eating chews | EC | Total number of trepidation bites and chews made while eating |
| Drinking gulps | DG | Total number of gulps consumed while drinking |
| Bolus | B | Number of boluses consumed during ruminating |
| Chews per minute | CM | Rumination chews per minute—chews for one minute |
| Chews per bolus | CB | Chews conducted between regurgitation and swallowing of one bolus during rumination |
| Activity | Act | The sum of all walking bouts presented as minutes over a specific recording period |
| Up time | UT | Time spent eating with the head elevated (min/h) |
| Down time | DT | Feeding time with the head positioned downwards (min/h) |
| Parameter and Abbreviation | THI Class | N | Mean | Std. Deviation | Std. Error Mean |
|---|---|---|---|---|---|
| Rumination time (RT) | THI < 72 | 86 | 24.36 | 14.761 | 1.592 |
| THI ≥ 72 | 62 | 19.72 | 15.523 | 1.971 | |
| Eating time (ET) | THI < 72 | 86 | 11.99 | 13.150 | 1.418 |
| THI ≥ 72 | 62 | 11.54 | 12.545 | 1.593 | |
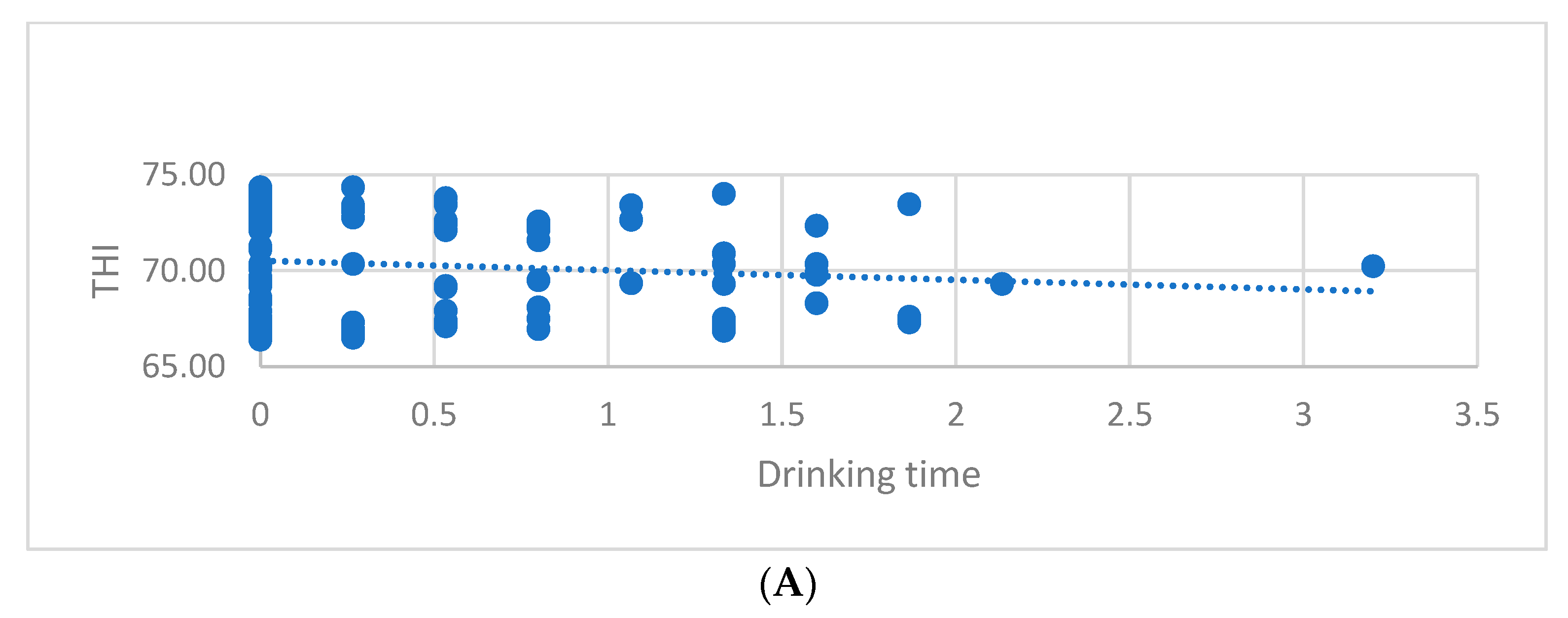
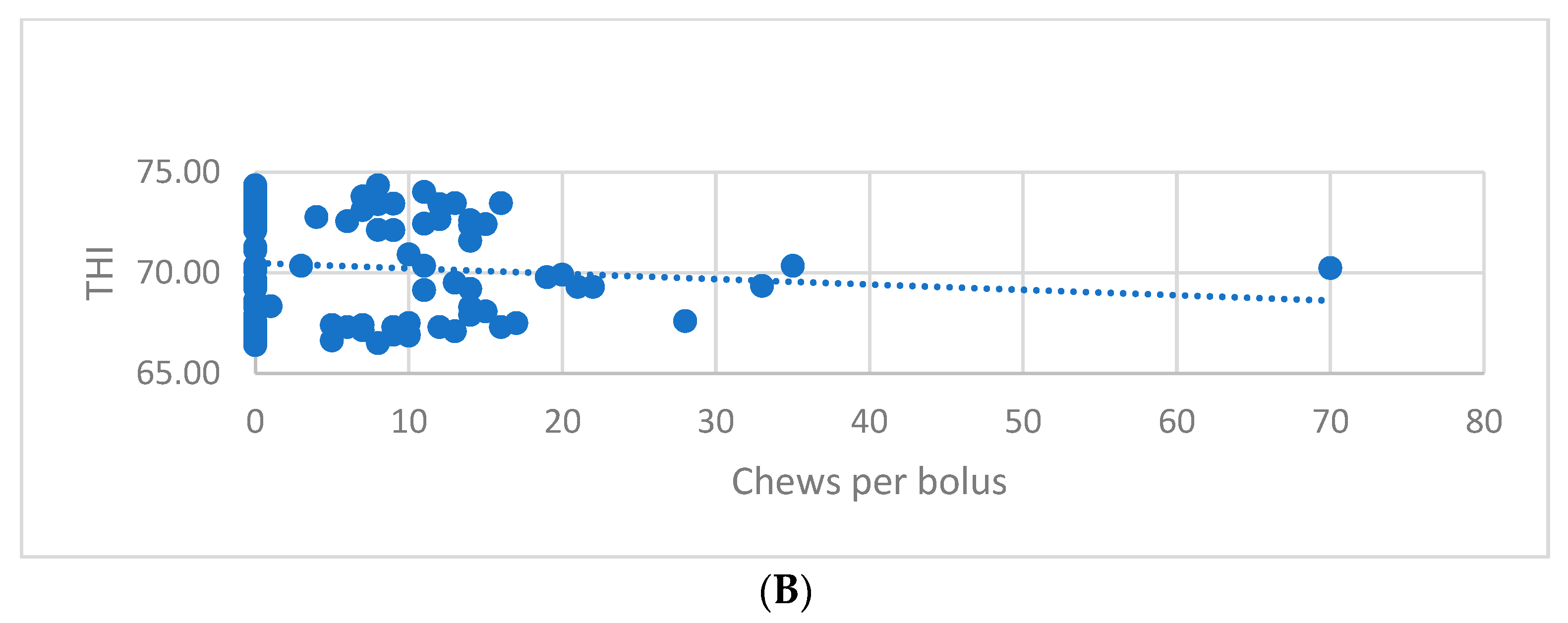
| Drinking time (DT) | THI < 72 | 86 | 0.43 A | 0.687 | 0.074 |
| THI ≥ 72 | 62 | 0.21 B | 0.394 | 0.050 | |
