Mitigating Human Impacts on Wild Animal Welfare
Simple Summary
Abstract
1. Background
2. Impacts of Wildlife Management on Animal Welfare
Welfare in wild Norway Rat Management
3. Impacts of Biodiversity Conservation Practice on Animal Welfare
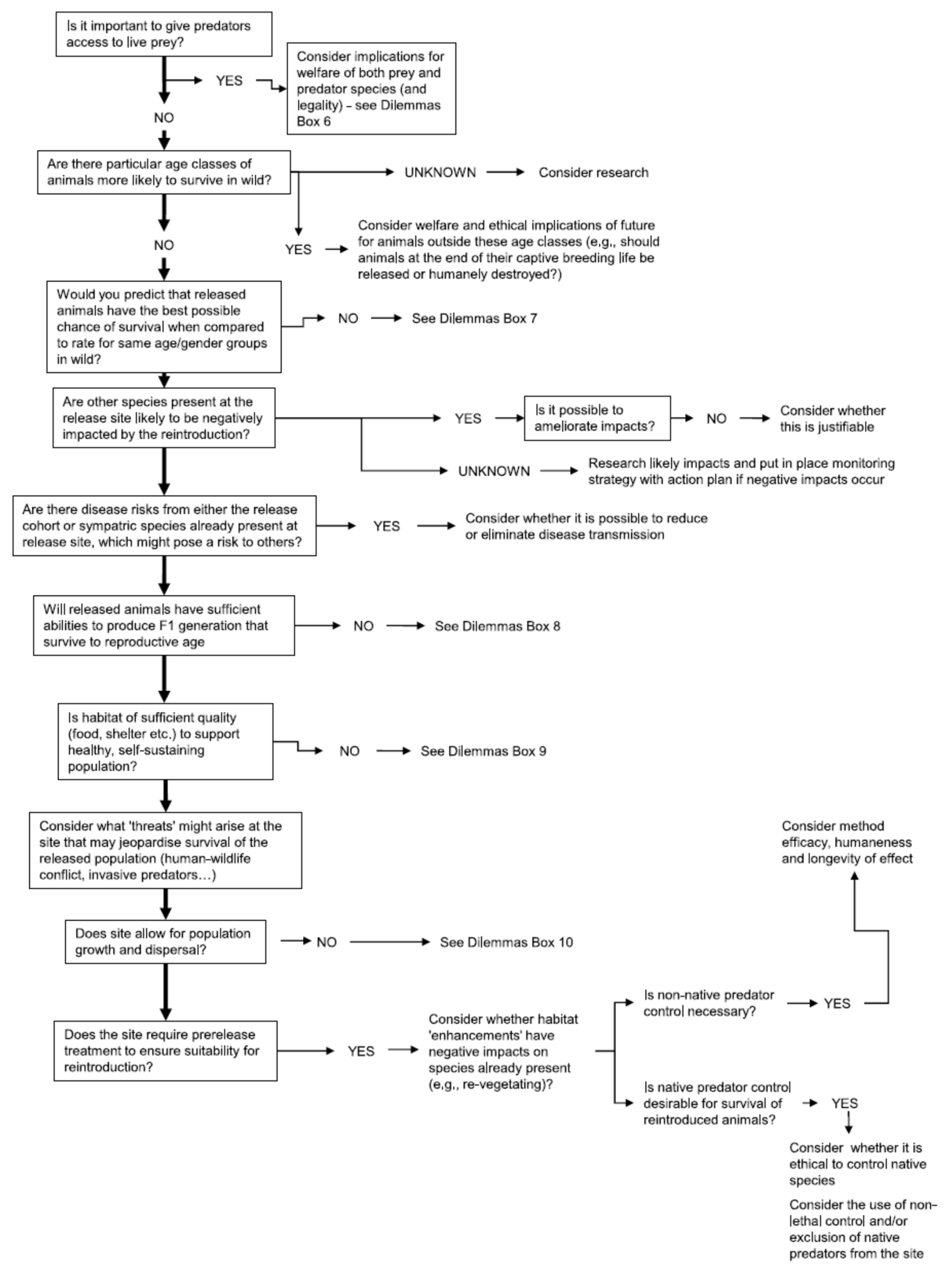
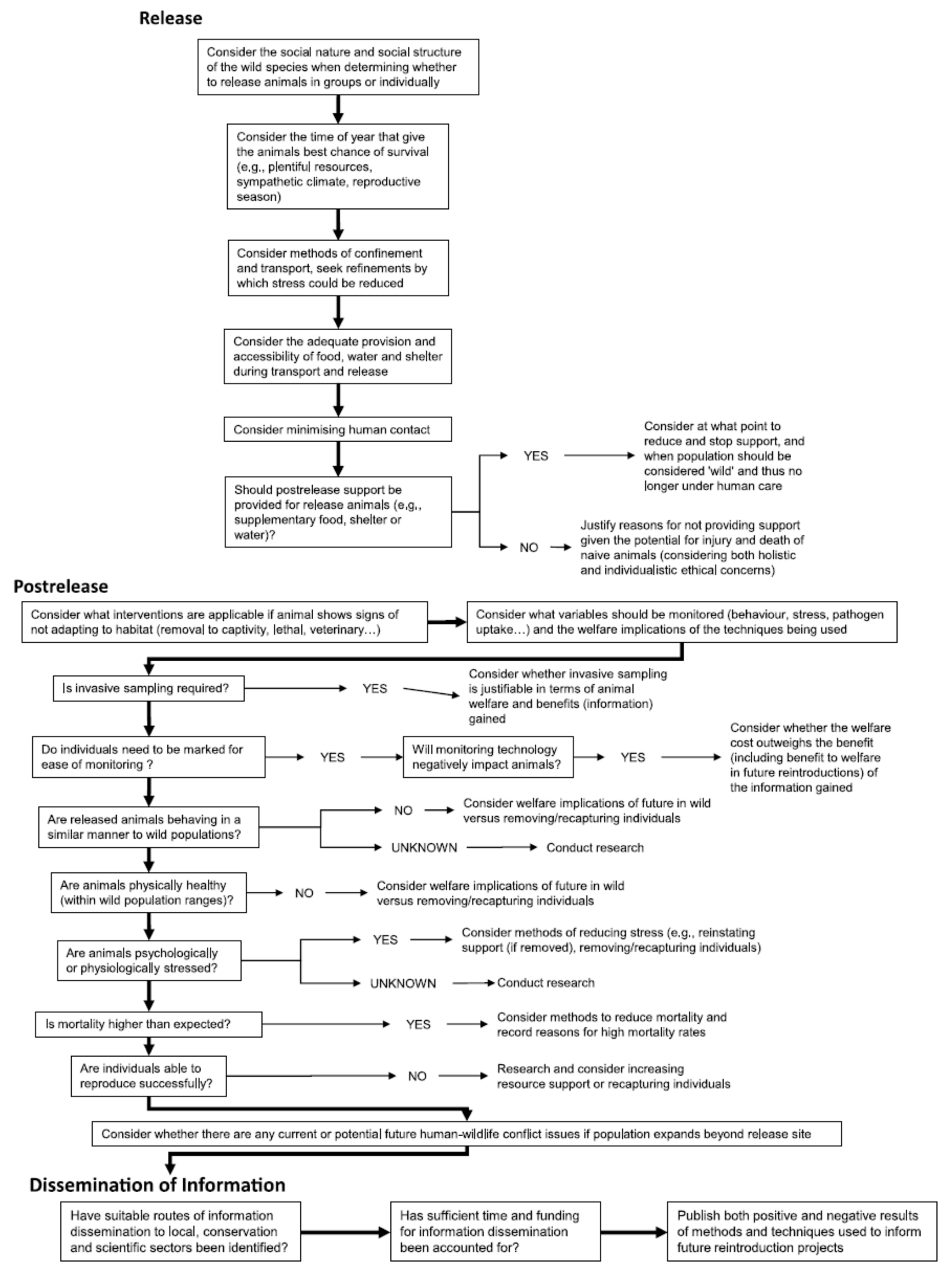
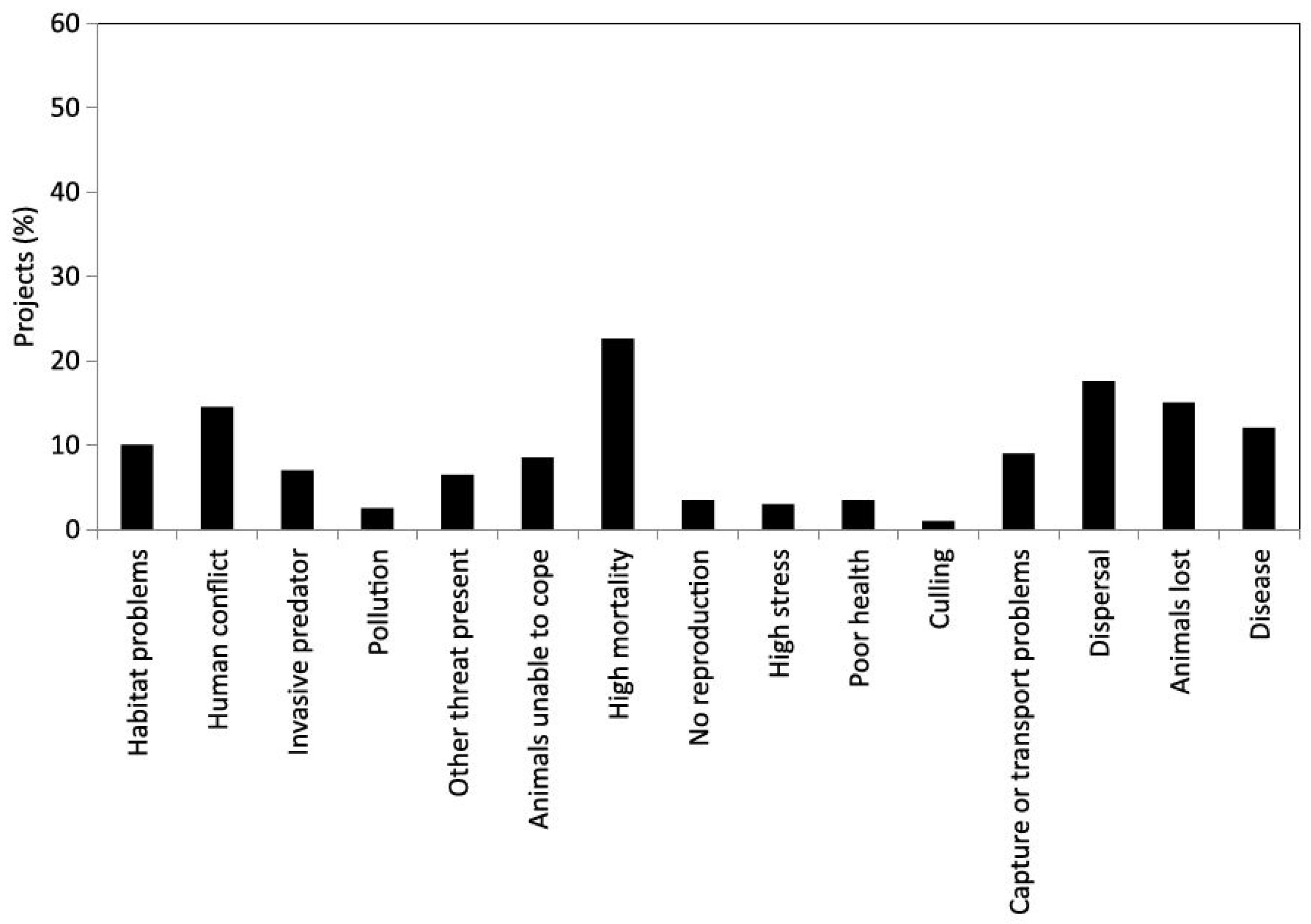
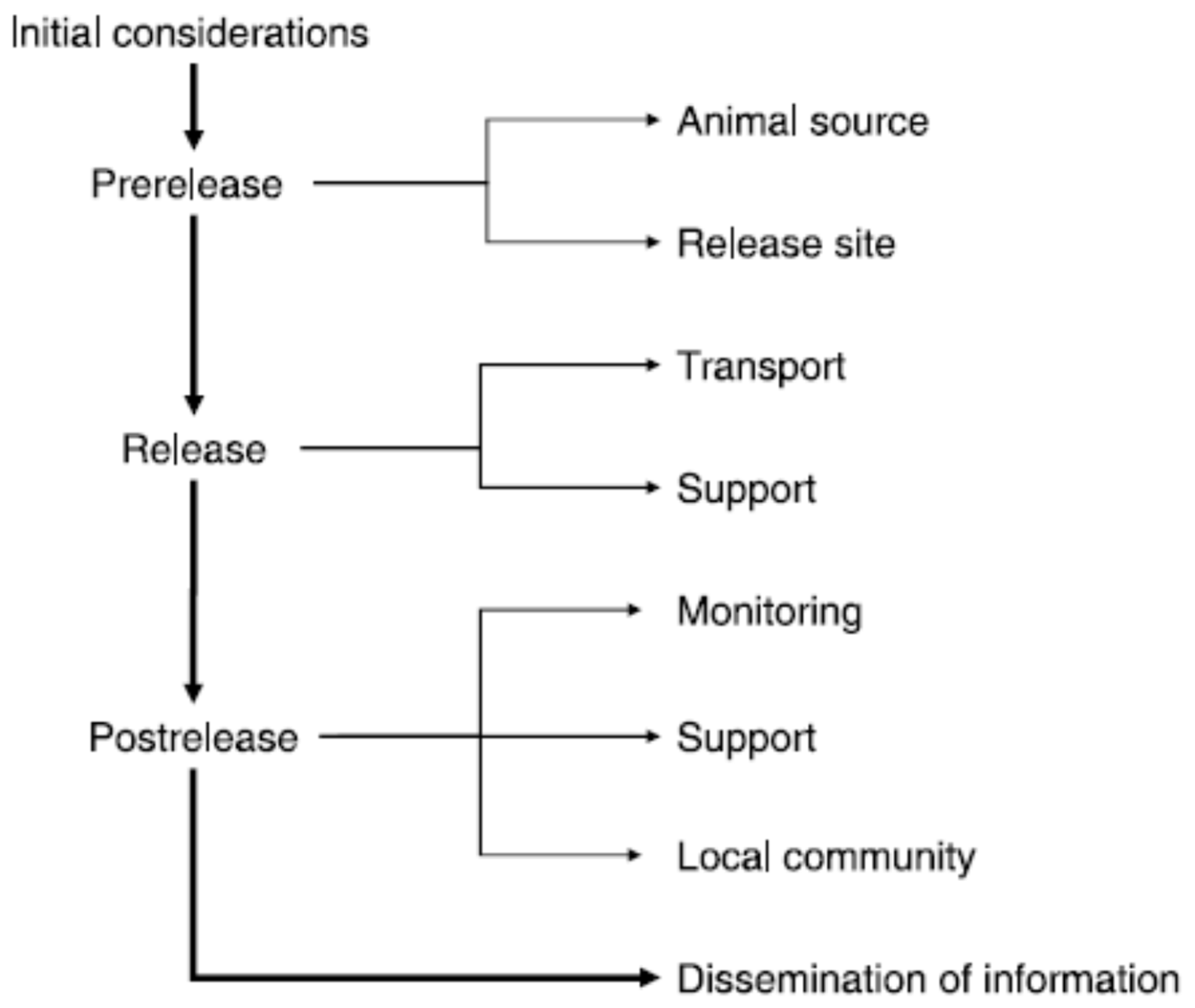
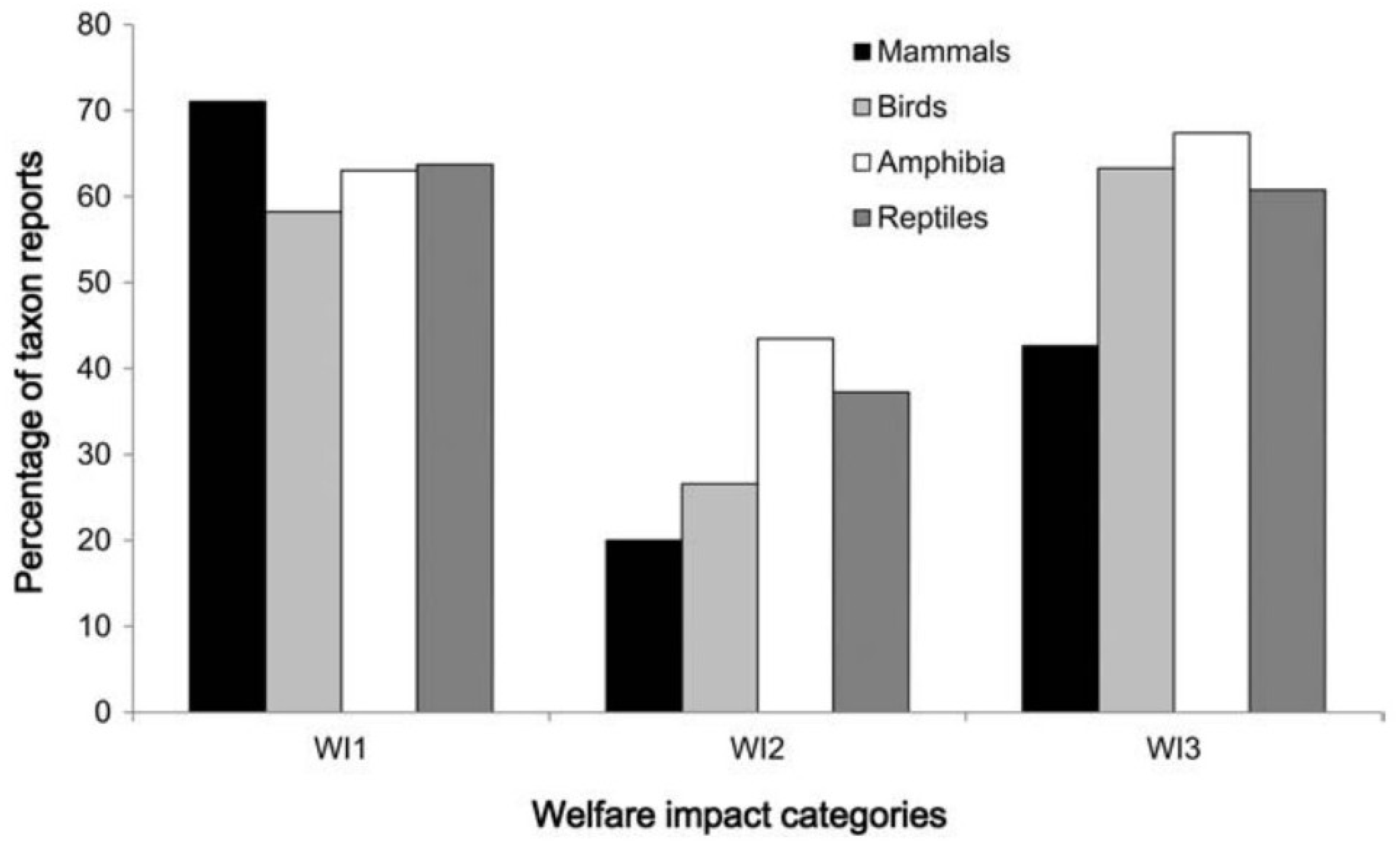
Welfare in Conservation Translocations
4. Other Impacts on Wild Animal Welfare
4.1. Wildlife Friendly Farming
4.2. Welfare in Wildlife Trade
4.3. Welfare in Wildlife Tourism
4.4. Urbanisation Impacts on the Welfare of Wild Animals
5. Synergies between Animal Welfare and Biodiversity Conservation
