The Effects of Dietary Protein Level on the Growth Performance, Body Composition, Intestinal Digestion and Microbiota of Litopenaeus vannamei Fed Chlorella sorokiniana as the Main Protein Source
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Diet Preparation
2.2. Feeding Trial
2.3. Sample Collection
2.4. Growth Performance Analysis
- SR (%) = 100 × (final shrimp number/initial shrimp number);
- WGR (%) = 100 × (final body weight − initial body weight)/initial body weight;
- SGR (%) = 100 × [ln (final body weight) − ln (initial body weight)]/day;
- FCR = feed intake/(final body weight − initial body weight);
2.5. Analysis of Body Composition
2.6. Analysis of Intestinal Digestive Enzymes Activities
2.7. Intestinal Microbial Analysis
2.8. Statistical Analysis
3. Results
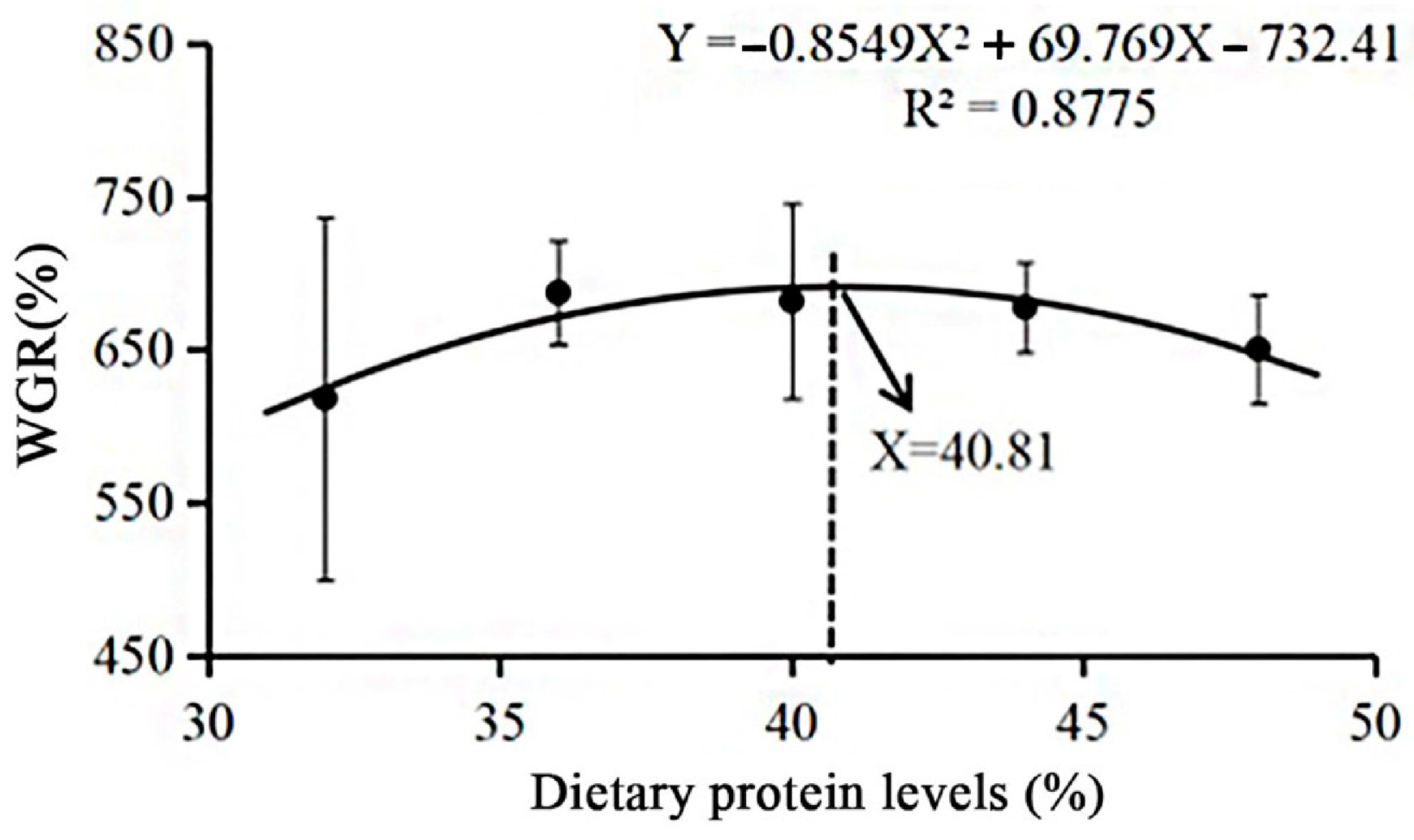
3.1. Growth Performance
3.2. Body Composition
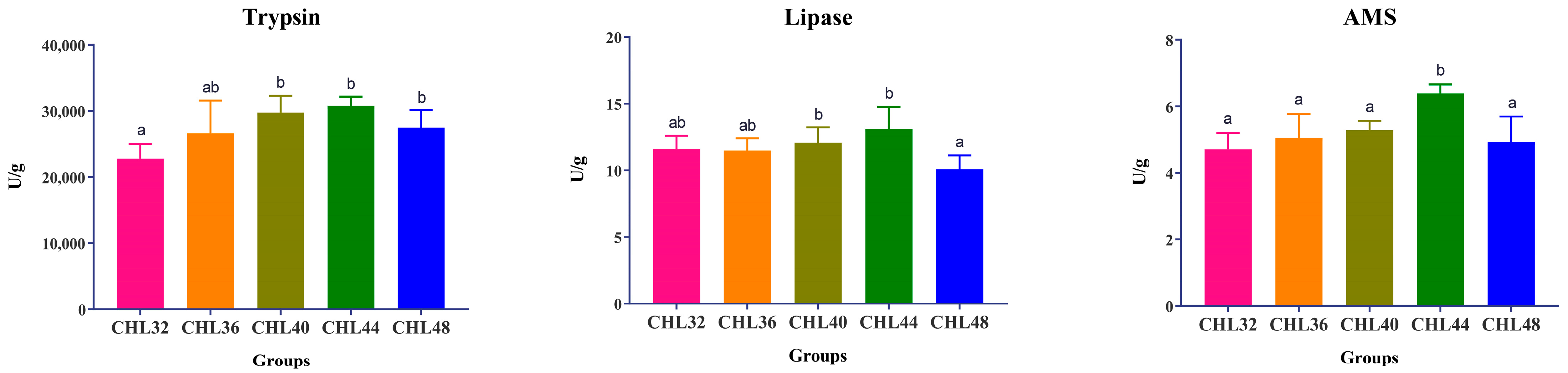
3.3. Digestive Enzyme Activities in the Intestine
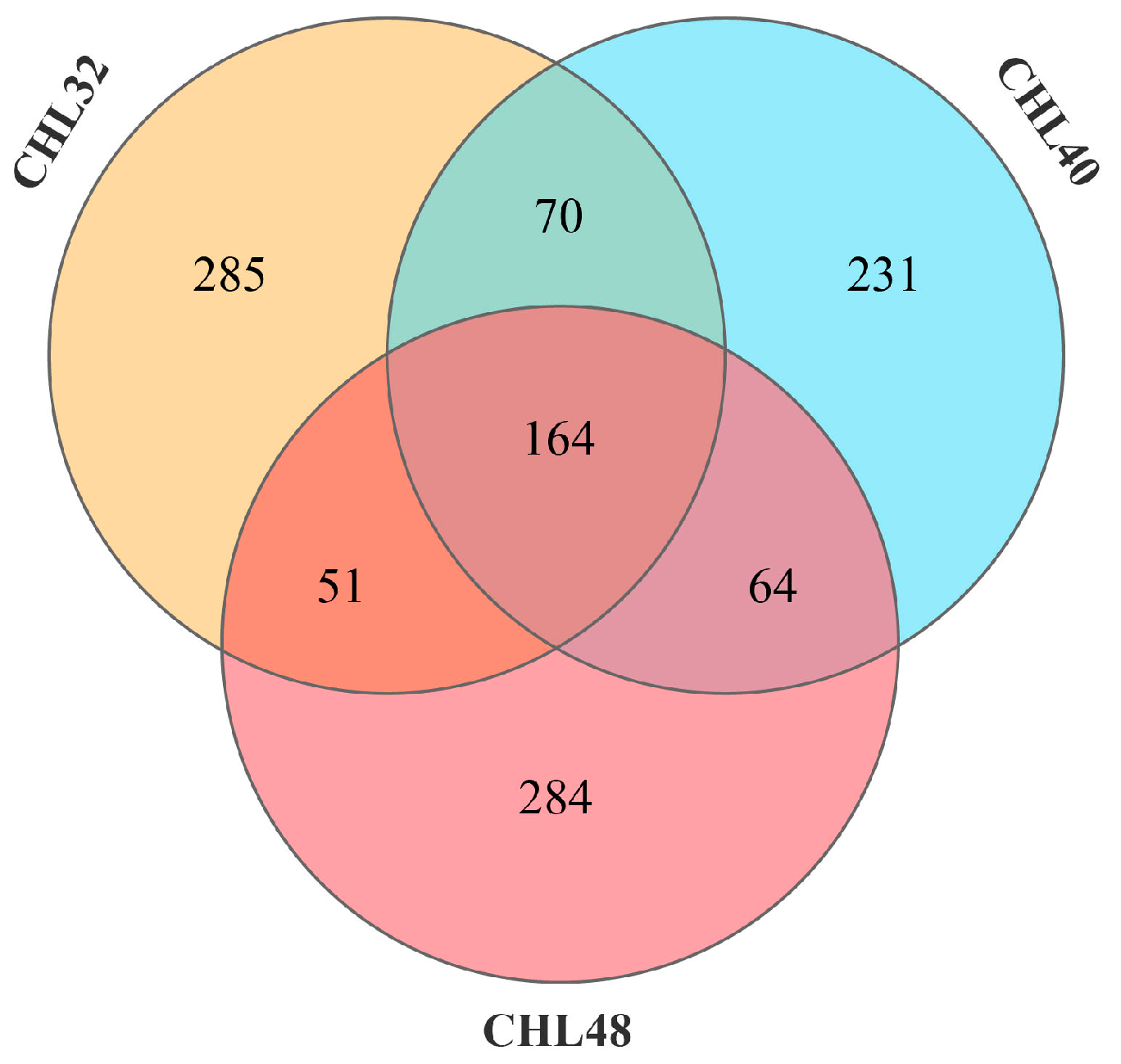
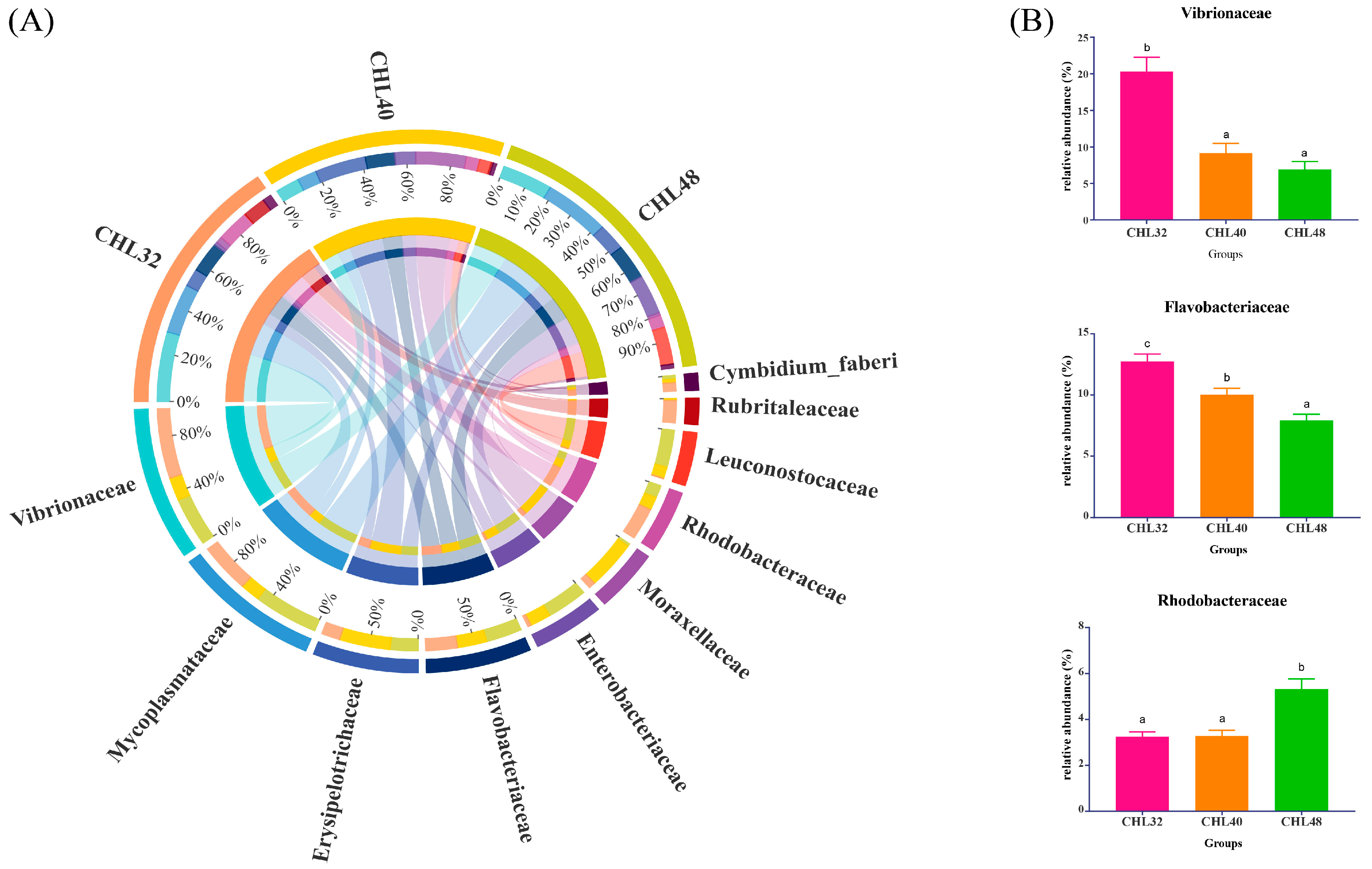
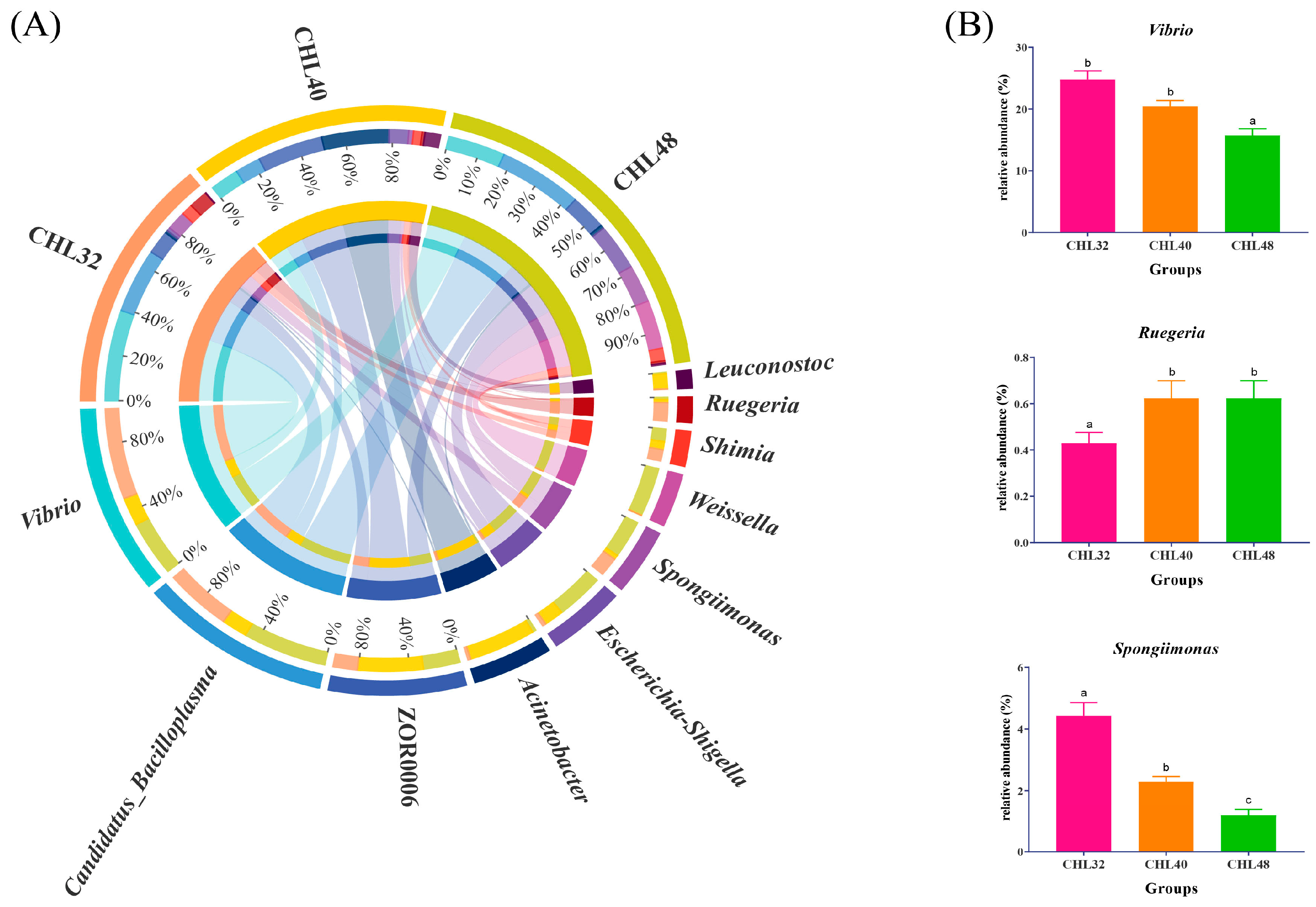
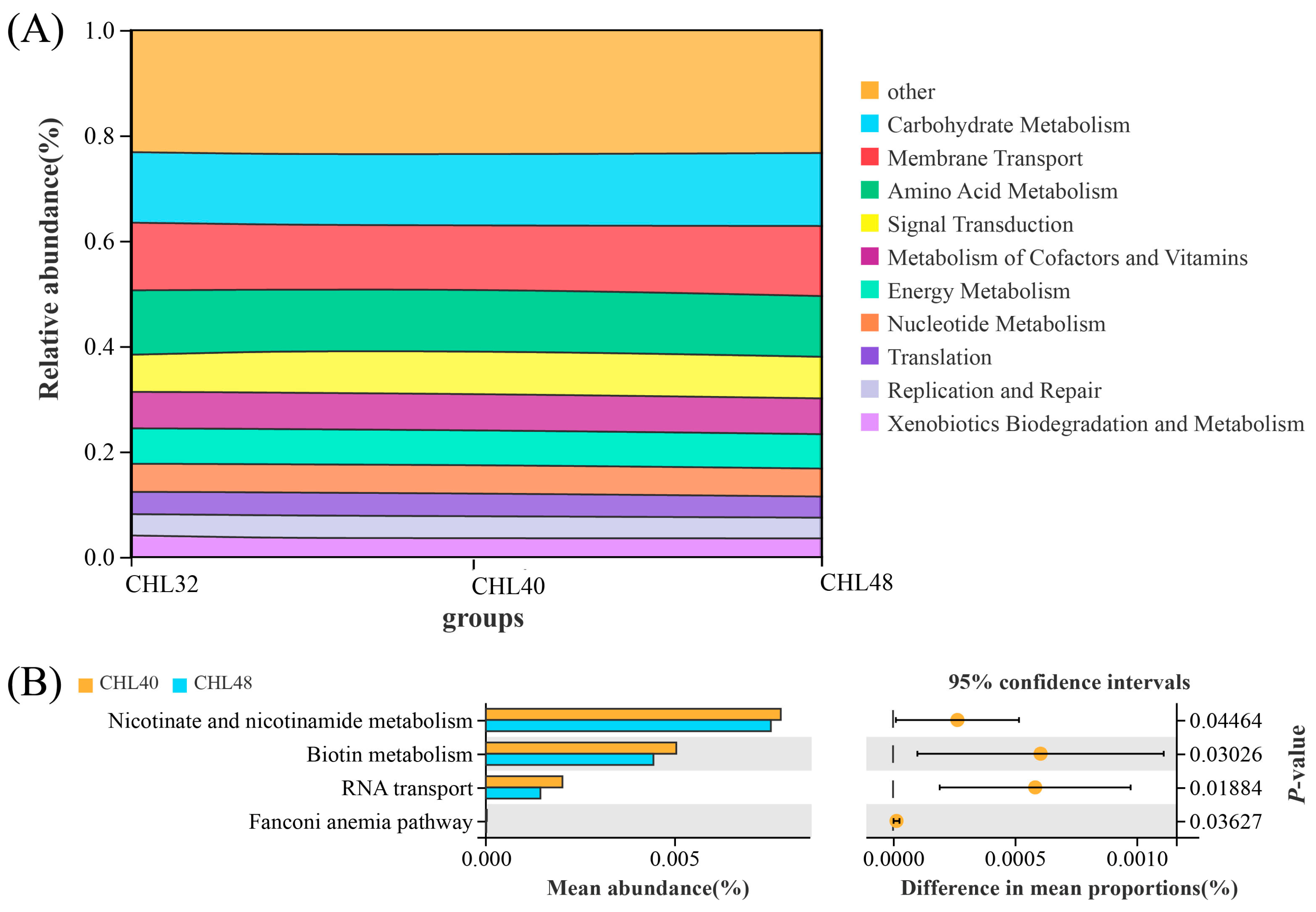
3.4. Intestinal Microbiota Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, Distribution, Impacts and Spatial Assessments—A Review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, D.; Jiang, Q. Chinese Freshwater Aquaculture: A Comparative Analysis of the Competitiveness on Regional Aquaculture Industries. Aquac. Fish. 2022, in press. [Google Scholar] [CrossRef]
- Tidwell, J.H.; Allan, G.L. Fish as Food: Aquaculture’s Contribution. Ecological and Economic Impacts and Contributions of Fish Farming and Capture Fisheries. EMBO Rep. 2001, 2, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H. Aquaculture: Farming Aquatic Animals and Plants. Afr. J. Aquat. Sci. 2005, 30, 213–214. [Google Scholar] [CrossRef]
- Pires, D.R.; Morais, A.; Coelho, C.; Marinho, A.F.; Saldanha, T. Nutritional Composition, Fatty Acids and Cholesterol Levels in Atlantic White Shrimp (Litopenaeus schimitti). Int. Food Res. J. 2018, 25, 151–157. [Google Scholar]
- Newman, S.G. Shrimp farming yesterday to tomorrow. Ref. Module Food Sci. 2022, 13, 235–266. [Google Scholar] [CrossRef]
- Wang, A.J.; Yang, Q.H.; Tang, B.P.; Xiao, W.W.; Jia, J.; Dong, X.H.; Chi, S.Y.; Liu, H.Y.; Zhang, S. Effects of Enzymolytic Soybean Meal on Growth Performance, Serum Biochemical Indices, Non-specific Immunity and Disease Resistance of Juvenile Litopenaeus vannamei. J. Guangdong Ocean Univ. 2018, 38, 14–21. [Google Scholar] [CrossRef]
- Suresh, A.V.; Kumaraguru Vasagam, K.P.; Nates, S. Attractability and Palatability of Protein Ingredients of Aquatic and Terrestrial Animal Origin, and Their Practical Value for Blue Shrimp, Litopenaeus stylirostris Fed Diets Formulated with High Levels of Poultry Byproduct Meal. Aquaculture 2011, 319, 132–140. [Google Scholar] [CrossRef]
- Yang, S.d.; Liou, C.H.; Liu, F.G. Effects of Dietary Protein Level on Growth Performance, Carcass Composition and Ammonia Excretion in Juvenile Silver Perch (Bidyanus bidyanus). Aquaculture 2002, 213, 363–372. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Bériault, R.; Lemire, J.; Singh, R.; Chénier, D.R.; Hamel, R.D.; Appanna, V.D. The Tricarboxylic Acid Cycle, an Ancient Metabolic Network with a Novel Twist. PLoS ONE 2007, 2, e690. [Google Scholar] [CrossRef]
- Wang, J.T.; Han, T.; Li, X.Y.; Yang, Y.X.; Yang, M.; Hu, S.X.; Jiang, Y.D.; Harpaz, S. Effects of Dietary Protein and Lipid Levels with Different Protein-to-Energy Ratios on Growth Performance, Feed Utilization and Body Composition of Juvenile Red-Spotted Grouper, Epinephelus akaara. Aquac. Nutr. 2017, 23, 994–1002. [Google Scholar] [CrossRef]
- Silva, P.; Andrade, C.A.P.; Timoteo Viriato, M.F.A.; Rocha, E.; Valente, L.M.P. Influence of Dietary Protein Level on Growth Performance and Body Composition of Juvenile Blackspot Seabream, Pagellus bogaraveo (Brunnich, 1768). J. Fish Biol. 2004, 65, 334–335. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Castro, P.L.; Torrecillas, S.; Montero, D.; Izquierdo, M.S.; Ginés, R. Effect of Combined Fishmeal and Fish Oil Replacement on Growth Performance, Flesh Quality and Shelf Life of European Sea Bass (Dicentrarchus labrax). Aquaculture 2022, 560, 738452. [Google Scholar] [CrossRef]
