Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. EEG Hardware and EEG Recordings
2.3. Standardized Thermal and Electrical Stimulation
2.4. EEG Data Processing
2.5. Physiological Anticipations
2.6. Statistics
2.7. Histological Procedures
3. Results
3.1. Basal EEG Activity
3.2. Electrical and Thermal Stimulation
3.3. Histological Verification
4. Discussion
4.1. Basal EEG
4.2. Electrical and Thermal Stimulation
4.3. Conscious Pain Perception
4.4. Selection of the Embryonal Timeframe for EEG Recordings
4.5. Histological Verification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reithmayer, C.; Mußhoff, O. Consumer preferences for alternatives to chick culling in Germany. Poult. Sci. 2019, 98, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Krautwald-Junghanns, M.E.; Cramer, K.; Fischer, B.; Förster, A.; Galli, R.; Kremer, F.; Mapesa, E.U.; Meissner, S.; Preisinger, R.; Preusse, G.; et al. Current approaches to avoid the culling of day-old male chicks in the layer industry, with special reference to spectroscopic methods. Poult. Sci. 2017, 97, 749–757. [Google Scholar] [CrossRef]
- Sandercock, D. Putative nociceptor responses to mechanical and chemical stimulation in skeletal muscles of the chicken leg. Brain Res. Rev. 2004, 46, 155–162. [Google Scholar] [CrossRef]
- Gentle, M.J.; Hunter, L.N. Physiological and behavioural responses associated with feather removal in Gallus gallus var domesticus. Res. Vet. Sci. 1990, 50, 95–101. [Google Scholar]
- Woolley, S.C.; Gentle, M.J. Physiological and behavioural responses in the hen (Gallus domesticus) to nociceptive stimulation. Comp. Biochem. Physiol. A Comp. Physiol. 1987, 88, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gentle, M.J.; Tilston, V.; McKeegan, D.E. Mechanothermal nociceptors in the scaly skin of the chicken leg. Neuroscience 2001, 106, 643–652. [Google Scholar] [CrossRef]
- Necker, R.; Reiner, B. Temperature-Sensitive Mechanoreceptors, Thermoreceptors and Heat Nociceptors in the Feathered Skin of Pigeons. J. Comp. Physiol. 1980, 135, 201–207. [Google Scholar]
- Mischkowski, D.; Palacios-Barrios, E.E.; Banker, L.; Dildine, T.C.; Atlas, L.Y. Pain or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses. Pain 2018, 159, 699–711. [Google Scholar] [CrossRef]
- Sneddon, L.U. Comparative physiology of nociception and pain. Physiology 2017, 33, 63–73. [Google Scholar]
