Sarcocystis spp. Macrocysts Infection in Wildfowl Species in Eastern Baltic Region: Trends in Prevalence in 2011–2022
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Molecular Examination of Macrocysts
2.3. Statistical Data Analysis
3. Results
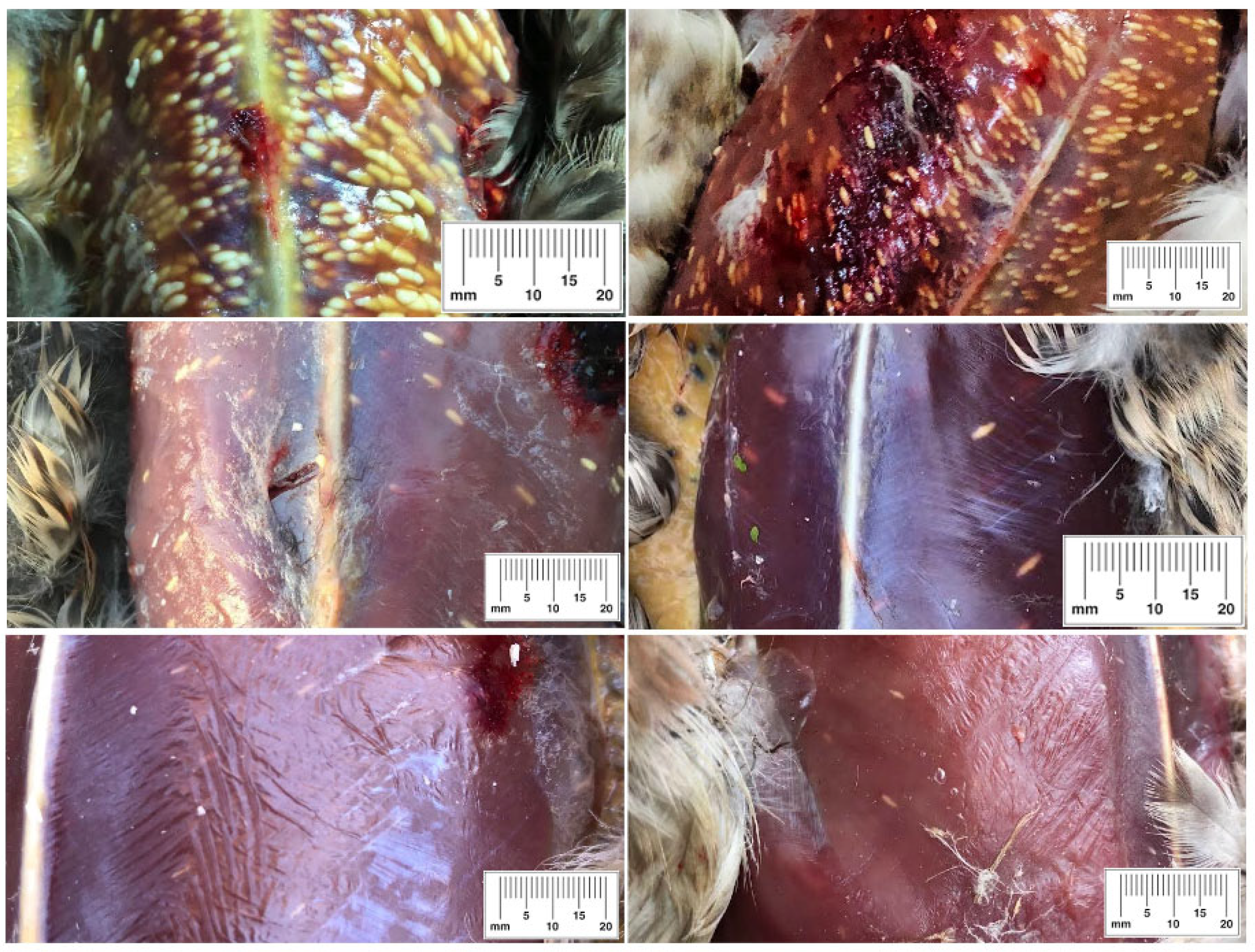
3.1. The Occurrence of Macrocysts in Wildfowl Species and Parasite Load
3.2. The Prevalence of Macrocysts of Sarcocystis in Wildfowl in Lithuania and Neighboring Countries
3.3. Factors Influencing the Prevalence of Macrocysts of Sarcocystis in the Mallard
3.4. Molecular Identification of S. rileyi in Mallards from Lithuania, Russia, Belarus, and Latvia
4. Discussion
4.1. The Genetic Identification and Variability of S. rileyi
4.2. The Distribution of Sarcocystis Macrocysts in Different Wildfowl Species
4.3. Factors Affecting the Prevalence of Macrocysts of Sarcocystis spp. in the Mallard in Lithuania
4.4. The Significance of Macrocyst Infection in Ducks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Fayer, R. Sarcocystis spp. in Human Infections. Clin. Microbiol. Rev. 2004, 17, 894–902. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. Sarcocystis cristata sp. nov. (Apicomplexa, Sarcocystidae) in the Imported Great Blue Turaco Corythaeola cristata (Aves, Musophagidae). Parasit. Vectors 2021, 14, 56. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, M.; Wu, Z.; Zeng, H.; Tao, J. Description of Sarcocystis platyrhynchosi n. sp. (Apicomplexa: Sarcocystidae) from Domestic Ducks Anas platyrhynchos (Anseriformes: Anatidae) in China. Parasit. Vectors 2023, 16, 50. [Google Scholar] [CrossRef]
- Smith, J.H.; Neill, P.J.; Box, E.D. Pathogenesis of Sarcocystis falcatula (Apicomplexa: Sarcocystidae) in the Budgerigar (Melopsittacus undulatus). III. Pathologic and Quantitative Parasitologic Analysis of Extrapulmonary Disease. J. Parasitol. 1989, 75, 270–287. [Google Scholar] [CrossRef]