6. Balancing Welfare with Other Priorities
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Expanded Version of Figure 2 Decision Tree

References
- Fischer, B.; Lamey, A. Field Deaths in Plant Agriculture. J. Agric. Environ. Ethics 2018, 31, 409–428. [Google Scholar] [CrossRef]
- Macdonald, D.W. Mammal conservation: Old problems, new perspectives, transdisciplinarity, and the coming of age of conservation geopolitics. Annu. Rev. Environ. Resour. 2019, 44, 61–88. [Google Scholar] [CrossRef]
- Fa, J.E.; Seymour, S.; Dupain, J.E.F.; Amin, R.; Albrechtsen, L.; Macdonald, D. Getting to grips with the magnitude of exploitation: Bushmeat in the Cross–Sanaga rivers region, Nigeria and Cameroon. Biol. Conserv. 2006, 129, 497–510. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Dahlsjö, C.A.L.; Baker, S.E.; D’Cruze, N.; Macdonald, D.W. The customer isn’t always right—Conservation and animal welfare implications of the increasing demand for wildlife tourism. PLoS ONE 2015, 10, e0138939. [Google Scholar] [CrossRef]
- Kirkwood, J.K. Wild animal welfare. Anim. Welf. 2013, 22, 147–148. [Google Scholar] [CrossRef]
- Fraser, D.; MacRae, A.M. Four types of activities that affect animals: Implications for animal welfare science and animal ethics philosophy. Anim. Welf. 2011, 20, 581–590. [Google Scholar] [CrossRef]
- Mellor, D.J. Updating animal welfare thinking: Moving beyond the ‘Five Freedoms’ towards ‘A life worth living’. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Mellor, D.J.; Reid, C.S.W. Concepts of animal well-being and predicting the impact of procedures on experimental animals. In Improving the Well-Being of Animals in the Research Environment; Baker, R.M., Jenkin, G., Mellor, D.J., Eds.; Marriott Hotel: Sydney, Australia; Australian and New Zealand Council for the Care of Animals in Research and Training (ANZCCART): Glen Osmond, Australia, 1994; pp. 3–18. [Google Scholar]
- Dawkins, M.S. Why Animals Matter, Animal Consciousness, Animal Welfare and Human Well-Being; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Veterinary Record Editorial. BVA council approves policy on animal sentience. Vet. Rec. 2020, 187, 468. [Google Scholar]
- Beausoleil, N.J. I Am a Compassionate Conservation Welfare Scientist: Considering the Theoretical and Practical Differences Between Compassionate Conservation and Conservation Welfare. Animals 2020, 10, 257. [Google Scholar] [CrossRef]
- House of Lords. Animal Welfare (Sentience) Bill. UK Parliament. 2021. Available online: https://bills.parliament.uk/bills/2867 (accessed on 27 July 2021).
- Lambert, H.; Carder, G.; D’Cruze, N. Given the Cold Shoulder: A review of the scientific literature for evidence of reptile sentience. Animals 2019, 9, 821. [Google Scholar] [CrossRef]
- Bruckner, D.W. Animal welfare science, varieties of value and philosophical methodology. Anim. Welf. 2020, 29, 387–397. [Google Scholar] [CrossRef]
- Coghlan, S. The role of ethical reflection and dialogue in conceptualising animal welfare. J. Agric. Environ. Ethics 2022, 35, 14. [Google Scholar] [CrossRef]
- Broom, D.M. The welfare of vertebrate pests in relation to their management. In Advances in Vertebrate Pest Management; Cowan, D.P., Feare, C.J., Eds.; Filander Verlag: Furth, Germany, 1999; pp. 309–329. [Google Scholar]
- Sharp, T.; Saunders, G. A Model for Assessing the Relative Humaneness of Pest Animal Control Methods, 2nd ed.; Australian Government Department of Agriculture, Fisheries and Forestry: Canberra, ACT, Australia, 2011. Available online: https://www.awe.gov.au/agriculture-land/animal/welfare/aaws/humaneness-of-pest-animal-control-methods (accessed on 1 August 2023).