| Rumination chews (RC) | THI < 72 | 86 | 1706.94 | 1103.742 | 119.020 |
| THI ≥ 72 | 62 | 1571.84 | 1237.684 | 157.186 | |
| Eating chews (EC) | THI < 72 | 86 | 860.13 | 960.611 | 103.585 |
| THI ≥ 72 | 62 | 814.81 | 925.396 | 117.525 | |
| Drinking gulps (DG) | THI < 72 | 86 | 643.58 | 730.619 | 78.785 |
| THI ≥ 72 | 62 | 624.24 | 702.293 | 89.191 | |
| Bolus (B) | THI < 72 | 86 | 29.36 | 18.753 | 2.022 |
| THI ≥ 72 | 62 | 23.76 | 18.709 | 2.376 | |
| Chews per minute (CM) | THI < 72 | 86 | 83.26 A | 30.512 | 3.290 |
| THI ≥ 72 | 62 | 72.51 B | 39.595 | 5.029 | |
| Chews per bolus (CB) | THI < 72 | 86 | 6.06 a | 10.686 | 1.152 |
| THI ≥ 72 | 62 | 2.97 b | 5.008 | 0.636 | |
| Activity (Act) | THI < 72 | 86 | 114.60 a | 71.832 | 7.746 |
| THI ≥ 72 | 62 | 138.06 b | 84.874 | 10.779 | |
| Up time (UT) | THI < 72 | 86 | 13.92 | 19.569 | 2.110 |
| THI ≥ 72 | 62 | 11.81 | 17.705 | 2.248 | |
| Down time (DT) | THI < 72 | 86 | 19.85 | 21.397 | 2.307 |
| THI ≥ 72 | 62 | 23.73 | 23.776 | 3.020 |
| Correlations | ||||
|---|---|---|---|---|
| THI Class | Drinking Time (DT) | Chews per Bolus (CB) | ||
| THI class | Pearson Correlation | 1 | −0.191 * | −0.172 * |
| Sig. (two-tailed) | 0.020 | 0.036 | ||
| N | 148 | 148 | 148 | |