- Olias, P.; Gruber, A.D.; Heydorn, A.O.; Kohls, A.; Mehlhorn, H.; Hafez, H.M.; Lierz, M. A Novel Sarcocystis-Associated Encephalitis and Myositis in Racing Pigeons. Avian Pathol. 2009, 38, 121–2128. [Google Scholar] [CrossRef] [PubMed]
- Wünschmann, A.; Rejmanek, D.; Cruz-Martinez, L.; Barr, B.C. Sarcocystis falcatula-Associated Encephalitis in a Bree-Ranging Great Horned Owl (Bubo virginianus). J. Vet. Diagn. Investig. 2009, 21, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Olias, P.; Gruber, A.D.; Heydorn, A.O.; Kohls, A.; Hafez, H.M.; Lierz, M. Unusual Biphasic Disease in Domestic Pigeons (Columba livia f. domestica) Following Experimental Infection with Sarcocystis calchasi. Avian Dis. 2010, 54, 1032–1037. [Google Scholar] [CrossRef]
- Olias, P.; Gruber, A.D.; Kohls, A.; Hafez, H.M.; Heydorn, A.O.; Mehlhorn, H.; Lierz, M. Sarcocystis Species Lethal for Domestic Pigeons. Emerg. Infect. Dis. 2010, 16, 497–499. [Google Scholar] [CrossRef]
- Wünschmann, A.; Rejmanek, D.; Conrad, P.A.; Hall, N.; Cruz-Martinez, L.; Vaughn, S.B.; Barr, B.C. Natural Fatal Sarcocystis falcatula Infections in Free-Ranging Eagles in North America. J. Vet. Diagn. Investig. 2010, 22, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Cawthorn, R.J.; Speer, C.A.; Wobeser, G.A. Redescription of the Sarcocysts of Sarcocystis rileyi (Apicomplexa: Sarcocystidae). J. Eukaryot. Microbiol. 2003, 50, 476–482. [Google Scholar] [CrossRef]
- Kutkienė, L.; Prakas, P.; Sruoga, A.; Butkauskas, D. Description of Sarcocystis anasi sp. nov. and Sarcocystis albifronsi sp. nov. in Birds of the Order Anseriformes. Parasitol. Res. 2012, 110, 1043–1046. [Google Scholar] [CrossRef]