- Rana, K.J.; Siriwardena, S.; Mohammed, R.H. Impact of Rising Feed Ingredient Prices on Aquafeeds and Aquaculture Production; FAO: Rome, Italy, 2009; ISBN 9789251064221. [Google Scholar]
- Zhou, Q.; Mai, K.; Liu, Y.; Tan, B. Advances in Animal and Plant Protein Sources in Place of Fish Meal. J. Fish. China 2005, 29, 404–410. [Google Scholar] [CrossRef]
- Merino, G.; Barange, M.; Mullon, C.; Rodwell, L. Impacts of Global Environmental Change and Aquaculture Expansion on Marine Ecosystems. Glob. Environ. Chang. 2010, 20, 586–596. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2007; Volume 4, pp. 40–41. ISBN 978-92-5-136364-5. [Google Scholar]
- Deutsch, L.; Gräslund, S.; Folke, C.; Troell, M.; Huitric, M.; Kautsky, N.; Lebel, L. Feeding Aquaculture Growth through Globalization: Exploitation of Marine Ecosystems for Fishmeal. Glob. Environ. Chang. 2007, 17, 238–249. [Google Scholar] [CrossRef]
- Szczepański, A.; Adamek-Urbańska, D.; Kasprzak, R.; Szudrowicz, H.; Śliwiński, J.; Kamaszewski, M. Lupin: A Promising Alternative Protein Source for Aquaculture Feeds? Aquac. Rep. 2022, 26, 101281. [Google Scholar] [CrossRef]
- Goda, A.M.A.S.; Wafa, M.E.; El-Haroun, E.R.; Kabir Chowdhury, M.A. Growth Performance and Feed Utilization of Nile Tilapia Oreochromis niloticus (Linnaeus, 1758) and Tilapia Galilae Sarotherodon galilaeus (Linnaeus, 1758) Fingerlings Fed Plant Protein-Based Diets. Aquac. Res. 2007, 38, 827–837. [Google Scholar] [CrossRef]
- Pahlow, M.; van Oel, P.R.; Mekonnen, M.M.; Hoekstra, A.Y. Increasing Pressure on Freshwater Resources Due to Terrestrial Feed Ingredients for Aquaculture Production. Sci. Total Environ. 2015, 536, 847–857. [Google Scholar] [CrossRef]
- Enyidi, U.; Pirhonen, J.; Vielma, J. Effects of Sesame Seed Meal and Bambaranut Meal on Growth, Feed Utilization and Body Composition of Juvenile African Catfish Clarias gariepinus. Iran J. Fish Sci. 2014, 13, 998–1013. [Google Scholar]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New Developments in Fish Amino Acid Nutrition: Towards Functional and Environmentally Oriented Aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Bureau, D.P.; Harris, A.M.; Bevan, D.J.; Simmons, L.A.; Azevedo, P.A.; Cho, C.Y. Feather Meals and Meat and Bone Meals from Different Origins as Protein Sources in Rainbow Trout (Oncorhynchus mykiss) Diets. Aquaculture 2000, 181, 281–291. [Google Scholar] [CrossRef]
- Chi, Q.L.; Qin, S.W.; Du, C.L.; Liu, J.H.; Hu, Q. Design and Evaluation of an Inclined Thin-layer Photobioreactor for Culture Effect of Microalgae. J. Guangdong Ocean Univ. 2021, 41, 27–32. [Google Scholar] [CrossRef]
- Ahmad, A.; Ashraf, S.S. Sustainable Food and Feed Sources from Microalgae: Food Security and the Circular Bioeconomy. Algal Res. 2023, 74, 103185. [Google Scholar] [CrossRef]
- Carneiro, W.F.; Castro, T.F.D.; Orlando, T.M.; Meurer, F.; de Jesus Paula, D.A.; Virote, B.d.C.R.; da Cunha Barreto Vianna, A.R.; Murgas, L.D.S. Replacing Fish Meal by Chlorella sp. Meal: Effects on Zebrafish Growth, Reproductive Performance, Biochemical Parameters and Digestive Enzymes. Aquaculture 2020, 528, 735612. [Google Scholar] [CrossRef]
- Alcántara, C.; Fernández, C.; García-Encina, P.A.; Muñoz, R. Mixotrophic Metabolism of Chlorella sorokiniana and Algal-Bacterial Consortia under Extended Dark-Light Periods and Nutrient Starvation. Appl. Microbiol. Biotechnol. 2015, 99, 2393–2404. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Wang, Y.; Xu, H.; Wang, S. Establishment and Characterization of a Outdoor Open Culture System for Microalgae Chlorella sorokiniana. J. Guangdong Ocean Univ. 2021, 41, 33–40. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. [Google Scholar] [CrossRef]
- Cecchin, M.; Benfatto, S.; Griggio, F.; Mori, A.; Cazzaniga, S.; Vitulo, N.; Delledonne, M.; Ballottari, M. Molecular Basis of Autotrophic vs Mixotrophic Growth in Chlorella sorokiniana. Sci. Rep. 2018, 8, 6465. [Google Scholar] [CrossRef]
- Chai, S.; Shi, J.; Huang, T.; Guo, Y.; Wei, J.; Guo, M.; Li, L.; Dou, S.; Liu, L.; Liu, G. Characterization of Chlorella sorokiniana Growth Properties in Monosaccharide-Supplemented Batch Culture. PLoS ONE 2018, 13, e0199873. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Bulynina, S.S.; Ziganshina, E.E.; Ziganshin, A.M. Growth Efficiency of Chlorella sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater. BioTech 2023, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G. Challenges and Opportunities to Increase Economic Feasibility and Sustainability of Mixotrophic Cultivation of Green Microalgae of the Genus Chlorella. Renew. Sustain. Rev. 2022, 160, 112284. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chang, C.H.; Chen, C.Y.; Chang, J.S.; Ng, I.S. Towards Protein Production and Application by Using Chlorella Species as Circular Economy. Bioresour. Technol. 2019, 289, 121625. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Buma, A.G.J. Opportunities and Challenges of Microalgal Cultivation on Wastewater, with Special Focus on Palm Oil Mill Effluent and the Production of High Value Compounds. Waste Biomass Valorization 2019, 10, 2079–2097. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana Growth in Anaerobic Digester Effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef]
- Shi, X.; Luo, Z.; Chen, F.; Wei, C.C.; Wu, K.; Zhu, X.M.; Liu, X. Effect of Fish Meal Replacement by Chlorella Meal with Dietary Cellulase Addition on Growth Performance, Digestive Enzymatic Activities, Histology and Myogenic Genes’ Expression for Crucian Carp Carassius auratus. Aquac. Res. 2017, 48, 3244–3256. [Google Scholar] [CrossRef]
- Maliwat, G.C.; Velasquez, S.; Robil, J.L.; Chan, M.; Traifalgar, R.F.; Tayamen, M.; Ragaza, J.A. Growth and Immune Response of Giant Freshwater Prawn Macrobrachium rosenbergii (De Man) Postlarvae Fed Diets Containing Chlorella vulgaris (Beijerinck). Aquac. Res. 2017, 48, 1666–1676. [Google Scholar] [CrossRef]
- Raji, A.A.; Junaid, O.Q.; Milow, P.; Taufek, N.M.; Fada, A.M.; Kolawole, A.A.; Alias, Z.; Razak, S.A. Partial Replacement of Fishmeal with Spirulina platensis and Chlorella vulgaris and Its Effect on Growth and Body Composition of African Catfish Clarias gariepinus (Burchell 1822). Indian J. Fish 2019, 66, 100–111. [Google Scholar] [CrossRef]
- Xi, L.; Lu, Q.; Liu, Y.; Su, J.; Chen, W.; Gong, Y.; Han, D.; Yang, Y.; Zhang, Z.; Jin, J.; et al. Effects of Fish Meal Replacement with Chlorella Meal on Growth Performance, Pigmentation, and Liver Health of Largemouth Bass (Micropterus salmoides). Anim. Nutr. 2022, 10, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, S.; Akbarzadeh, A.; Sajjadi, M.M.; Hajimoradloo, A.; Noori, F. Chlorella vulgaris Meal Improved Growth Performance, Digestive Enzyme Activities, Fatty Acid Composition and Tolerance of Hypoxia and Ammonia Stress in Juvenile Pacific White Shrimp Litopenaeus vannamei. Aquac. Nutr. 2018, 24, 594–604. [Google Scholar] [CrossRef]
- Jin, H.; Chuai, W.; Li, K.; Hou, G.; Wu, M.; Chen, J.; Wang, H.; Jia, J.; Han, D.; Hu, Q. Ultrahigh-Cell-Density Heterotrophic Cultivation of the Unicellular Green Alga Chlorella sorokiniana for Biomass Production. Biotechnol. Bioeng. 2021, 118, 4138–4151. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Meena, D.K.; Sahoo, A.K.; Das, B.K.; Sen, R. Effective Valorization of Microalgal Biomass for the Production of Nutritional Fish-Feed Supplements. J. Clean. Prod. 2020, 243, 118697. [Google Scholar] [CrossRef]
- Jayant, M.; Hassan, M.A.; Srivastava, P.P.; Meena, D.K.; Kumar, P.; Kumar, A.; Wagde, M.S. Brewer’s Spent Grains (BSGs) as Feedstuff for Striped Catfish, Pangasianodon hypophthalmus fingerlings: An Approach to Transform Waste into Wealth. J. Clean. Prod. 2018, 199, 716–722. [Google Scholar] [CrossRef]
- USPCD Madrid DSpace CEU. Official Methods of Analysis of AOAC International. Volume I. In Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Xu, C.J.; Su, G.H.; Zhao, K.S.; Kong, X.H.; Wang, H.X.; Xu, X.Q.; Li, Z.Q.; Zhang, M.; Xu, J. Societal benefits and environmental performance of Chinese aquaculture. J. Clean. Produnc. 2023, 422, 138645. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lee, S.M.; Park, H.G.; Choi, J. Effects of Dietary Inclusion of Chlorella Vulgaris on Growth, Blood Biochemical Parameters, and Antioxidant Enzyme Activity in Olive Flounder, Paralichthys Olivaceus. J. World Aquac. Soc. 2017, 48, 103–112. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of Various Forms of Dietary Chlorella supplementation on Growth Performance, Immune Characteristics, and Intestinal Microflora Population of Broiler Chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Coelho, D.; Pestana, J.; Almeida, J.M.; Alfaia, C.M.; Fontes, C.M.G.A.; Moreira, O.; Prates, J.A.M. A High Dietary Incorporation Level of Chlorella vulgaris Improves the Nutritional Value of Pork Fat without Impairing the Performance of Finishing Pigs. Animals 2020, 10, 2384. [Google Scholar] [CrossRef]
- Shao, R.; Qi, Z.T.; Peng, J.Q.; Qiu, M.; Gao, Z. Effect of Dietary Chlorella on the Growth Performance and Physiological Parameters of Gibel Carp, Carassius auratus gibelio. Turk. J. Fish. Aquat. Sci. 2014, 14, 7. [Google Scholar] [CrossRef]
- Strebel, L.M.; Nguyen, K.; Araujo, A.; Corby, T.; Rhodes, M.; Beck, B.H.; Roy, L.A.; Davis, D.A. On Demand Feeding and the Response of Pacific White Shrimp (Litopenaeus vannamei) to Varying Dietary Protein Levels in Semi-Intensive Pond Production. Aquaculture 2023, 574, 739698. [Google Scholar] [CrossRef]
- Tian, J.; Xu, Q.; Liu, J.; Luo, M.; Hu, W.; Tian, L.; Gao, W. Optimal Dietary Protein Level for the White Shrimp (Litopenaeus vannamei) in Low Salinity Water. Isr. J. Aquac. 2016, 68, 20798. [Google Scholar] [CrossRef]
- Lu, X.; Wen, H.; Li, Q.; Wang, G.; Li, P.; Chen, J.; Sun, Y.; Yang, C.; Wu, F. Comparative Analysis of Growth Performance and Liver Transcriptome Response of Juvenile Ancherythroculter nigrocauda Fed Diets with Different Protein Levels. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100592. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I. Effect of Dietary Protein Levels on Growth Performance, Hematological Profile and Biochemical Composition of Fingerlings Rainbow Trout, Oncorhynchus mykiss Reared in Indian Himalayan Region. Aquac. Rep. 2020, 16, 100268. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Zhan, W.; Han, M.; Liu, F.; Xie, Q.; Guo, D.; Chen, L.; Lou, B. Effects of Dietary Protein Levels on Growth Performance, Muscle Composition and Fiber Recruitment of Juvenile Small Yellow Croaker (Larimichthys polyactis). Aquac. Rep. 2022, 27, 101335. [Google Scholar] [CrossRef]
- Rueda-López, S.; Lazo, J.P.; Reyes, G.C.; Viana, M.T. Effect of Dietary Protein and Energy Levels on Growth, Survival and Body Composition of Juvenile Totoaba macdonaldi. Aquaculture 2011, 319, 385–390. [Google Scholar] [CrossRef]
- Nguyen, H.Y.N.; Trinh, T.L.; Baruah, K.; Lundh, T.; Kiessling, A. Growth and Feed Utilisation of Nile Tilapia (Oreochromis niloticus) Fed Different Protein Levels in a Clear-Water or Biofloc-RAS System. Aquaculture 2021, 536, 736404. [Google Scholar] [CrossRef]
- Safari, O.; Paolucci, M.; Motlagh, H.A. Dietary Supplementation of Chlorella vulgaris Improved Growth Performance, Immunity, Intestinal Microbiota and Stress Resistance of Juvenile Narrow Clawed Crayfish, Pontastacus leptodactylus eschscholtz, 1823. Aquaculture 2022, 554, 738138. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, J.; Chen, G.; Huang, J.; Wang, Z.; Tang, B.; Zhuo, H. Combined Effects of Breeding Density, Feeding Frequency and Feeding Level on Specific Growth Rate, Feed Conversion Rate and Pepsin Activity of Juvenile Hybrid Groupers (Epinephelus fuscoguttatus × E. Lanceolatus). J. Guangdong Ocean Univ. 2018, 38, 22–31. [Google Scholar] [CrossRef]
- Vergara, J.M.; Fernández-Palacios, H.; Robainà, L.; Jauncey, K.; De La Higuera, M.; Izquierdo, M. The Effects of Varying Dietary Protein Level on the Growth, Feed Efficiency, Protein Utilization and Body Composition of Gilthead Sea Bream Fry. Fish Sci. 1996, 62, 620–623. [Google Scholar] [CrossRef]
- Goda, A.M.A.S. Effect of Dietary Protein and Lipid Levels and Protein–Energy Ratio on Growth Indices, Feed Utilization and Body Composition of Freshwater Prawn, Macrobrachium Rosenbergii (de Man 1879) Post Larvae. Aquac. Res. 2008, 39, 891–901. [Google Scholar] [CrossRef]
- Fournier, V.; Gouillou-Coustans, M.F.; Métailler, R.; Vachot, C.; Guedes, M.J.; Tulli, F.; Oliva-Teles, A.; Tibaldit, E.; Kaushik, S.J. Protein and Arginine Requirements for Maintenance and Nitrogen Gain in Four Teleosts. Br. J. Nutr. 2002, 87, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; Ai, Q.; Mai, K.; Xu, W.; Qi, G.; Luo, Y. Synergistic Effects of Dietary Cholesterol and Taurine on Growth Performance and Cholesterol Metabolism in Juvenile Turbot (Scophthalmus maximus L.) Fed High Plant Protein Diets. Aquaculture 2012, 324–325, 85–91. [Google Scholar] [CrossRef]