- Woo, C.-W.; Schmidt, L.; Krishnan, A.; Jepma, M.; Roy, M.; Lindquist, M.A.; Atlas, L.Y.; Wager, T.D. Quantifying cerebral contributions to pain beyond nociception. Nat. Commun. 2017, 8, 14211. [Google Scholar]
- Livingston, A. Physiological basis for pain perception in animals. J. Vet. Anaesth. 1994, 21, 73–77. [Google Scholar]
- Johnson, C.B. Research Tools for the Measurement of Pain and Nociception. Animals 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Murrell, C.; Johnson, C.B. Neurophysiological techniques to assess pain in animals. J. Vet. Pharmacol. Therap. 2006, 29, 325–335. [Google Scholar]
- O’Donovan, M.S.E.; Sholomenko, G.; Ho, S.; Antal, M.; Yee, W. Development of Spinal Motor Networks in the Chick Embryo. J. Exp. Zool. 1992, 261, 261–273. [Google Scholar] [PubMed]
- Bellairs, R.; Osmond, M. The Atlas of Chick Development, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2014; p. 693. [Google Scholar]
- Eide, A.L.; Glover, J.C. Development of the Longitudinal Projection Patterns of Lumbar Primary Sensory Afferents in the Chicken Embryo. J. Comp. Neurol. 1995, 353, 247–259. [Google Scholar] [CrossRef]
- Peters, J.J.; Vonderahe, A.R.; Schmid, D. Onset of cerebral electrical activity associated with behavioral sleep and attention in the developing chick. J. Exp. Zool. 1965, 160, 255–261. [Google Scholar] [CrossRef]
- Katori, M. The development of the spontaneous electrical activity in the brain of a chick embryo and the effects of several drugs on it. Jpn. J. Pharmacol. 1962, 12, 9–25. [Google Scholar] [CrossRef]
- Garcia-Austt, E., Jr. Development of Electrical Activity in Cerebral Hemispheres of the Chick Embryo. Proc. Soc. Exp. Biol. Med. 1954, 86, 348–352. [Google Scholar]
- Hamburger, V.; Hamilton, L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar]
- Corner, M.A.; Bakhius, W.L. Developmental patterns in the central nervous system of birds. V. Cerebral electrical activity, forebrain function and behavior in the chick at the time of hatching. Brain Res. 1969, 13, 541–555. [Google Scholar] [CrossRef]
- Corner, M.A.; Schade, J.P.; Sedlácek, J.; Stoeckart, R.; Bot, A.P. Developmental patterns in the central nervous system of birds. I. Electrical activity in the cerebral hemisphere, optic lobe and cerebellum. Prog. Brain Res. 1966, 26, 145–192. [Google Scholar] [CrossRef]
- Schwitalla, J.C.; Pakusch, J.; Mücher, B.; Brückner, A.; Depke, D.A.; Fenzl, T.; De Zeeuw, C.I.; Kros, L.; Hoebeek, F.E.; Mark, M.D. Controlling absence seizures from the cerebellar nuclei via activation of the Gq signaling pathway. Cell. Mol. Life Sci. 2022, 79, 197. [Google Scholar] [CrossRef]