- Kutkienė, L.; Prakas, P.; Sruoga, A.; Butkauskas, D. The mallard duck (Anas platyrhynchos) as intermediate host for Sarcocystis wobeseri sp. nov. from the barnacle goose (Branta leucopsis). Parasitol. Res. 2010, 107, 879–888. [Google Scholar] [CrossRef]
- Kutkienė, L.; Prakas, P.; Sruoga, A.; Butkauskas, D. Identification of Sarcocystis rileyi from the Mallard Duck (Anas platyrhynchos) in Europe: Cyst Morphology and Results of DNA Analysis. Parasitol. Res. 2011, 108, 709–714. [Google Scholar] [CrossRef]
- Prakas, P.; Oksanen, A.; Butkauskas, D.; Sruoga, A.; Kutkienė, L.; Švažas, S.; Isomursu, M.; Liaugaudaitė, S. Identification and Intraspecific Genetic Diversity of Sarcocystis rileyi from Ducks, Anas spp., in Lithuania and Finland. J. Parasitol. 2014, 100, 657–661. [Google Scholar] [CrossRef]
- Dubey, J.P.; Rosenthal, B.M.; Felix, T.A. Morphologic and Molecular Characterization of the Sarcocysts of Sarcocystis rileyi (Apicomplexa: Sarcocystidae) from the Mallard duck (Anas platyrhynchos). J. Parasitol. 2010, 96, 765–770. [Google Scholar] [CrossRef]
- Kalisinska, E.; Betlejewska, K.M.; Schmidt, M.; Gozdzicka-Jozefiak, A.; Tomczyk, G. Protozoal Macrocysts in the Skeletal Muscle of a Mallard duck in Poland: The First Recorded Case. Acta Parasitol. 2003, 48, 1–5. [Google Scholar]
- Padilla-Aguilar, P.; Romero-Callejas, E.; Osorio-Sarabia, D.; Ramírez-Lezama, J.; Cigarroa-Toledo, N.; Machain-Williams, C.; Manterola, C.; Zarza, H. Detection and Molecular Identification of Sarcocystis rileyi (Apicomplexa: Sarcocystidae) From a Northern Shoveler (Anas clypeata) in Mexico. J. Wildl. Dis. 2016, 52, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Cromie, R.; Ellis, M. Sarcocystis Survey. Sarcocystis Survey Feedback Report—The UK Wildfowl Sarcocystis Survey. 2019. Available online: www.sarcocystissurvey.org.uk/2015-2018-feedback-report/ (accessed on 15 December 2022).