- Ballestrazzi, R.; Lanari, D.; D’Agaro, E.; Mion, A. The Effect of Dietary Protein Level and Source on Growth, Body Composition, Total Ammonia and Reactive Phosphate Excretion of Growing Sea Bass (Dicentrarchus labrax). Aquaculture 1994, 127, 197–206. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. Effect of Dietary Protein and Lipid Level on Metabolic Utilization of Diets by European Sea Bass (Dicentrarchus labrax) Juveniles. Fish Physiol. Biochem. 2001, 25, 269–275. [Google Scholar] [CrossRef]
- Tu, Y.; Xie, S.; Han, D.; Yang, Y.; Jin, J.; Liu, H.; Zhu, X. Growth Performance, Digestive Enzyme, Transaminase and GH-IGF-I Axis Gene Responsiveness to Different Dietary Protein Levels in Broodstock Allogenogynetic Gibel Carp (Carassius auratus gibelio) CAS III. Aquaculture 2015, 446, 290–297. [Google Scholar] [CrossRef]
- Wang, F.; Han, H.; Wang, Y.; Ma, X. Growth, Feed Utilization and Body Composition of Juvenile Golden Pompano Trachinotus ovatus Fed at Different Dietary Protein and Lipid Levels. Aquac. Nutr. 2013, 19, 360–367. [Google Scholar] [CrossRef]
- Skalli, A.; Hidalgo, M.C.; Abellán, E.; Arizcun, M.; Cardenete, G. Effects of the Dietary Protein/Lipid Ratio on Growth and Nutrient Utilization in Common Dentex (Dentex dentex L.) at Different Growth Stages. Aquaculture 2004, 235, 1–11. [Google Scholar] [CrossRef]
- Kim, L.O.; Lee, S.M. Effects of the Dietary Protein and Lipid Levels on Growth and Body Composition of Bagrid Catfish, Pseudobagrus fulvidraco. Aquaculture 2005, 243, 323–329. [Google Scholar] [CrossRef]
- Gawat, M.H.; Mao, B.; Singh, J.; Kaur, L. Food Protein Digestion and Absorption: Current Assessment Protocols. In Processing Technologies and Food Protein Digestion; Academic Press: Cambridge, MA, USA, 2023; pp. 51–80. [Google Scholar] [CrossRef]
- Wu, W.; Ji, H.; Yu, H.; Sum, J.; Zhou, J. Effect of refeeding dietary containing different protein and lipid levels on growth performance, body composition, digestive enzyme activities and metabolic related gene expression of grass carp (Ctenopharyngodon idellus) after overwinter starvation. Aquaculture 2020, 523, 735196. [Google Scholar] [CrossRef]
- Fang, G. Effect of Protein Levels on Growth Performance, Body Composition and Digestive Enzyme Activity of Culter mongolicus. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2014; pp. 35–36. [Google Scholar]
- Tu, Y.; Han, D.; Zhu, X.; Yang, Y.; Xie, S. Effects of Dietary Protein and Lipid Levels on Growth, Feed Utilization and Body Composition of Cyprinus longipectoralis. Acta Hydrobiol. Sin. 2013, 36, 843–850. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Shi, Y.; Yang, Y. Optimum Dietary Protein Level of Juvenile Pseudobagrus ussuriensis. Chin. J. Anim. Nutr. 2012, 24, 176–182. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.P.; Ranjan, A. Feeding De-Oiled Rice Bran (DORB) to Rohu, Labeo rohita: Effect of Varying Dietary Protein and Lipid Level on Growth, Body Composition, and Insulin like Growth Factor (IGF) Expression. Aquaculture 2018, 492, 59–66. [Google Scholar] [CrossRef]
- He, J.; Feng, P.; Lv, C.; Lv, M.; Ruan, Z.; Yang, H.; Ma, H.; Wang, R. Effect of a Fish–Rice Co-Culture System on the Growth Performance and Muscle Quality of Tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100367. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, W.D.; Wu, P.; Liu, Y.; Zeng, Y.Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Wang, S.W.; et al. Soybean Isoflavones Improve the Health Benefits, Flavour Quality Indicators and Physical Properties of Grass Carp (Ctenopharygodon idella). PLoS ONE 2019, 14, e0209570. [Google Scholar] [CrossRef]
- Methven, L. Natural Food and Beverage Flavour Enhancer. In Natural Food Additives, Ingredients and Flavourings; Woodhead Publishing: Sawston, UK, 2012; pp. 76–99. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, X.; Guo, C.R.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Luo, W.; Huang, X.L.; Chen, D.F.; et al. Dietary Isoleucine Affects Muscle Fatty Acid and Amino Acid Profiles through Regulating Lipid Metabolism and Autophagy in Hybrid Catfish (Pelteobagrus vachelli × Leiocassis longirostris). Anim. Nutr. 2022, 11, 369–380. [Google Scholar] [CrossRef]
- Carrillo-Farnés, O.; Forrellat-Barrios, A.; Guerrero-Galván, S.; Vega-Villasante, F. A Review of Digestive Enzyme Activity in Penaeid Shrimps. Crustaceana 2007, 80, 257–275. [Google Scholar] [CrossRef]