| Drinking time (DT) | Pearson Correlation | −0.191 * | 1 | 0.865 ** |
| Sig. (two-tailed) | 0.020 | <0.001 | ||
| N | 148 | 148 | 148 | |
| Chews per bolus (CB) | Pearson Correlation | −0.172 * | 0.865 ** | 1 |
| Sig. (two-tailed) | 0.036 | <0.001 | ||
| N | 148 | 148 | 148 | |
| THI | Hours | Bolus | Chews per Minute | Rumination Time | ||
|---|---|---|---|---|---|---|
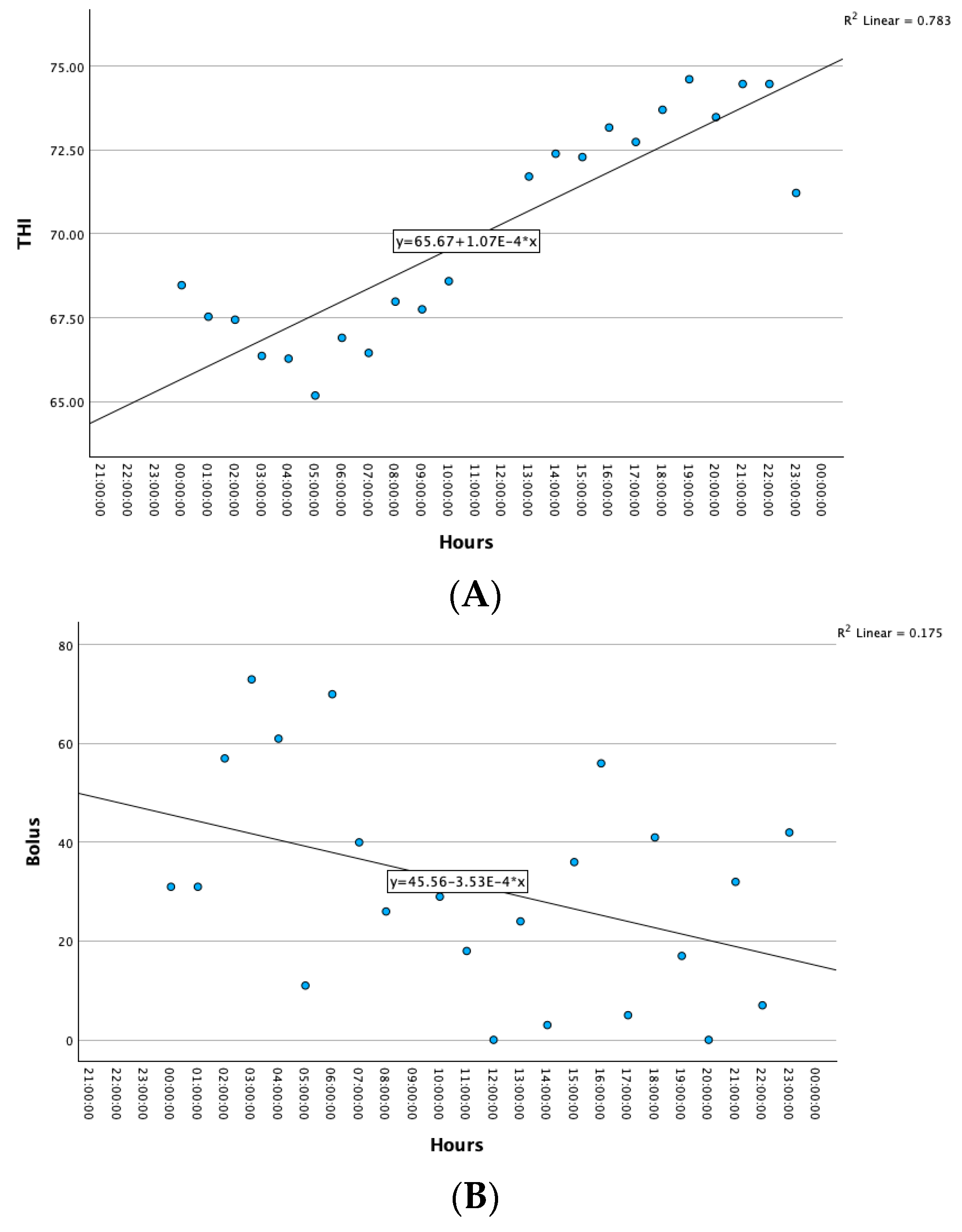
| THI | Pearson Correlation | 1 | 0.432 ** | −0.214 ** | −0.191 * | −0.212 ** |
| Sig. (two-tailed) | <0.001 | 0.009 | 0.020 | 0.010 | ||
| N | 148 | 148 | 148 | 148 | 148 | |
| Correlations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rumination Time | Eating Time | Drinking Time | Rumination Chew | Eating Chews | Drinking Gulps | Bolus | Chews per Minute | Activity | ||
| Rumination time | Pearson Correlation | 1 | −0.666 ** | −0.172 * | 0.734 ** | −0.660 ** | −0.671 ** | 0.991 ** | 0.671 ** | −0.655 ** |
| Sig. (1-tailed) | <0.001 | 0.018 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| N | 148 | 148 | 148 | 148 | 148 | 148 | 148 | 148 | 148 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antanaitis, R.; Džermeikaitė, K.; Bespalovaitė, A.; Ribelytė, I.; Rutkauskas, A.; Japertas, S.; Baumgartner, W. Assessment