- Szekeres, S.; Juhász, A.; Kondor, M.; Takács, N.; Sugár, L.; Hornok, S. Sarcocystis rileyi Emerging in Hungary: Is Rice Breast Disease Underreported in the Region? Acta Vet. Hung. 2019, 67, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.; Ellis, M.; Blake, D.P.; Chantrey, J.; Strong, E.A.; Reeves, J.P.; Cromie, R.L. Sarcocystis rileyi in UK Free-living Wildfowl (Anatidae): Surveillance, Histopathology and First Molecular Characterisation. Vet. Rec. 2020, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Sørensen, S.R.; Kania, P.W.; Buchmann, K. Sarcocystis rileyi (Apicomplexa) in Anas platyrhynchos in Europe with a Potential for Spread. Int. J. Parasitol. Parasites Wildl. 2021, 15, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Gower, C. A New Host and Locality Record for Sarcocystis rileyi (Stiles, 1803). J. Parasitol. 1938, 24, 378. [Google Scholar] [CrossRef]
- Erickson, A.B. Sarcocystis in Birds. Auk 1940, 57, 514–519. [Google Scholar] [CrossRef]
- Wichware, A.B. Sarcosporidiosis in a Black Duck. Can. J. Comp. Med. 1944, 8, 15–17. [Google Scholar]
- Cornwell, G. New Waterfowl Host Records for Sarcocystis rileyi and a Review of Sarcosporidiosis in Birds. Avian Dis. 1963, 7, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Chabreck, R.H. Sarcosporidiosis in Ducks in Louisiana. Trans. North Am. Wildl. Conf. 1965, 30, 174–184. [Google Scholar]
- Hoppe, D.M. Prevalence of Macroscopically Detectable Sarcocystis in North Dakota ducks. J. Wildl. Dis. 1976, 12, 27–29. [Google Scholar] [CrossRef]
- Broderson, D.; Canaris, A.G.; Bristol, J.R. Parasites of Waterfowl from Southwest Texas: II. The Shoveler, Anas clypeata. J. Wildl. Dis. 1977, 13, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Drouin, T.E.; Mahrt, J.L. The Prevalence of Sarcocystis Lankester, 1882, in some Bird Species in Western Canada, with Notes on its Life Cycle. Can. J. Zool. 1979, 57, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Canaris, A.G.; Mena, A.C.; Bristol, J.R. Parasites of Waterfowl from Southwest Texas: III. The Green-Winged Teal, Anas crecca. J. Wildl. Dis. 1981, 17, 57–64. [Google Scholar] [CrossRef]
- Costanzo, G.R. Sarcocystis in American Black Ducks Wintering in New Jersey. J. Wildl. Dis. 1990, 26, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Moorman, T.E.; Baldassarre, G.A.; Richard, D.M. The Frequency of Sarcocystis spp. and its Effect on Winter Carcass Composition of Mottled Ducks. J. Wildl. Dis. 1991, 27, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Fedynich, A.M.; Pence, D.B. Sarcocystis in Mallards on the Southern High Plains of Texas. Avian Dis. 1992, 36, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Hermanovská, L.; Čurlíl, J.; Hurníková, Z.; Brunčák, J.; Oberhauserová, K.; Dvorožňáková, E. Sarcocystosis of Mallards in Slovak Respublic. Folia Vet. 2013, 57, 45–48. [Google Scholar]
- Gjerde, B. Molecular Characterisation of Sarcocystis rileyi from a Common eider (Somateria mollissima) in Norway. Parasitol. Res. 2014, 113, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, R.J.; Rainnie, D.; Wobeser, G. Experimental Transmission of Sarcocystis sp. (Protozoa: Sarcocystidae) between the Shoveler (Anas clypeata) Duck and the Striped skunk (Mephitis mephitis). J. Wildl. Dis. 1981, 17, 389–394. [Google Scholar] [CrossRef]
- Wicht, R.J. Transmission of Sarcocystis rileyi to the Striped skunk (Mephitis mephitis). J. Wildl. Dis. 1981, 17, 387–388. [Google Scholar] [CrossRef]
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2005. [Google Scholar]
- Moré, G.; Maksimov, A.; Conraths, F.J.; Schares, G. Molecular Identification of Sarcocystis spp. in Foxes (Vulpes vulpes) and Raccoon dogs (Nyctereutes procyonoides) from Germany. Vet. Parasitol. 2016, 220, 9–14. [Google Scholar] [CrossRef]
- Prakas, P.; Liaugaudaitė, S.; Kutkienė, L.; Sruoga, A.; Švažas, S. Molecular Identification of Sarcocystis rileyi Sporocysts in Red foxes (Vulpes vulpes) and raccoon dog (Nyctereutes procyonoides) in Lithuania. Parasitol. Res. 2015, 114, 1671–1676. [Google Scholar] [CrossRef]
- Prakas, P.; Moskaliova, D.; Šneideris, D.; Juozaitytė-Ngugu, E.; Maziliauskaitė, E.; Švažas, S.; Butkauskas, D. Molecular Identification of Sarcocystis rileyi and Sarcocystis sp. (Closely Related to Sarcocystis wenzeli) in Intestines of Mustelids from Lithuania. Animals 2023, 13, 467. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Švažas, S.; Stanevičius, V. Morphological and Genetic Characterisation of Sarcocystis halieti from the Great Cormorant (Phalacrocorax carbo). Parasitol. Res. 2018, 117, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, V.; Prakas, P.; Calero-Bernal, R.; Gavarāne, I.; Fernández-García, J.L.; Martínez-González, M.; Rudaitytė-Lukošienė, E.; Martínez-Estéllez, M.Á.H.; Butkauskas, D.; Kirjušina, M. Identification and Genetic Characterization of Sarcocystis arctica and Sarcocystis lutrae in Red Foxes (Vulpes vulpes) from Baltic States and Spain. Parasit. Vectors 2018, 11, 173. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 17, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Reiczigel, J. Confidence Intervals for the Binomial Parameter: Some New Considerations. Stat. Med. 2003, 22, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Reiczigel, J.; Abonyi-Tóth, Z.; Singer, J. An Exact Confidence Set for two Binomial Proportions and Exact Unconditional Confidence Intervals for the Difference and Ratio of Proportions. Comput. Stat. Data Anal. 2008, 52, 5046–5053. [Google Scholar] [CrossRef]
- Rózsa, L.; Reiczigel, J.; Majoros, G. Quantifying Parasites in Samples of Hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef] [PubMed]
- El-Morsey, A.; El-Seify, M.; Desouky, A.R.; Abdel-Aziz, M.M.; El-Dakhly, K.M.; Kasem, S.; Abdo, W.; Haridy, M.; Sakai, H.; Yanai, T. Morphologic and Molecular Characteristics of Sarcocystis atraii n. sp. (Apicomplexa: Sarcocystidae) infecting the Common Coot (Fulica atra) from Egypt. Acta Parasitol. 2015, 60, 691–699. [Google Scholar] [CrossRef]
- Pan, J.; Ma, C.; Huang, Z.; Ye, Y.; Zeng, H.; Deng, S.; Hu, J.; Tao, J. Morphological and Molecular Characterization of Sarcocystis wenzeli in Chickens (Gallus gallus) in China. Parasit. Vectors 2020, 13, 512. [Google Scholar] [CrossRef]
- Stiles, C.W. On the presence of sarcosporidia in birds. USDA Bureau Anim. Ind. Bull. 1893, 3, 79–89. [Google Scholar]
- Dylko, N.I. Distribution of Sarcosporidia among Wild Vertebrates in Belorussia. Dokl. Akad. Nauk BSSR 1962, 6, 399–400. [Google Scholar]
- Golubkov, V.I. Infection of the Dog and Cat with Sarcocysts from the Chicken and Duck. Veterinariya 1979, 1, 55–56. [Google Scholar]
- Prakas, P.; Butkauskas, D.; Juozaitytė-Ngugu, E. Molecular Identification of four Sarcocystis Species in the Herring Gull, Larus argentatus, from Lithuania. Parasit. Vectors 2020, 13, 2. [Google Scholar] [CrossRef]
- Lee, F.C.H.; Muthu, V. From 18S to 28S rRNA Gene: An Improved Targeted Sarcocystidae PCR Amplification, Species Identification with Long DNA Sequences. Am. J. Trop. Med. Hyg. 2021, 104, 1388–1393. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Juozaitytė-Ngugu, E. Molecular and Morphological Description of Sarcocystis kutkienae sp. nov. from the Common Raven (Corvus corax). Parasitol. Res. 2020, 119, 4205–4210. [Google Scholar] [CrossRef]
- Šukytė, T.; Butkauskas, D.; Juozaitytė-Ngugu, E.; Švažas, S.; Prakas, P. Molecular Confirmation of Accipiter Birds of Prey as Definitive Hosts of Numerous Sarcocystis Species, including Sarcocystis sp., Closely Related to Pathogenic S. calchasi. Pathogens 2023, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Juozaitytė-Ngugu, E.; Prakas, P. The Richness of Sarcocystis Species in the Common Gull (Larus canus) and Black-Headed Gull (Larus ridibundus) from Lithuania. Parasitologia 2023, 3, 172–180. [Google Scholar] [CrossRef]
- Gondim, L.F.P.; Soares, R.M.; Tavares, A.S.; Borges-Silva, W.; de Jesus, R.F.; Llano, H.A.B.; Gondim, L.Q. Sarcocystis falcatula-like derived from Opossum in Northeastern Brazil: In Vitro Propagation in Avian Cells, Molecular Characterization and Bioassay in Birds. Int. J. Parasitol. Parasites Wildl. 2019, 10, 132–137. [Google Scholar] [CrossRef]
- Llano, H.A.B.; Zavatieri Polato, H.; Borges Keid, L.; Ferreira de Souza Oliveira, T.M.; Zwarg, T.; de Oliveira, A.S.; Sanches, T.C.; Joppert, A.M.; Gondim, L.F.P.; Martins Soares, R. Molecular screening for Sarcocystidae in Muscles of Wild Birds from Brazil suggests a Plethora of Intermediate Hosts for Sarcocystis falcatula. Int. J. Parasitol. Parasites Wildl. 2022, 17, 230–238. [Google Scholar] [CrossRef]
- Sato, A.P.; da Silva, T.C.E.; de Pontes, T.P.; Sanches, A.W.D.; Prakas, P.; Locatelli-Dittrich, R. Molecular Characterization of Sarcocystis spp. in Seabirds from Southern Brazil. Parasitol. Int. 2022, 90, 102595. [Google Scholar] [CrossRef] [PubMed]
- Kutkienė, L.; Sruoga, A. Sarcocystis spp. in Birds of the Order Anseriformes. Parasitol. Res. 2004, 92, 171–172. [Google Scholar] [CrossRef]
- Munday, B.L.; Hartley, W.J.; Harrigan, K.E.; Presidente, P.J.; Obendorf, D.L. Sarcocystis and related organisms in Australian wildlife: II. Survey findings in birds, reptiles, amphibians and fish. J. Wildl. Dis. 1979, 15, 57–73. [Google Scholar] [CrossRef]
- Pak, S.M.; Eshtokina, V.N. Sarcosporidians of Birds. In Sarcosporidians of Animals in Kazakhstan; Panin, J.V., Ed.; Nauka: Alma-Ata, Kazakhstan, 1984. [Google Scholar]
- Hirschfeld, A.; Heyd, A. Mortality of Migratory Birds caused by Hunting in Europe: Bag Statistics and Proposals for the Conservation of Birds and Animal Welfare. Berichte Vogelschutz 2005, 42, 47–74. [Google Scholar]
- Wetlands International. Waterbird Population Estimates. 2020. Available online: www.wpe.wetlands.org (accessed on 7 April 2023).
- Bright, P.W. Lessons from Lean Beasts: Conservation Biology of the Mustelids. Mamm. Rev. 2000, 30, 217–226. [Google Scholar] [CrossRef]
- Zschille, J.; Stier, N.; Roth, M.; Mayer, R. Feeding Habits of Invasive American mink (Neovison vison) in Northern Germany—Potential Implications for Fishery and Waterfowl. Acta Theriol. 2013, 59, 25–34. [Google Scholar] [CrossRef]
- Švažas, S.; Meissner, W.; Serebryakov, V.; Kozulin, A.; Grishanov, G. Changes of Wintering Sites of Waterfowl in Central and Eastern Europe; OMPO Vilnius & Institute of Ecology: Vilnius, Lithuania, 2001. [Google Scholar]
- Švažas, S.; Kozulin, A. Waterbirds of Large Fishponds of Belarus and Lithuania; OMPO Vilnius & Institute of Ecology: Vilnius, Lithuania, 2002. [Google Scholar]
- Viksne, J.; Švažas, S.; Czajkowski, A.; Janaus, M.; Mischenko, A.; Kozulin, A.; Kuresoo, A.; Serebryakov, V. Atlas of Duck Populations in Europe; Akstis Press: Vilnius, Lithuania, 2010. [Google Scholar]
- Tuggle, B.N. Sarcocystis. In Field Guide to Wildlife Diseases-General Field Procedures and Diseases of Migratory Birds; Friend, M., Ed.; Resource Publication 167; United States Fish and Wildlife Service: Washington, DC, USA, 1987. [Google Scholar]
- Andrew, R.; Newman, P.P. Sarcosporidiosis. J. La. State Med. Soc. 1961, 113, 224–226. [Google Scholar]
- Keawcharoen, J.; Van Riel, D.; Van Amerongen, G.; Bestebroer, T.; Beyer, W.; Van Lavieren, R.; Osterhaus, A.; Fouchier, R.; Kuiken, T. Wild Ducks as Long-Distance Vectors of highly Pathogenic Avian Influenza Virus (H5N1). Emerg. Infect. Dis. 2008, 14, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Söderquist, P. Large-Scale Releases of Native Species: The Mallard as a Predictive Model System. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2015. [Google Scholar]


| Year | Lithuania | Kaliningrad Region (Russia) | Belarus | Latvia |
|---|---|---|---|---|
| 2012 | 2/158 (1.3%) b,e | 10/107 (9.4%) c,f | 6/72 (8.3%) g | 3/98 (3.1%) |
| 2013 | 1/21 (4.8%) | 2/74 (2.7%) | 5/62 (8.1%) | |
| 2014 | 9/66 (13.6%) a,h | 4/138 (2.9%) d,i | 1/68 (1.5%) j | |
| Overall | 12/245 (4.9%) | 16/319 (5.0%) | 12/202 (5.9%) | 3/98 (3.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakas, P.; Stankevičiūtė, J.; Švažas, S.; Juozaitytė-Ngugu, E.; Butkauskas, D.; Vaitkevičiūtė-Balčė, R. Sarcocystis spp. Macrocysts Infection in Wildfowl Species in Eastern Baltic Region: Trends in Prevalence in 2011–2022. Animals 2023, 13, 2875. https://doi.org/10.3390/ani13182875
Prakas P, Stankevičiūtė J, Švažas S, Juozaitytė-Ngugu E, Butkauskas D, Vaitkevičiūtė-Balčė R. Sarcocystis spp. Macrocysts Infection in Wildfowl Species in Eastern Baltic Region: Trends in Prevalence in 2011–2022. Animals. 2023; 13(18):2875. https://doi.org/10.3390/ani13182875
Chicago/Turabian StylePrakas, Petras, Jolanta Stankevičiūtė, Saulius Švažas, Evelina Juozaitytė-Ngugu, Dalius Butkauskas, and Rasa Vaitkevičiūtė-Balčė. 2023. "Sarcocystis spp. Macrocysts Infection in Wildfowl Species in Eastern Baltic Region: Trends in Prevalence in 2011–2022" Animals 13, no. 18: 2875. https://doi.org/10.3390/ani13182875
APA StylePrakas, P., Stankevičiūtė, J., Švažas, S., Juozaitytė-Ngugu, E., Butkauskas, D., & Vaitkevičiūtė-Balčė, R. (2023). Sarcocystis spp. Macrocysts Infection in Wildfowl Species in Eastern Baltic Region: Trends in Prevalence in 2011–2022. Animals, 13(18), 2875. https://doi.org/10.3390/ani13182875