- Van Wormhoudt, A.; Favrel, P. Electrophoretic Characterization of Palaemon elegans (Crustacea, Decapoda) α Amylase System: Study of Amylase Polymorphism during the Intermolt Cycle. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 89, 201–207. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Dong, H.; Zheng, X.; Wang, Y.; Li, H.; Liu, Q.; Zhang, J. Effect of Dietary Poly-β-Hydroxybutyrate (PHB) on Growth Performance, Intestinal Health Status and Body Composition of Pacific White Shrimp Litopenaeus vannamei (Boone, 1931). Fish Shellfish Immunol. 2017, 60, 520–528. [Google Scholar] [CrossRef]
- Javahery, S.; Noori, A.; Hoseinifar, S.H. Growth Performance, Immune Response, and Digestive Enzyme Activity in Pacific White Shrimp, Penaeus vannamei Boone, 1931, Fed Dietary Microbial Lysozyme. Fish Shellfish Immunol. 2019, 92, 528–535. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, Y.W.; Yi, L.; Shi, M.Y.; Deng, D. Effects of Different Diets on Growth Performance, Digestive Enzyme Activities and Intestinal Bacterial Community of Juvenile Chinese Giant Salamander (Andrias davidianus). Aquac. Rep. 2023, 30, 101612. [Google Scholar] [CrossRef]
- Lu, X.; Peng, D.; Chen, X.; Wu, F.; Jiang, M.; Tian, J.; Liu, W.; Yu, L.; Wen, H.; Wei, K. Effects of Dietary Protein Levels on Growth, Muscle Composition, Digestive Enzymes Activities, Hemolymph Biochemical Indices and Ovary Development of Pre-Adult Red Swamp Crayfish (Procambarus clarkii). Aquac. Rep. 2020, 18, 100542. [Google Scholar] [CrossRef]
- He, B.; Zhou, B.; Xie, H.; Hu, Z.-T.; Wang, B.; Zhang, J.-L.; Li, Q.-Z.; Zhao, F.-Q.; Liu, X.; Li, Q.-D.; et al. Effect of Feeding Level on Growth, Digestive and Metabolic Enzymes and Antioxidant Capacity in Juvenile Yangtze Sturgeon (Acipenser dabryanus). Aquaculture 2023, 567, 739265. [Google Scholar] [CrossRef]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; van der Giezen, M. Understanding the Role of the Shrimp Gut Microbiome in Health and Disease. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, W.; Liao, Y.; Yang, C.; Wang, Q. Community Structure of Culturable Bacterial Communities in the Intestine of Pinctada Fucata Martensii and Its Aquaculture Water. J. Guangdong Ocean Univ 2021, 41, 8. [Google Scholar] [CrossRef]
- Simon, C.J.; Truong, H.H.; Noble, T.H.; Osborne, S.A.; Wynne, J.W.; Wade, N.M. Microbial Biomass, Marine Invertebrate Meals and Feed Restriction Influence the Biological and Gut Microbiota Response of Shrimp Penaeus monodon. Aquaculture 2020, 520, 734679. [Google Scholar] [CrossRef]
- He, Y.; Chi, S.; Tan, B.; Zhang, H.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S. Effect of Yeast Culture on Intestinal Microbiota of Litopenaeus vannamei. J. Guangdong Ocean Univ. 2017, 37, 21–27. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, Å.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S.; et al. Effect of Dietary Components on the Gut Microbiota of Aquatic Animals. A Never-Ending Story? Aquac. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef]
- Hartviksen, M.; Vecino, J.L.G.; Ringø, E.; Bakke, A.M.; Wadsworth, S.; Krogdahl, Å.; Ruohonen, K.; Kettunen, A. Alternative Dietary Protein Sources for Atlantic Salmon (Salmo salar L.) Effect on Intestinal Microbiota, Intestinal and Liver Histology and Growth. Aquac. Nutr. 2014, 20, 381–398. [Google Scholar] [CrossRef]
- Yu, X.; Luo, K.; Rao, W.; Chen, P.; Lei, K.; Liu, C.; Cui, Z.; Zhang, W.; Mai, K. Effects of Replacing Dietary Fish Meal with Enzyme-Treated Soybean Meal on Growth Performance, Intestinal Microbiota, Immunity and MTOR Pathway in Abalone Haliotis Discus hannai. Fish Shellfish Immunol. 2022, 130, 9–21. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Gómez Del Pulgar, E.M.; Kjølbæk, L.; Brahe, L.K.; Astrup, A.; Larsen, L.H.; Sanz, Y. Impact of Dietary Fiber and Fat on Gut Microbiota Re-Modeling and Metabolic Health. Trends Food Sci. Technol. 2016, 57, 201–212. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; MacKey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Garibay-Valdez, E.; Martínez-Porchas, M.; Calderón, K.; Vargas-Albores, F.; Gollas-Galván, T.; Martínez-Córdova, L. Taxonomic and Functional Changes in the Microbiota of the White Shrimp (Litopenaeus vannamei) Associated with Postlarval Ontogenetic Development. Aquaculture 2020, 518, 734842. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Wang, Y.; Chen, W.; Jin, Z.; Yao, Z.; Zhang, D. Strain-Specific Changes in the Gut Microbiota Profiles of the White Shrimp Litopenaeus vannamei in Response to Cold Stress. Aquaculture 2019, 503, 357–366. [Google Scholar] [CrossRef]
- Bertucci, A.; Hoede, C.; Dassié, E.; Gourves, P.Y.; Suin, A.; Le Menach, K.; Budzinski, H.; Daverat, F. Impact of Environmental Micropollutants and Diet Composition on the Gut Microbiota of Wild European Eels (Anguilla anguilla). Environ. Pollut. 2022, 314, 120207. [Google Scholar] [CrossRef]
- Miura, N.; Motone, K.; Takagi, T.; Aburaya, S.; Watanabe, S.; Aoki, W.; Ueda, M. Ruegeria sp. Strains Isolated from the Reef-Building Coral Galaxea Fascicularis Inhibit Growth of the Temperature-Dependent Pathogen Vibrio coralliilyticus. Mar. Biotechnol. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Z.; Tang, Y.; Kuang, J.; Duan, Y.; Lin, H.; Jiang, S.; Shu, H.; Huang, J. Effects on Development and Microbial Community of Shrimp Litopenaeus vannamei Larvae with Probiotics Treatment. AMB Express 2020, 10, 109. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Yilmaz, E.; Dawood, M.A.O.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp Vibriosis and Possible Control Measures Using Probiotics, Postbiotics, Prebiotics, and Synbiotics: A Review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Hou, D.; Zhou, R.; Zeng, S.; Wei, D.; Deng, X.; Xing, C.; Yu, L.; Deng, Z.; Wang, H.; Weng, S.; et al. Intestine Bacterial Community Composition of Shrimp Varies Under Low- and High-Salinity Culture Conditions. Front. Microbiol. 2020, 11, 589164. [Google Scholar] [CrossRef]
- Mudarris, M.; Austin, B.; Segers, P.; Vancanneyt, M.; Hoste, B.; Bernardet, J.F. Flavobacterium Scophthalmum sp. Nov., a Pathogen of Turbot (Scophthalmus maximus L.). Int. J. Syst. Evol. Microbiol. 1994, 44, 447–453. [Google Scholar] [CrossRef]
- Costello, E.K.; Gordon, J.I.; Secor, S.M.; Knight, R. Postprandial Remodeling of the Gut Microbiota in Burmese Pythons. ISME J. 2010, 4, 1375–1385. [Google Scholar] [CrossRef]
- Yu, G.; Xu, C.; Zhang, D.; Ju, F.; Ni, Y. MetOrigin: Discriminating the Origins of Microbial Metabolites for Integrative Analysis of the Gut Microbiome and Metabolome. IMeta 2022, 1, e10. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.A.; West, A.A.; Gayle, A.; Lucas, L.K.; Yan, J.; Jiang, X.; Malysheva, O.; Caudill, M.A. Pregnancy and Lactation Alter Biomarkers of Biotin Metabolism in Women Consuming a Controlled Diet. J. Nutr. 2014, 144, 1977–1984. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Groups | ||||
|---|---|---|---|---|---|
| CHL32 | CHL36 | CHL40 | CHL44 | CHL48 | |
| Brown fish meal | 150 | 150 | 150 | 150 | 150 |
| Casein protein | 80 | 80 | 80 | 80 | 80 |
| Corn starch | 150 | 150 | 150 | 150 | 150 |
| C. sorokiniana powder | 251 | 321 | 390 | 460 | 530 |
| Fish oil | 20.2 | 18.25 | 16.35 | 14.45 | 12.5 |
| Corn oil | 20.2 | 18.25 | 16.35 | 14.45 | 12.5 |
| Soybean lecithin | 10 | 10 | 10 | 10 | 10 |
| Vitamin and Mineral premix a | 12 | 12 | 12 | 12 | 12 |
| Choline chloride | 5 | 5 | 5 | 5 | 5 |
| Antioxidants | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Attractant | 1 | 1 | 1 | 1 | 1 |
| Vitamin C | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Calcium | 12 | 12 | 12 | 12 | 12 |
| Cellulose | 287.6 | 221.5 | 156.3 | 90.1 | 24 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 |
| Nutrient composition (DM, %) | |||||
| Crude protein | 33.02 | 37.98 | 41.37 | 45.51 | 48.92 |
| Crude lipids | 6.77 | 7.59 | 7.66 | 7.95 | 7.45 |
| Ash | 7.15 | 7.56 | 7.11 | 7.03 | 8.21 |
| Dry matter | 91.56 | 92.92 | 91.52 | 91.24 | 89.74 |
| Indexes | Groups | ||||
|---|---|---|---|---|---|
| CHL32 | CHL36 | CHL40 | CHL44 | CHL48 | |
| IBW (g) | 0.83 ± 0.02 | 0.83 ± 0.02 | 0.83 ± 0.02 | 0.83 ± 0.02 | 0.83 ± 0.02 |
| FBW (g) | 5.96 ± 0.04 a | 6.54 ± 0.03 b | 6.48 ± 0.20 b | 6.46 ± 0.16 b | 6.23 ± 0.03 ab |
| WGR (%) | 618.21 ± 3.14 a | 687.29 ± 5.01 c | 681.90 ± 4.60 c | 677.67 ± 6.79 c | 650.46 ± 17.55 b |
| SGR (%/day) | 3.52 ± 0.09 | 3.68 ± 0.08 | 3.67 ± 0.15 | 3.65 ± 0.23 | 3.60 ± 0.08 |
| FCR | 1.73 ± 0.04 c | 1.54 ± 0.01 ab | 1.69 ± 0.02 b | 1.55 ± 0.08 b | 1.48 ± 0.06 a |
| SR (%) | 97.5 ± 2.5 | 99.17 ± 1.44 | 97.5 ± 2.5 | 98.33 ± 1.44 | 98.33 ± 1.81 |
| Indexes | Groups | ||||
|---|---|---|---|---|---|
| CHL32 | CHL36 | CHL40 | CHL44 | CHL48 | |
| Whole body composition | |||||
| Crude protein (%) | 73.02 ± 0.62 a | 73.35 ± 0.62 a | 74.73 ± 0.44 b | 74.46± 0.62 b | 73.45± 0.29 a |
| Crude lipid (%) | 7.17 ± 0.25 | 7.60 ± 0.40 | 8.18 ± 0.38 | 7.96 ± 0.55 | 7.37 ± 0.31 |
| Ash (%) | 12.18 ± 0.97 | 12.63 ± 1.00 | 11.60 ± 1.10 | 13.87 ± 1.85 | 12.37 ± 0.71 |
| Essential amino acids | |||||
| Thr | 0.76 ± 0.05 | 0.72 ± 0.02 | 0.79 ± 0.03 | 0.73 ± 0.03 | 0.74 ± 0.04 |
| Val | 0.82 ± 0.03 ab | 0.81 ± 0.03 ab | 0.87 ± 0.06 b | 0.78 ± 0.04 ab | 0.75 ± 0.04 a |
| Met | 0.59 ± 0.05 | 0.57 ± 0.02 | 0.58 ± 0.04 | 0.57 ± 0.06 | 0.52 ± 0.03 |
| Ile | 0.8 ± 0.04 | 0.77 ± 0.05 | 0.82 ± 0.06 | 0.74 ± 0.02 | 0.71 ± 0.03 |
| Leu | 1.55 ± 0.06 | 1.49 ± 0.05 | 1.55 ± 0.07 | 1.46 ± 0.1 | 1.36 ± 0.05 |
| Phe | 0.84 ± 0.05 | 0.81 ± 0.01 | 0.84 ± 0.05 | 0.79 ± 0.06 | 0.73 ± 0.01 |
| Lys | 1.67 ± 0.09 | 1.62 ± 0.04 | 1.7 ± 0.1 | 1.61 ± 0.1 | 1.49 ± 0.07 |
| His | 0.43 ± 0.01 b | 0.4 ± 0.03 b | 0.42 ± 0.01 b | 0.40 ± 0.01 b | 0.34 ± 0.0 a |
| Arg | 2.01 ± 0.26 | 2.09 ± 0.13 | 2.29 ± 0.08 | 2.19 ± 0.06 | 2.00 ± 0.23 |
| Non-essential amino acids | |||||
| Asp | 2.05 ± 0.08 | 1.98 ± 0.05 | 2.09 ± 0.1 | 1.96 ± 0.08 | 1.88 ± 0.09 |
| Glu | 3.39 ± 0.11 | 3.33 ± 0.09 | 3.4 ± 0.15 | 3.22 ± 0.22 | 3.11 ± 0.14 |
| Ala | 1.94 ± 0.05 | 2.06 ± 0.03 | 2.08 ± 0.16 | 2.07 ± 0.1 | 1.95 ± 0.06 |
| Gly | 1.37 ± 0.04 | 1.31 ± 0.07 | 1.39 ± 0.09 | 1.33 ± 0.02 | 1.32 ± 0.1 |
| Serine | 0.76 ± 0.05 | 0.72 ± 0.02 | 0.79 ± 0.03 | 0.73 ± 0.03 | 0.70 ± 0.04 |
| Tyr | 0.83 ± 0.13 | 0.80 ± 0.06 | 0.80 ± 0.05 | 0.81 ± 0.12 | 0.70 ± 0.03 |
| Pro | 1.16 ± 0.05 | 1.07 ± 0.03 | 1.11 ± 0.03 | 0.96 ± 0.17 | 0.96 ± 0.15 |
| Total amino acids (TAAs) | 20.93 ± 0.87 | 20.57 ± 0.21 | 21.5 ± 1.04 | 20.37 ± 1.06 | 19.23 ± 0.76 |
| ∑EAA | 9.46 ± 0.53 ab | 9.28 ± 0.07 ab | 9.85 ± 0.5 b | 9.28 ± 0.46 ab | 8.61 ± 0.46 a |
| ∑NEAA | 11.49 ± 0.36 | 11.28 ± 0.18 | 11.64 ± 0.54 | 11.08 ± 0.61 | 10.64 ± 0.32 |
| EAA/TAAs | 45.20 | 45.11 | 45.81 | 45.56 | 44.77 |
| ∑FlavorAA | 8.75 ± 0.15 | 8.69 ± 0.19 | 8.97 ± 0.5 | 8.58 ± 0.28 | 8.26 ± 0.28 |
| Sample Name | Indexes | ||||||
|---|---|---|---|---|---|---|---|
| Raw PE | Clean PE | Raw Tags | Clean Tags | Chimera | Effective Tags | Effective Ratio (%) | |
| CHL32-1 | 122,664 | 122,022 | 106,842 | 105,726 | 446 | 105,280 | 85.83 |
| CHL32-2 | 120,260 | 119,749 | 110,074 | 107,945 | 4668 | 103,277 | 85.88 |
| CHL32-3 | 120,728 | 120,167 | 109,686 | 108,565 | 571 | 107,994 | 89.45 |
| CHL40-1 | 130,754 | 130,212 | 119,650 | 117,333 | 5527 | 111,806 | 85.51 |
| CHL40-2 | 135,666 | 135,206 | 126,534 | 125,580 | 1125 | 124,455 | 91.74 |
| CHL40-3 | 129,857 | 129,236 | 116,454 | 115,410 | 497 | 114,913 | 88.49 |
| CHL48-1 | 129,159 | 128,678 | 119,762 | 118,160 | 1996 | 116,164 | 89.94 |
| CHL48-2 | 130,380 | 129,817 | 119,996 | 119,073 | 5492 | 113,581 | 87.12 |
| CHL48-3 | 126,165 | 125,662 | 115,396 | 114,278 | 1587 | 112,691 | 89.32 |
| Indexes | Groups | ||
|---|---|---|---|
| CHL36 | CHL40 | CHL44 | |
| Sobs | 433.33 ± 18.17 | 430.33 ± 12.09 | 457.67 ± 17.01 |
| ACE | 723.39 ± 2.45 | 744.53 ± 26.78 | 734.80 ± 4.63 |
| Chao1 | 758.89 ± 2.47 b | 769.60 ± 13.91 b | 647.45 ± 16.56 a |
| Shannon | 3.79 ± 0.12 | 4.04 ± 0.76 | 4.04 ± 0.36 |
| Simpson | 0.85 ± 0.04 | 0.91 ± 0.02 | 0.83 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Song, W.; Tan, J.; Zheng, Y.; Wang, H.; Shi, L.; Zhang, S. The Effects of Dietary Protein Level on the Growth Performance, Body Composition, Intestinal Digestion and Microbiota of Litopenaeus vannamei Fed Chlorella sorokiniana as the Main Protein Source. Animals 2023, 13, 2881. https://doi.org/10.3390/ani13182881
Yuan H, Song W, Tan J, Zheng Y, Wang H, Shi L, Zhang S. The Effects of Dietary Protein Level on the Growth Performance, Body Composition, Intestinal Digestion and Microbiota of Litopenaeus vannamei Fed Chlorella sorokiniana as the Main Protein Source. Animals. 2023; 13(18):2881. https://doi.org/10.3390/ani13182881
Chicago/Turabian StyleYuan, Hang, Wanlin Song, Jianqiang Tan, Yudong Zheng, Hongming Wang, Lili Shi, and Shuang Zhang. 2023. "The Effects of Dietary Protein Level on the Growth Performance, Body Composition, Intestinal Digestion and Microbiota of Litopenaeus vannamei Fed Chlorella sorokiniana as the Main Protein Source" Animals 13, no. 18: 2881. https://doi.org/10.3390/ani13182881