- Littin, K.E.; Mellor, D.J.; Warburton, B.; Eason, C.T. Animal welfare and ethical issues relevant to the humane control of vertebrate pests. N. Z. Vet. J. 2004, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cowan, P.; Warburton, B. Animal welfare and ethical issues in island pest eradication. In Island Invasives: Eradication and Management; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 418–421. [Google Scholar]
- Hampton, J.O.; Fisher, P.M.; Warburton, B. Reconsidering humaneness. Conserv. Biol. 2020, 34, 1107–1113. [Google Scholar] [CrossRef]
- Beausoleil, N.J.; Mellor, D.J.; Baker, L.; Baker, S.E.; Bellio, M.; Clarke, A.; Dale, A.; Garlick, S.; Jones, B.; Harvey, A.; et al. Feelings and Fitness’ not ‘Feelings or Fitness’—The raison d’etre of Conservation Welfare, which aligns conservation and animal welfare objectives. Front. Vet. Sci. Spec. Wild Anim. Welf. 2018, 5, 296. [Google Scholar] [CrossRef]
- Vucetich, J.A.; Macdonald, E.A.; Burnham, D.; Bruskotter, J.T.; Macdonald, D.W. Finding purpose in the conservation of biodiversity by the commingling of science and ethics. Animals 2021, 11, 837. [Google Scholar] [CrossRef]
- Sainsbury, A.W.; Bennett, P.M.; Kirkwood, J.K. The welfare of free-living wild animals in Europe: Harm caused by human activities. Anim. Welf. 1995, 4, 183–206. [Google Scholar] [CrossRef]
- Littin, K. Animal welfare and pest control: Meeting both conservation and animal welfare goals. Anim. Welf. 2010, 19, 171–176. [Google Scholar] [CrossRef]
- Fraser, D. Understanding Animal Welfare, The Science in Its Cultural Context; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Berdoy, M.; Drickamer, L.C. Comparative Social Organization and Life History of Rattus and Mus. In Rodent Societies: An Ecological and Evolutionary Perspective; Wolff, J.O., Sherman, P.W., Eds.; University of Chicago Press: Chicago, IL, USA, 2007; pp. 380–392. [Google Scholar]
- Macdonald, D.W.; Berdoy, M.L.; Webster, J.P. Brown rats on farmland: Ecological citizens or subsidized carpet-baggers? In Wildlife Conservation on Farmland Volume 2: Conflict in the Countryside; Macdonald, D.W., Feber, R.E., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 222–244. [Google Scholar]
- Birch, J.; Burn, C.; Schnell, A.; Browning, H.; Crump, A. Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans. 2021. Available online: https://www.wellbeingintlstudiesrepository.org/af_gen/2/ (accessed on 1 August 2023).