of Ruminating, Eating, and Locomotion Behavior during Heat Stress in Dairy Cattle by Using Advanced Technological Monitoring. Animals 2023, 13, 2825. https://doi.org/10.3390/ani13182825
Antanaitis R, Džermeikaitė K, Bespalovaitė A, Ribelytė I, Rutkauskas A, Japertas S, Baumgartner W. Assessment of Ruminating, Eating, and Locomotion Behavior during Heat Stress in Dairy Cattle by Using Advanced Technological Monitoring. Animals. 2023; 13(18):2825. https://doi.org/10.3390/ani13182825
Chicago/Turabian StyleAntanaitis, Ramūnas, Karina Džermeikaitė, Agnė Bespalovaitė, Ieva Ribelytė, Arūnas Rutkauskas, Sigitas Japertas, and Walter Baumgartner. 2023. "Assessment of Ruminating, Eating, and Locomotion Behavior during Heat Stress in Dairy Cattle by Using Advanced Technological Monitoring" Animals 13, no. 18: 2825. https://doi.org/10.3390/ani13182825
APA StyleAntanaitis, R., Džermeikaitė, K., Bespalovaitė, A., Ribelytė, I., Rutkauskas, A., Japertas, S., & Baumgartner, W. (2023). Assessment of Ruminating, Eating, and Locomotion Behavior during Heat Stress in Dairy Cattle by Using Advanced Technological Monitoring. Animals, 13(18), 2825. https://doi.org/10.3390/ani13182825