- Fritz, E.M.; Kreuzer, M.; Altunkaya, A.; Singewald, N.; Fenzl, T. Altered sleep behavior in a genetic mouse model of impaired fear extinction. Sci. Rep. 2021, 11, 8978. [Google Scholar] [PubMed]
- Kreuzer, M.; Polta, S.; Gapp, J.; Schuler, C.; Kochs, E.F.; Fenzl, T. Sleep scoring made easy—Semi-automated sleep analysis software and manual rescoring tools for basic sleep research in mice. MethodsX 2015, 2, 232–240. [Google Scholar] [CrossRef]
- Polta, S.; Fenzl, T.; Jakubcakova, V.; Kimura, M.; Yassouridis, A.; Wotjak, C. Prognostic and Symptomatic Aspects of Rapid Eye Movement Sleep in a Mouse Model of Posttraumatic Stress Disorder. Front. Behav. Neurosci. 2013, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Romanowski, C.P.N.; Becker, A.; Wetter, T.C.; Kimura, M.; Fenzel, T. Rapid eye movements during sleep in mice: High trait-like stability qualifies rapid eye movement density for characterization of phenotypic variation in sleep patterns of rodents. BMC Neurosci. 2011, 12, 110. [Google Scholar] [CrossRef]
- Fenzl, T.; Romanowski, C.P.N.; Flachskamm, C.; Honsberg, K.; Boll, E.; Hoehne, A.; Kimura, M. Fully automated sleep deprivation in mice as a tool in sleep research. J. Neurosci. Methods 2007, 166, 229–235. [Google Scholar] [CrossRef]
- Sharma, K.; Dua, S.; Singh, B.; Anand, B. Electro-ontogenesis of cerebral and cardiac activities in the chick embryo. Electroencephalogr. Clin. Neurophysiol. 1964, 16, 503–509. [Google Scholar] [CrossRef]
- Hothersall, B.; Caplen, G.; Nicol, C.J.; Taylor, P.M.; Waterman-Pearson, A.E.; Weeks, C.A.; Murrell, J.C. Development of mechanical and thermal nociceptive threshold testing devices in unrestrained birds (broiler chickens). J. Neurosci. Methods 2011, 201, 220–227. [Google Scholar] [CrossRef]
- Hothersall, B.; Caplen, G.; Parker, R.M.A.; Nicol, C.J.; Waterman-Pearson, A.E.; Weeks, C.A.; Murrell, J.C. Thermal nociceptive threshold testing detects altered sensory processing in broiler chickens with spontaneous lameness. PLoS ONE 2014, 9, e97883. [Google Scholar] [CrossRef]
- Bogdanov, O.V.; Smetankin, A.A.; Saraev, S.Y.; Mikhailenok, E.L.; Ved, V.V. Electrical activity of the chick embryo brain during development of stable rearrangements of movement. Neurosci. Behav. Physiol. 1984, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ring, C.; Kavussanu, M.; Willoughby, A.R. Emotional modulation of pain-related evoked potentials. Biol. Psychol. 2013, 93, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, T.; Schuller, G. Periaqueductal gray and the region of the paralemniscal area have different functions in the control of vocalization in the neotropical bat, Phyllostomus discolor. Eur. J. Neurosci. 2002, 16, 1974–1986. [Google Scholar] [PubMed]
- Fenzl, T.; Schuller, G. Echolocation calls and communication calls are controlled differentially in the brainstem of the bat Phyllostomus discolor. BMC Biol. 2005, 3, 17. [Google Scholar] [CrossRef]
- Fenzl, T.; Schuller, G. Dissimilarities in the vocal control over communication and echolocation calls in bats. Behav. Brain Res. 2007, 182, 173–179. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsu, S.H.; Pion-Tonachini, L.; Jung, T.P. Evaluation of Artifact Subspace Reconstruction for Automatic EEG Artifact Removal. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 1242–1245. [Google Scholar] [CrossRef]
- Anders, M.; Anders, B.; Dreismickenbecker, E.; Hight, D.; Kreuzer, M.; Walter, C.; Zinn, S. EEG responses to standardised noxious stimulation during clinical anaesthesia: A pilot study. BJA Open 2023, 5, 100118. [Google Scholar]
- Anders, M.; Dreismickenbecker, E.; Fleckenstein, J.; Walter, C.; Enax-Krumova, E.K.; Fischer, M.J.; Kreuzer, M.; Zinn, S. EEG-based sensory testing reveals altered nociceptive processing in elite endurance athletes. Exp. Brain Res. 2022, 241, 341–354. [Google Scholar]
- Grandchamp, R.; Delorme, A. Single-trial normalization for event-related spectral decomposition reduces sensitivity to noisy trials. Front. Psychol. 2011, 2, 236. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Rach, S.; Vosskuhl, J.; Strüber, D. Time-frequency analysis of event-related potentials: A brief tutorial. Brain Topogr. 2014, 27, 438–450. [Google Scholar] [CrossRef]
- Anders, M.; Anders, B.; Kreuzer, M.; Zinn, S.; Walter, C. Application of referencing techniques in EEG-based Recordings of Contact Heat Evoked Potentials (CHEPS). Front. Hum. Neurosci. 2020, 14, 527. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, Y.; Kumar, T.; Singh, R.; Jha, K. Electroencephalographic characterization of a case of infantile spasm with atypical presentation. Indian J. Child Health 2019, 6, 42–45. [Google Scholar]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG-and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Kuenzel, W.; Masson, M. A Stereotaxic Atlas of the Brain of the Chick (Gallus domesticus). Poult. Sci. Fac. Publ. Present. 1988. Available online: https://scholarworks.uark.edu/poscpub/1/ (accessed on 1 July 2023).
- Hunter, M.; Batillana, M.; Bragg, T. EEG as a Measure of Developmental Changes in the Chicken Brain. Dev. Psychobiol. 2000, 36, 23–28. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef]
- Franken, P.; Dijk, D.-J.; Tobler, I.; Borbély, A.A. Sleep deprivation in rats: Effects on EEG power spectra, vigilance states, and cortical temperature. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1991, 261, R198–R208. [Google Scholar] [CrossRef]
- Dijk, D.-J. EEG slow waves and sleep spindles: Windows on the sleeping brain. Behav. Brain Res. 1995, 69, 109–116. [Google Scholar] [CrossRef]
- Fenzl, T.; Touma, C.; Romanowski, C.P.; Ruschel, J.; Holsboer, F.; Landgraf, R.; Kimura, M.; Yassouridis, A. Sleep disturbances in highly stress reactive mice: Modeling endophenotypes of major depression. BMC Neurosci. 2011, 12, 29. [Google Scholar] [CrossRef]
- Peters, J.V.R.; Powers, T.H. The functional chronology in the developing chick nervous system. J. Exp. Zool. 1956, 133, 505–518. [Google Scholar] [CrossRef]
- Mellor, D.J.; Diesch, T.J. Birth and hatching: Key events in the onset of awareness in the lamb and chick. New Zealand Vet. J. 2007, 55, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Vonderahe, A.R.; McDonough, J.J. Electrical changes in brain and eye of the developing chick during hyperthermia. Amer. J. Physiol. 1964, 207, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.J.; Vonderahe, A.R.; Huesman, A.A. Chronological development of electrical activity in the optic lobes, cerebellum, and cerebrum of the chick embryo. Physiol. Zool. 1960, 33, 225–231. [Google Scholar] [CrossRef]
- Kuhlenbeck, H. The ontogenetic development of the diencephalic centers in a bird’s brain (chick) and comparison with the reptilian and mammalian diencephalon. J. Comp. Neurol. 1937, 66, 23–75. [Google Scholar] [CrossRef]
- Kanz, K.-G.; Kay, M.V.; Biberthaler, P.; Russ, W.; Wessel, S.; Lackner, C.K.; Mutschler, W. Susceptibility of automated external defibrillators to train overhead lines and metro third rails. Resuscitation 2004, 62, 189–198. [Google Scholar] [CrossRef]
- Hadrian, W. Die magnetischen Stoerfelder des elektrischen Bahnbetriebes. Elektrotechnik Und Informationstechnik Ei 2006, 1, 46–49. [Google Scholar] [CrossRef]