- Browning, H.; Birch, J. Animal sentience. Philos. Compass 2022, 17, e12822. [Google Scholar] [CrossRef]
- Horvath, K.; Angeletti, D.; Nascetti, G.; Carere, C. (Eds.) Invertebrate welfare: An overlooked issue. Ann. Dell’istituto Super. Di Sanità 2013, 49, 9–17. [Google Scholar]
- Carere, C.; Mather, J. The Welfare of Invertebrate Animals; Springer: Berlin/Heidelberg, Germany, 2019; Volume 18. [Google Scholar]
- Kirkwood, J.K.; Sainsbury, A.W.; Bennett, P.M. The welfare of free-living wild animals—Methods of assessment. Anim. Welf. 1994, 3, 257–273. [Google Scholar] [CrossRef]
- Mathews, F. Wild animal conservation and welfare in agricultural systems. Anim. Welf. 2010, 19, 159–170. [Google Scholar] [CrossRef]
- Faria, C.; Horta, O. Welfare biology. In The Routledge Handbook of Animal Ethics; Routledge: Oxfordshire, UK, 2019; pp. 455–466. [Google Scholar]
- Hayward, M.W.; Callen, A.; Allen, B.L.; Ballard, G.; Broekhuis, F.; Bugir, C.; Clarke, R.H.; Clulow, J.; Clulow, S.; Daltry, J.C.; et al. Deconstructing compassionate conservation. Conserv. Biol. 2019, 33, 760–768. [Google Scholar] [CrossRef]
- Coghlan, S.; Cardilini, A.P. A critical review of the compassionate conservation debate. Conserv. Biol. 2022, 36, e13760. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.E.; Sharp, T.M.; Macdonald, D.W. Assessing animal welfare impacts in the management of European rabbits (Oryctolagus cuniculus), European moles (Talpa europaea) and Carrion Crows (Corvus corone). PLoS ONE 2016, 1, e0146298. [Google Scholar] [CrossRef]
- Mason, G.; Littin, K. The humaneness of rodent pest control. Anim. Welf. 2003, 12, 1–37. [Google Scholar] [CrossRef]
- Iossa, G.; Soulsbury, C.D.; Harris, S. Mammal trapping: A review of animal welfare standards of killing and restraining traps. Anim. Welf. 2007, 16, 335–352. [Google Scholar] [CrossRef]
- Baker, S.E.; Ellwood, S.A.; Tagarielli, V.L.; Macdonald, D.W. Mechanical Performance of Rat, Mouse and Mole Spring Traps, and Possible Implications for Welfare Performance. PLoS ONE 2012, 7, e39334. [Google Scholar] [CrossRef]
- Littin, K.; Fisher, P.; Beausoleil, N.J.; Sharp, T. Welfare aspects of vertebrate pest control and culling: Ranking control techniques for humaneness. Rev. Sci. Tech. (Int. Off. Epizoot.) 2014, 33, 281–289. [Google Scholar] [CrossRef]
- Hampton, J.O.; Jones, B.; Perry, A.L.; Miller, C.J.; Hart, Q. Integrating animal welfare into wild herbivore management: Lessons from the Australian Feral Camel Management Project. Rangel. J. 2016, 38, 163–171. [Google Scholar] [CrossRef]
- Baker, S.E.; Ayers, M.; Beausoleil, N.J.; Belmain, S.R.; Berdoy, M.; Buckle, A.P.; Cagienard, C.; Cowan, D.; Fearn-Daglish, J.; Goddard, P.; et al. An assessment of animal welfare impacts in wild Norway rat (Rattus norvegicus) management. Anim. Welf. 2022, 31, 51–68. [Google Scholar] [CrossRef]
- Harrington, L.A.; Moehrenschlager, A.; Gelling, M.; Atkinson, R.; Hughes, J.; Macdonald, D.W. Conflicting and complementary ethics of animal welfare considerations in reintroductions. Conserv. Biol. 2013, 27, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, F.; Rasmussen, M.H.; Lusseau, D. Inferring energy expenditure from respiration rates in minke whales to measure the effects of whale watching boat interactions. J. Exp. Mar. Biol. Ecol. 2014, 459, 96–104. [Google Scholar] [CrossRef]
- Teerlink, S.; Horstmann, L.; Witteveen, B. Humpback whale (Megaptera novaeangliae) blubber steroid hormone concentration to evaluate chronic stress response from whale-watching vessels. Aquat. Mamm. 2018, 44, 411–425. [Google Scholar] [CrossRef]
- Baker, S.E.; Cain, R.; van Kesteren, F.; Zommers, Z.A.; D’Cruze, N.; Macdonald, D.W. Rough Trade: Animal Welfare in the Global Wildlife Trade. Bioscience 2013, 63, 928–938. [Google Scholar]
- Bush, E.R.; Baker, S.E.; Macdonald, D.W. