Reduction in Cold Stress in an Innovative Metabolic Cage Housing System Increases Animal Welfare in Laboratory Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals and Housing Conditions
2.3. Description of Metabolic Cages
2.4. Study Design
2.5. Humane Endpoints: Termination Criteria
2.6. Food Intake
2.7. Body Weight and Body Composition
2.8. Determination of Cage and Body Surface Temperature with Thermal Imaging
2.9. Expression of Uncoupling Protein 1 in Brown Adipose Tissue
2.10. Assessment of Grooming State by Using the Fur Score
2.11. Determination of Fecal Corticosterone Metabolites
2.12. Statistical Analyses
3. Results
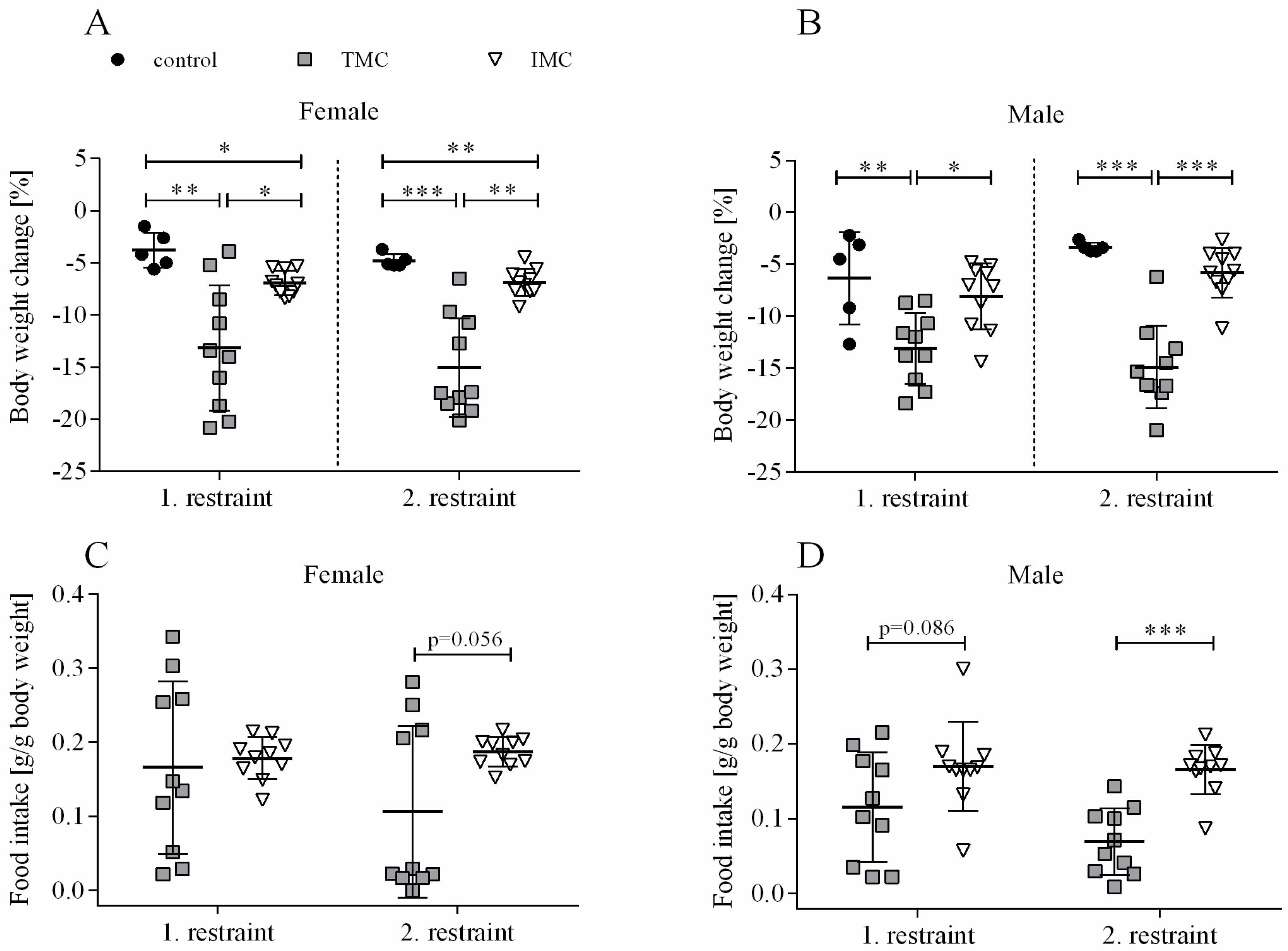
3.1. Changes in Body Weight, Body Composition, and Food Intake Are Dependent on the Metabolic Cage Type

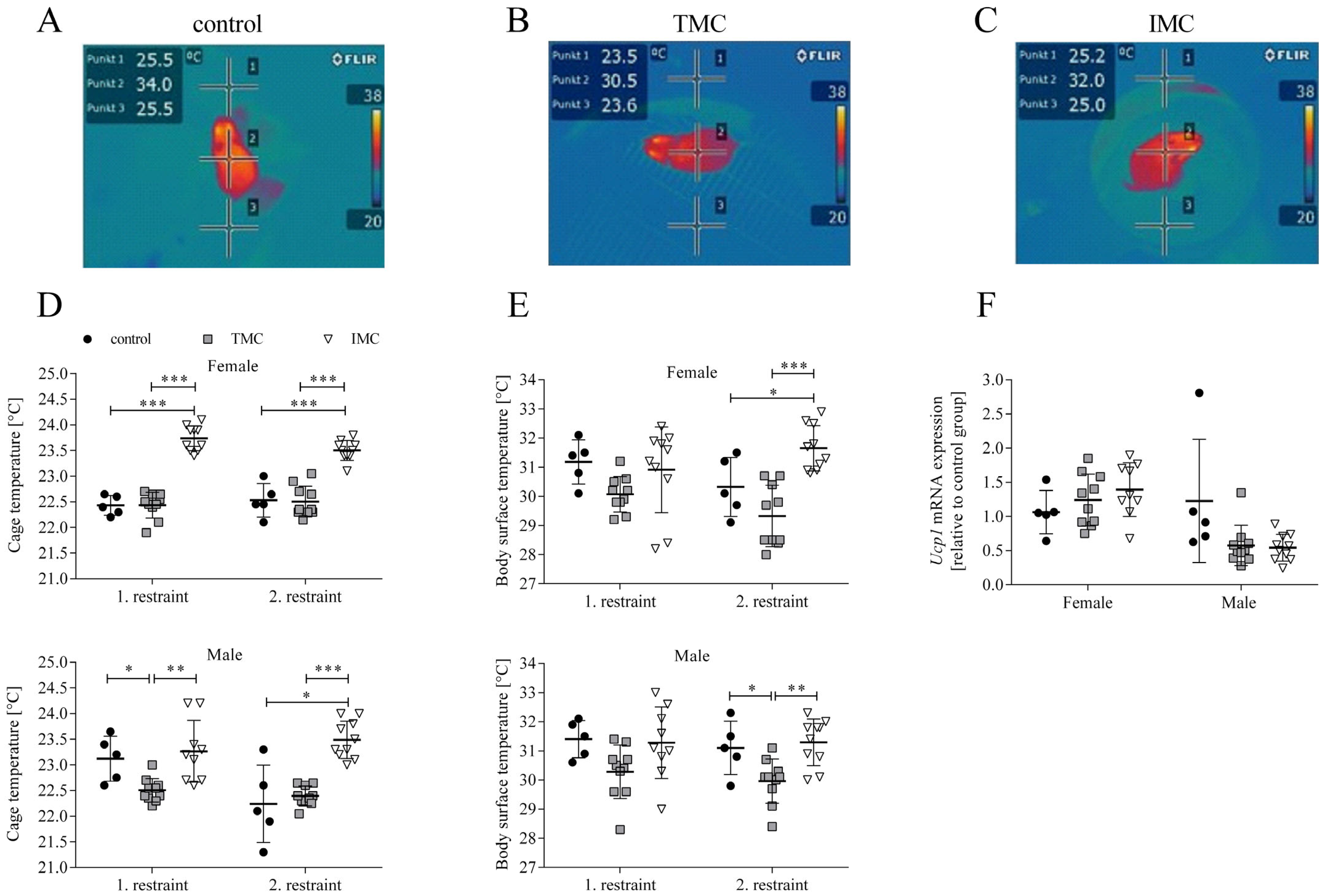
3.2. The Cold Stress for Mice Is Attenuated during Restraint in the Innovative Metabolic Cage
3.3. Excretion of Corticosterone Metabolites in the Feces Is Decreased by Restraining Mice in the Innovative Metabolic Cage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalliokoski, O.; Jacobsen, K.R.; Darusman, H.S.; Henriksen, T.; Weimann, A.; Poulsen, H.E.; Hau, J.; Abelson, K.S.P. Mice do not habituate to metabolism cage housing-a three week study of male BALB/c mice. PLoS ONE 2013, 8, e58460. [Google Scholar] [CrossRef]
- Kovalčíková, A.; Gyurászová, M.; Gardlík, R.; Boriš, M.; Celec, P.; Tóthová, Ľ. The effects of sucrose on urine collection in metabolic cages. Lab Anim. 2019, 53, 180–189. [Google Scholar] [CrossRef]
- Hennessy, M.B.; Levine, S. Effects of various habituation procedures on pituitary-adrenal responsiveness in the mouse. Physiol. Behav. 1977, 18, 799–802. [Google Scholar] [CrossRef]
- Speakman, J.R.; Keijer, J. Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans. Mol. Metab. 2012, 2, 5–9. [Google Scholar] [CrossRef]
- Seeley, R.J.; MacDougald, O.A. Mice as experimental models for human physiology: When several degrees in housing temperature matter. Nat Metab. 2021, 3, 443–445. [Google Scholar] [CrossRef]
- Karp, C.L. Unstressing intemperate models: How cold stress undermines mouse modeling. J. Exp. Med. 2012, 209, 1069–1074. [Google Scholar] [CrossRef]
- Vialard, F.; Olivier, M. Thermoneutrality and Immunity: How Does Cold Stress Affect Disease. Front. Immunol. 2020, 11, 588387. [Google Scholar] [CrossRef]
- Fischer, A.W.; Cannon, B.; Nedergaard, J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol. Metab. 2018, 7, 161–170. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Sahin, Z.; Solak, H.; Koc, A.; Ozen Koca, R.; Ozkurkculer, A.; Cakan, P.; Gormus, Z.I.S.; Kutlu, S.; Kelestimur, H. Long-term metabolic cage housing increases anxiety/depression-related behaviours in adult male rats. Arch. Physiol. Biochem. 2019, 125, 122–127. [Google Scholar] [CrossRef]
- Hoppe, C.C.; Moritz, K.M.; Fitzgerald, S.M.; Bertram, J.F.; Evans, R.G. Transient Hypertension and Sustained Tachycardia in Mice Housed Individually in Metabolism Cages. Physiol. Res. 2009, 58, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Z. Metabolic cages can be used for social isolation stress rather than metabolism studies with their current form. Türk. Bilimsel Derlemeler Derg. 2022, 15, 115–120. [Google Scholar]
- Prior, H.; Blunt, H.; Crossman, L.; McGuire, A.; Stow, R.; Sewell, F. Refining Procedures within Regulatory Toxicology Studies: Improving Animal Welfare and Data. Animals 2021, 11, 3057. [Google Scholar] [CrossRef]
- Meyer, C.W.; Ootsuka, Y.; Romanovsky, A.A. Body Temperature Measurements for Metabolic Phenotyping in Mice. Front. Physiol. 2017, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef]
- Bienboire-Frosini, C.; Wang, D.; Marcet-Rius, M.; Villanueva-García, D.; Gazzano, A.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Hernndez-Ávalos, I.; Lezama-García, K.; Verduzco-Mendoza, A.; et al. The Role of Brown Adipose Tissue and Energy Metabolism in Mammalian Thermoregulation during the Perinatal Period. Animals 2023, 13, 2173. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mota-Rojas, D.; Martínez-Burnes, J.; Villanueva-García, D.; Domínguez-Oliva, A.; Gómez-Prado, J.; Mora-Medina, P.; Casas-Alvarado, A.; Olmos-Hernández, A.; Soto, P.; et al. Strategies for Hypothermia Compensation in Altricial and Precocial Newborn Mammals and Their Monitoring by Infrared Thermography. Vet. Sci. 2022, 9, 246. [Google Scholar] [CrossRef]
- Payne, S.; Macintosh, A.; Stock, J. Body size and body composition effects on heat loss from the hands during severe cold exposure. Am. J. Phys. Anthropol. 2018, 166, 313–322. [Google Scholar] [CrossRef]
- Kingma, B.; Frijns, A.; van Marken Lichtenbelt, W. The thermoneutral zone: Implications for metabolic studies. Front. Biosci. 2012, 4, 1975–1985. [Google Scholar] [CrossRef]
- Guillen, J. FELASA Guidelines and Recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar]
- Mähler Convenor, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 2014, 48, 178–192. [Google Scholar] [CrossRef]
- Busch, M.; Chourbaji, S.; Dammann, P.; Gerold, S.; Haemisch, A.; Jirkof, P.; Osterkamp, A.; Ott, S.; Peters, S.; Spekl, K. Tiergerechte Haltung von Labormäusen. Ausschuss für Tiergerechte Labortierhaltung; GV-SOLAS Gesellschaft für Versuchstierkunde: Freiburg, Germany, 2014; pp. 1–25. [Google Scholar]
- Henkel, J.; Alfine, E.; Saín, J.; Jöhrens, K.; Weber, D.; Castro, J.P.; König, J.; Stuhlmann, C.; Vahrenbrink, M.; Jonas, W.; et al. Soybean Oil-Derived Poly-Unsaturated Fatty Acids Enhance Liver Damage in NAFLD Induced by Dietary Cholesterol. Nutrients 2018, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Prasol, D.J.; Belzung, C.; Crusio, W.E. Agonistic Behavior and Unpredictable Chronic Mild Stress in Mice. Behav. Genet. 2003, 33, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Touma, C.; Palme, R.; Sachser, N. Analyzing corticosterone metabolites in fecal samples of mice: A noninvasive technique to monitor stress hormones. Horm. Behav. 2004, 45, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Brown adipose tissue: Updates in cellular and molecular biology. Tissue Cell 2016, 48, 452–460. [Google Scholar] [CrossRef]
- Nedergaard, J.; Golozoubova, V.; Matthias, A.; Asadi, A.; Jacobsson, A.; Cannon, B. UCP1: The only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta 2001, 1504, 82–106. [Google Scholar] [CrossRef]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar]
- Keubler, L.M.; Hoppe, N.; Potschka, H.; Talbot, S.R.; Vollmar, B.; Zechner, D.; Häger, C.; Bleich, A. Where are we heading? Challenges in evidence-based severity assessment. Lab Anim. 2020, 54, 50–62. [Google Scholar] [CrossRef]
- Högberg, H.; Engblom, L.; Ekdahl, A.; Lidell, V.; Walum, E.; Alberts, P. Temperature dependence of O2 consumption; opposite effects of leptin and etomoxir on respiratory quotient in mice. Obesity 2006, 14, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Stechman, M.J.; Ahmad, B.N.; Loh, N.Y.; Reed, A.A.C.; Stewart, M.; Wells, S.; Hough, T.; Bentley, L.; Cox, R.D.; Brown, S.D.M.; et al. Establishing normal plasma and 24-hour urinary biochemistry ranges in C3H, BALB/c and C57BL/6J mice following acclimatization in metabolic cages. Lab. Anim. 2010, 44, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Schweizerische Eidgenossenschaft, Bundesamt für Lebensmittelsicherheit und Veterinärwesen BLV. Fachinformation Tierversuche. Das Halten von Versuchstieren in Stoffwechselkäfigen und Stoffwechselboxen 2.06. 2017. Available online: https://www.blv.admin.ch/blv/de/home/tiere/rechts--und-vollzugsgrundlagen/hilfsmittel-und-vollzugsgrundlagen/fachinformationen-und-merkblaetter.html (accessed on 8 August 2023).
- Škop, V.; Guo, J.; Liu, N.; Xiao, C.; Hall, K.D.; Gavrilova, O.; Reitman, M.L. Mouse Thermoregulation: Introducing the Concept of the Thermoneutral Point. Cell Rep. 2020, 31, 107501. [Google Scholar] [CrossRef]
- Franco, N.H.; Gerós, A.; Oliveira, L.; Olsson, I.A.S.; Aguiar, P. ThermoLabAnimal—A high-throughput analysis software for non-invasive thermal assessment of laboratory mice. Physiol. Behav. 2019, 207, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.-E.; Kozak, L.P. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Spiegelman, B.M. Towards a molecular understanding of adaptive thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.F.; Fan, A.X.; Neuendorff, E.H.; Vergara, V.B.; Kalinich, J.F. Hydrophobic Sand Versus Metabolic Cages: A Comparison of Urine Collection Methods for Rats (Rattus norvegicus). J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 51–57. [Google Scholar]
- Ortín-Piqueras, V.; Freitag, T.L.; Andersson, L.C.; Lehtonen, S.H.; Meri, S.K.; Spillmann, T.; Frias, R. Urinary Excretion of Iohexol as a Permeability Marker in a Mouse Model of Intestinal Inflammation: Time Course, Performance and Welfare Considerations. Animals 2021, 11, 79. [Google Scholar] [CrossRef]

| Criteria for Animal Welfare Assessment | Score |
|---|---|
| Reduced grooming | 1 |
| Body weight loss < 5% | |
| Feces: slight changes in shape (pasty, still shaped), consistency (soft), smell, color | |
| Significantly reduced grooming | 2 |
| Gummy eyes | |
| Blocked nose | |
| Fecal contamination | |
| Body weight loss 5–15% | |
| No grooming | 3 |
| Half-closed, pale, gummy eyes | |
| Curved back | |
| Crouched posture | |
| Positive skin fold test | 4 |
| Feces: absence of defecation, major change in shape (unformed)/ consistency (liquid)/striking smell or color | |
| Body weight loss 20% | 5 |
| Disorientation |
| Cage Type | No. of Restraint | Mean Fur Score (Standard Deviation) | Statistics | |||
|---|---|---|---|---|---|---|
| Female n = 25 | Male n = 25 | Female n = 25 | Male n = 25 | |||
| control | baseline | 1.00 (0.00) | 1.00 (0.00) | control vs. TMC | p = 1.000 | p = 1.000 |
| TMC | 1.00 (0.00) | 1.00 (0.00) | control vs. IMC | p = 1.000 | p = 1.000 | |
| IMC | 1.00 (0.00) | 1.00 (0.00) | TMC vs. IMC | p = 1.000 | p = 1.000 | |
| control | first | 1.00 (0.00) | 1.00 (0.00) | control vs. TMC | *** | *** |
| TMC | 2.00 (0.00) | 2.75 (0.26) | control vs. IMC | *** | *** | |
| IMC | 2.00 (0.00) | 2.00 (0.00) | TMC vs. IMC | p = 1.000 | *** | |
| control | second | 1.00 (0.00) | 1.00 (0.00) | control vs. TMC | *** | *** |
| TMC | 2.00 (0.00) | 2.50 (0.53) | control vs. IMC | *** | *** | |
| IMC | 2.00 (0.00) | 2.00 (0.00) | TMC vs. IMC | p = 1.000 | p = 0.063 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittek, L.; Touma, C.; Nitezki, T.; Laeger, T.; Krämer, S.; Raila, J. Reduction in Cold Stress in an Innovative Metabolic Cage Housing System Increases Animal Welfare in Laboratory Mice. Animals 2023, 13, 2866. https://doi.org/10.3390/ani13182866
Wittek L, Touma C, Nitezki T, Laeger T, Krämer S, Raila J. Reduction in Cold Stress in an Innovative Metabolic Cage Housing System Increases Animal Welfare in Laboratory Mice. Animals. 2023; 13(18):2866. https://doi.org/10.3390/ani13182866
Chicago/Turabian StyleWittek, Laura, Chadi Touma, Tina Nitezki, Thomas Laeger, Stephanie Krämer, and Jens Raila. 2023. "Reduction in Cold Stress in an Innovative Metabolic Cage Housing System Increases Animal Welfare in Laboratory Mice" Animals 13, no. 18: 2866. https://doi.org/10.3390/ani13182866
APA StyleWittek, L., Touma, C., Nitezki, T., Laeger, T., Krämer, S., & Raila, J. (2023). Reduction in Cold Stress in an Innovative Metabolic Cage Housing System Increases Animal Welfare in Laboratory Mice. Animals, 13(18), 2866. https://doi.org/10.3390/ani13182866