- Corner, M.; Schadé, J. Developmental Patterns in the Central Nervous System of Birds: IV. Cellular and Molecular Bases of Functional Activity. Prog. Brain Res. 1967, 26, 237–250. [Google Scholar]
- McIlhone, A.E.; Beausoleil, N.J.; Kells, N.J.; Mellor, D.J.; Johnson, C.B. Effects of noxious stimuli on the electroencephalogram of anaesthetised chickens (Gallus gallus domesticus). PLoS ONE 2018, 13, e0196454. [Google Scholar] [CrossRef]
- Gentle, M.J. Pain in birds. Anim. Welf. 1992, 1, 235–247. [Google Scholar] [CrossRef]
- Legrain, V.; Iannetti, G.D.; Plaghki, L.; Mouraux, A. The pain matrix reloaded—A salience detection system for the body. Prog. Neurobiol. 2010, 93, 111–124. [Google Scholar] [CrossRef]
- Butler, A.B.; Cotterill, R.M. Mammalian and Avian neuroanatomy and the question of consciousness in birds. Biol. Bull. 2006, 211, 106–127. [Google Scholar] [CrossRef]
- Kuenzel, W. Neurobiological basis of sensory perception: Welfare implications of beak trimming. Poult. Sci. 2007, 86, 1273–1282. [Google Scholar] [CrossRef]
- Reiner, A.; Yamamoto, K.; Karten, H.J. Organization and Evolution of the avian forebrain. Anat. Rep. Part A 2005, 287A, 1080–1102. [Google Scholar] [CrossRef]
- González, G.; Puelles, L.; Medina, L. Organization of the mouse dorsal thalamus based on topology, calretinin immnunostaining, and gene expression. Brain Res. Bull. 2002, 57, 439–442. [Google Scholar] [CrossRef]
- Butler, A.B. The dorsal thalamus of jawed vertebrates: A comparative viewpoint. Brain Behav. Evol. 1995, 46, 209–223. [Google Scholar] [CrossRef]
- Butler, A.B. The evolution of the dorsal pallium in the telencephalon of amniotes: Cladistic analysis and a new hypothesis. Brain Res. Rev. 1994, 19, 66–101. [Google Scholar] [CrossRef]
- Wild, J.M. The avian somatosensory system: Connections of regions of body representation in the forebrain of the pigeon. Brain Res. 1987, 412, 205–223. [Google Scholar] [CrossRef]
- Delius, J.D.; Bennetto, K. Cutaneous sensory projections to the avian forebrain. Brain Res. 1972, 37, 205–221. [Google Scholar] [CrossRef]
- Lierz, M.; Korbel, R. Anesthesia and analgesia in birds. J. Exot. Pet Med. 2012, 21, 44–58. [Google Scholar] [CrossRef]
- Bromm, B. Pain Measurement in Man: Neurophysiological Correlates of Pain; Elsevier Publishing Company: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Gibson, T.; Johnson, C.; Stafford, K.; Mitchinson, S.; Mellor, D. Validation of the acute electroencephalographic responses of calves to noxious stimulus with scoop dehorning. New Zealand Vet. J. 2007, 55, 152–157. [Google Scholar] [CrossRef]
- Johnson, C.B.; Sylvester, S.P.; Stafford, K.J.; Mitchinson, S.L.; Ward, R.N.; Mellor, D.J. Effects of age on the electroencephalographic response to castration in lambs anaesthetized with halothane in oxygen from birth to 6 weeks old. Vet. Anaesth. Analg. 2009, 36, 273–279. [Google Scholar] [CrossRef]
- Johnson, C.B.; Wilson, P.R.; Woodbury, M.R.; Caulkett, N.A. Comparison of analgesic techniques for antler removal in halothane-anaesthetized red deer (Cervus elaphus): Electroencephalographic responses. Vet. Anaesth. Analg. 2005, 32, 61–71. [Google Scholar] [CrossRef]
- Kells, N.J.; Beausoleil, N.J.; Chambers, J.P.; Sutherland, M.A.; Morrison, R.S.; Johnson, C.B. Electroencephalographic responses of anaesthetized pigs (Sus scrofa) to tail docking using clippers or cautery iron performed at 2 or 20 days of age. Vet. Anaesth. Analg. 2017, 44, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Murrell, J.C.; Johnson, C.B.; White, K.L.; Taylor, P.M.; Haberham, Z.L.; Waterman–Pearson, A.E. Changes in the EEG during castration in horses and ponies anaesthetized with halothane. Vet. Anaesth. Analg. 2003, 30, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Diesch, T.J. Onset of sentience: The potential for suffering in fetal and newborn farm animals. Appl. Anim. Behav. Sci. 2006, 100, 48–57. [Google Scholar] [CrossRef]
- McMahan, J. Killing embryos for stem cell research. Metaphilosophy 2007, 38, 170–189. [Google Scholar] [CrossRef]
- Zeman, A. What in the world is consciousness? Prog. Brain Res. 2005, 150, 1–10. [Google Scholar]
- Dehaene, S.; Lau, H.; Kouider, S. What is consciousness, and could machines have it? Robot. AI Humanit. Sci. Ethics Policy 2021, 43–56. [Google Scholar] [CrossRef]
- Koch, C. What is consciousness. Nature 2018, 557, S8–S12. [Google Scholar] [CrossRef]
- Wallace, R. What is Consciousness? Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Solms, M. What is consciousness? J. Am. Psychoanal. Assoc. 1997, 45, 681–703. [Google Scholar] [CrossRef]
- Douglas, J.M.; Guzman, D.S.-M.; Paul-Murphy, J.R. Pain in Birds: The anatomical and physiological basis. Vet. Clin. Exot. Anim. Pract. 2018, 21, 17–31. [Google Scholar] [CrossRef]
- Rosenbruch, M. [The sensitivity of chicken embryos in incubated eggs][Article in German]. ALTEX-Altern. Anim. Exp. 1997, 14, 111–113. [Google Scholar]

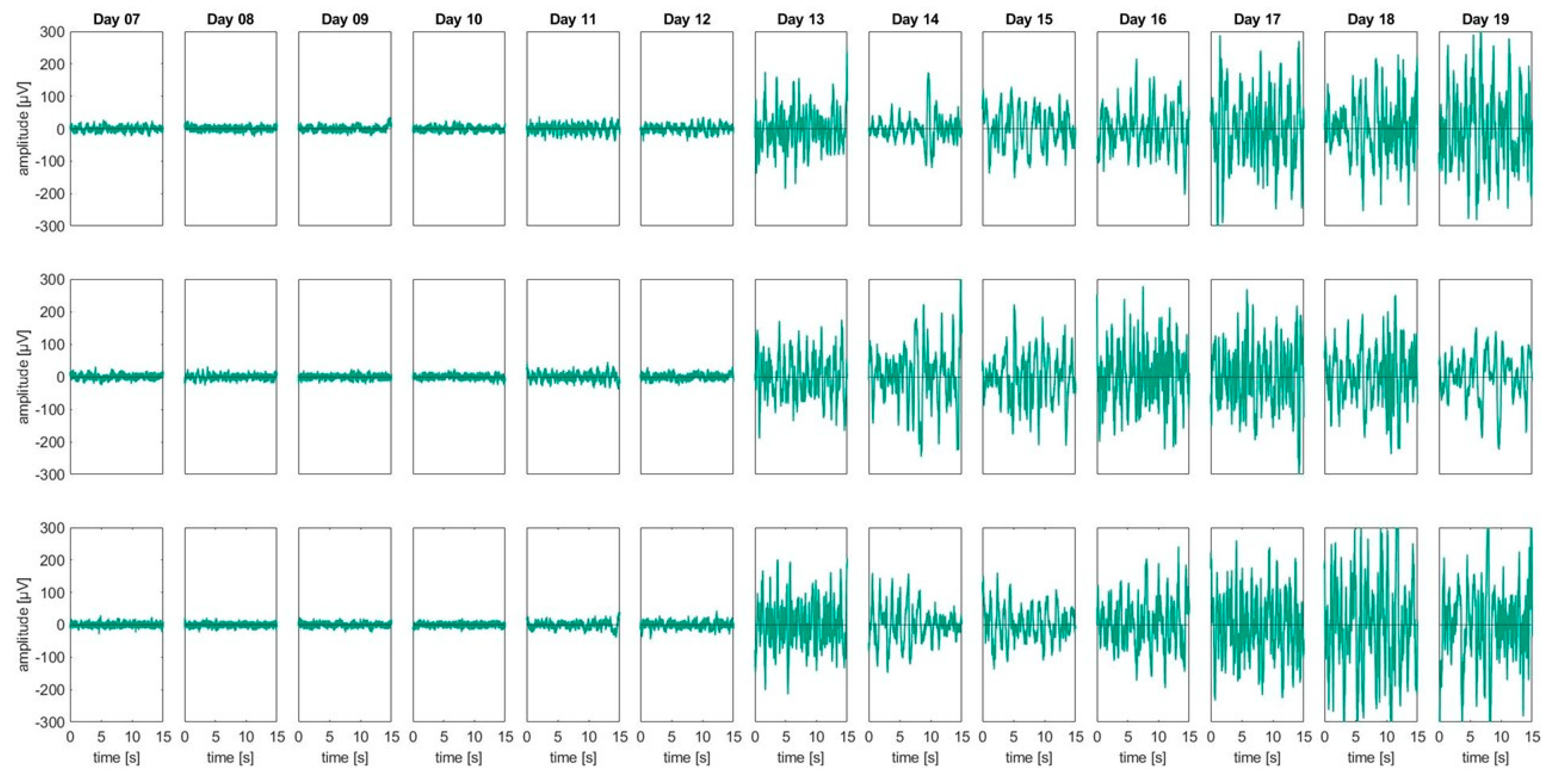

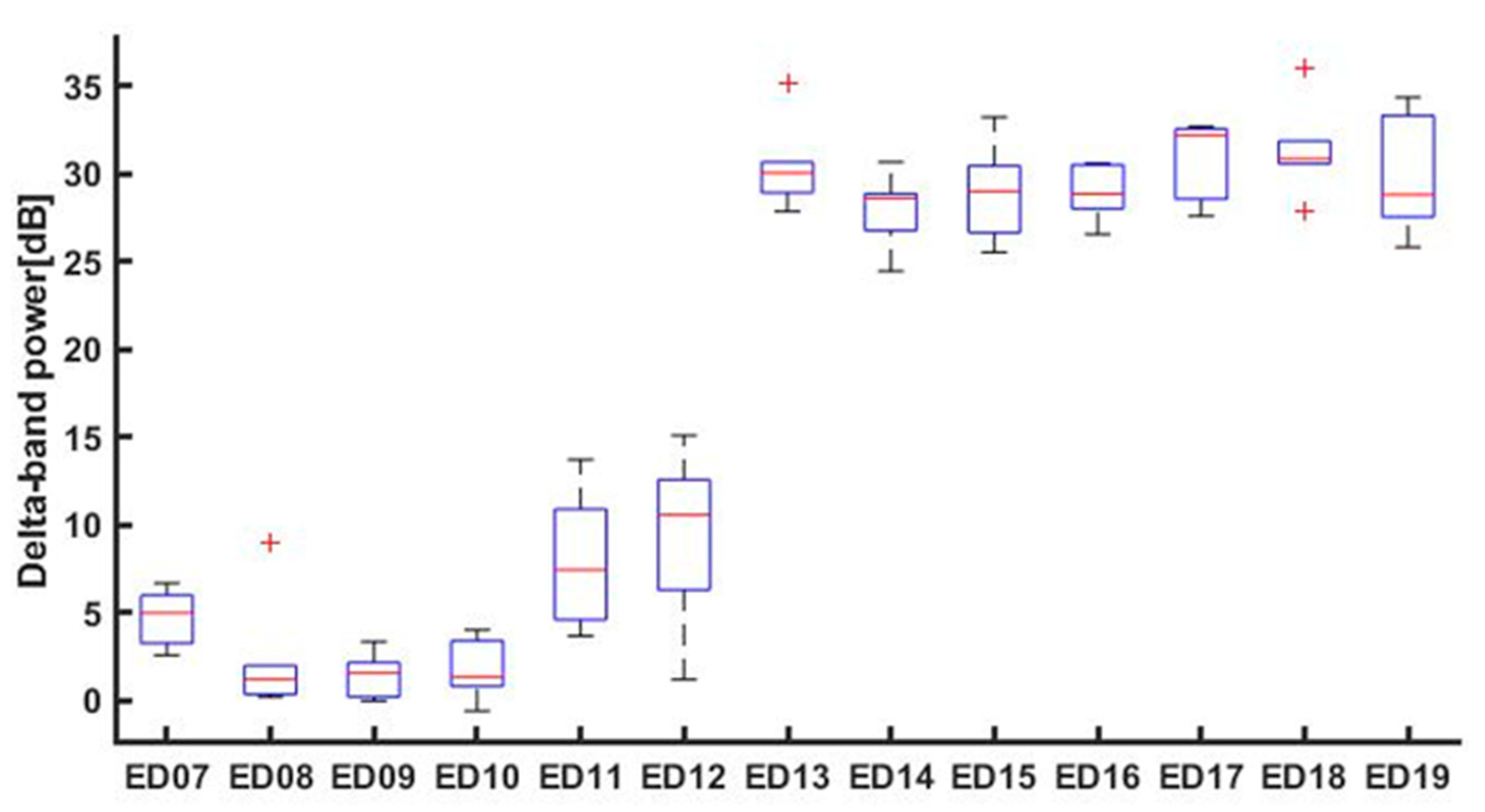
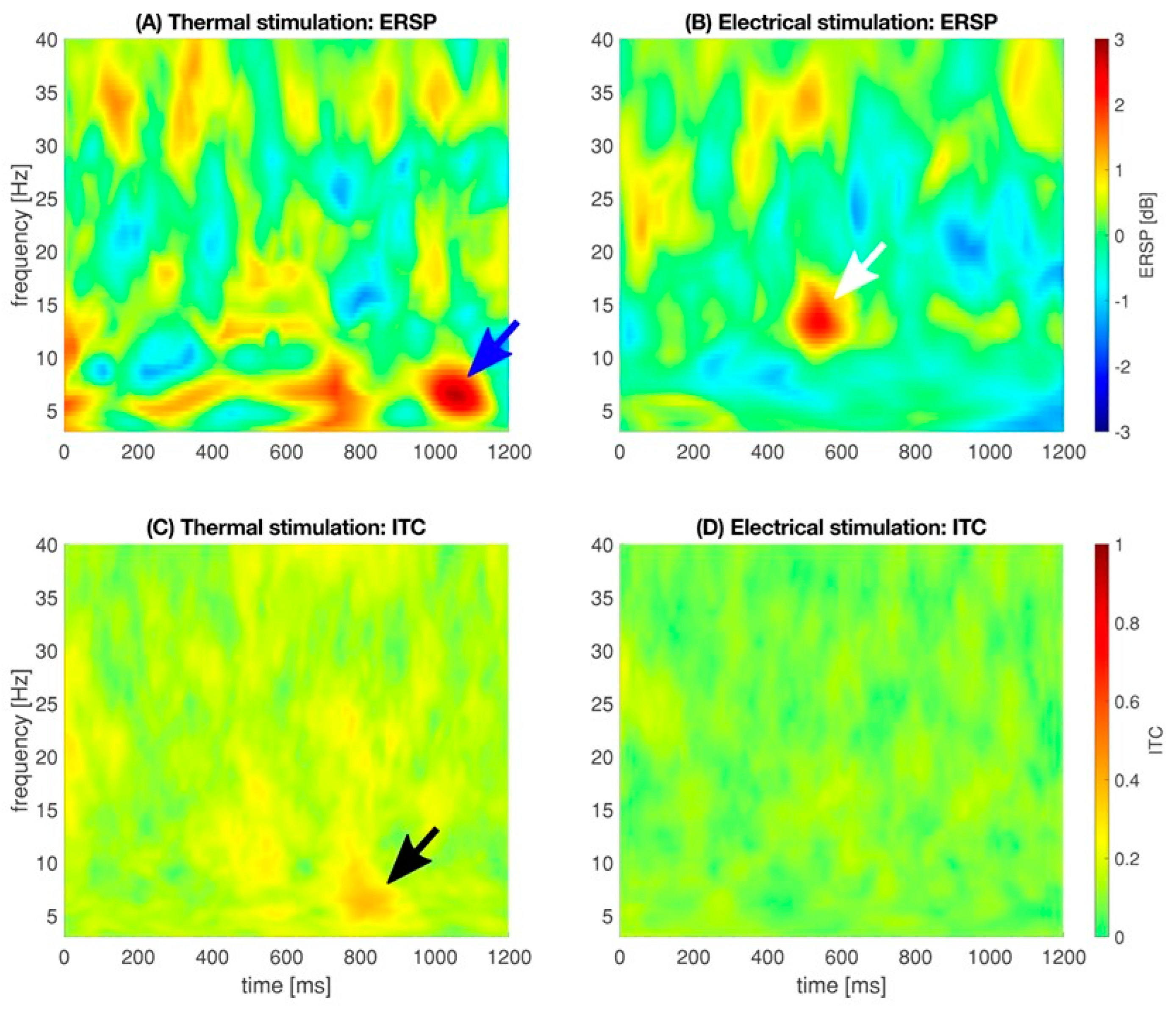
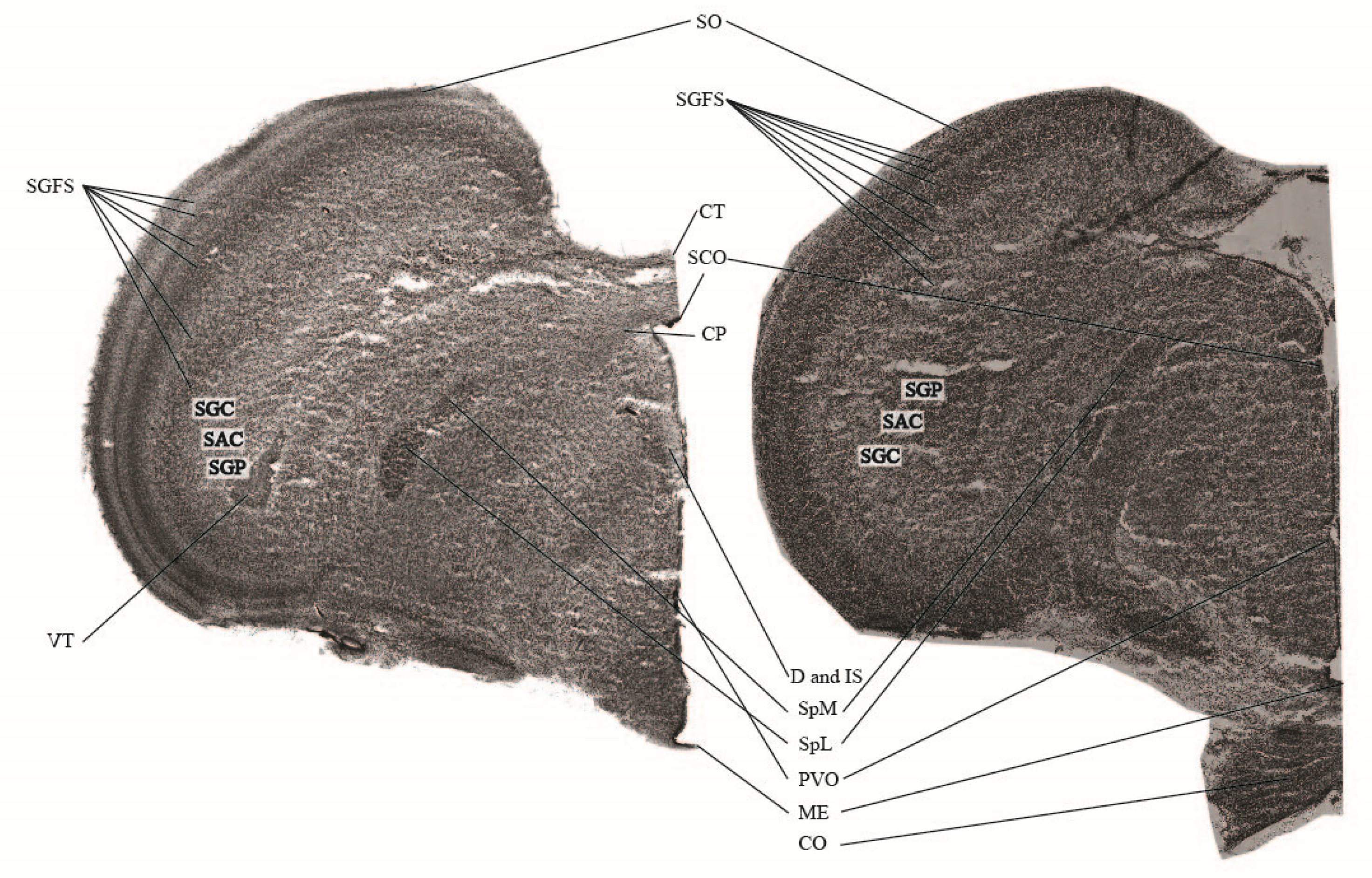
| Stage (ED) | ED7 | ED8 | ED9 | ED10 | ED11 | ED12 | ED13 | ED14 | ED15 | ED16 | ED17 | ED18 | ED19 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animals total | 36 | 9 | 8 | 9 | 18 | 56 | 57 | 14 | 13 | 17 | 18 | 40 | 66 | 361 |
| Discarded | 5 | - | - | - | 5 | 12 | 9 | 1 | 2 | 3 | 1 | 16 | 22 | 76 |
| Evaluated | 10 | 9 | 8 | 9 | 13 | 21 | 32 | 13 | 9 | 14 | 17 | 21 | 29 | 205 1 |
| Random EEG | - | - | - | - | - | - | - | - | - | - | - | 2 | 13 | 15 |
| Onset EEG | - | - | - | - | - | - | 12 | 14 | - | - | - | - | - | 26 |
| Electrical stimulation | 8 | 6 | 4 | 5 | 9 | 6 | 15 | 6 | 4 | 7 | 11 | 12 | 10 | 103 |
| Thermal stimulation | 2 | 3 | 4 | 4 | 4 | 3 | 3 | 7 | 5 | 7 | 6 | 7 | 6 | 61 |
| Histology | 21 | - | - | - | - | 23 | 16 | - | 2 | - | - | 3 | 15 | 80 |
| STAGE (ED) | ED07 | ED08 | ED09 | ED10 | ED11 | ED12 | ED13 | ED14 | ED15 | ED16 | ED17 | ED18 | ED19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEDIAN DELTA POWER | 4.970 | 1.194 | 1.532 | 1.322 | 7.427 | 10.555 | 30.032 | 28.602 | 28.988 | 28.846 | 32.167 | 30.848 | 28.796 |
| [25%] PERCENTILE [75%] PERCENTILE | 3.231 5.972 | 0.338 1.968 | 0.181 2.141 | 0.788 3.377 | 4.582 10.873 | 6.274 12.567 | 28.906 30.656 | 26.767 28.842 | 26.640 30.442 | 28.003 30.497 | 28.551 32.530 | 30.564 31.861 | 27.526 33.304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kollmansperger, S.; Anders, M.; Werner, J.; Saller, A.M.; Weiss, L.; Süß, S.C.; Reiser, J.; Schneider, G.; Schusser, B.; Baumgartner, C.; et al. Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception. Animals 2023, 13, 2839. https://doi.org/10.3390/ani13182839
Kollmansperger S, Anders M, Werner J, Saller AM, Weiss L, Süß SC, Reiser J, Schneider G, Schusser B, Baumgartner C, et al. Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception. Animals. 2023; 13(18):2839. https://doi.org/10.3390/ani13182839
Chicago/Turabian StyleKollmansperger, Sandra, Malte Anders, Julia Werner, Anna M. Saller, Larissa Weiss, Stephanie C. Süß, Judith Reiser, Gerhard Schneider, Benjamin Schusser, Christine Baumgartner, and et al. 2023. "Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception" Animals 13, no. 18: 2839. https://doi.org/10.3390/ani13182839






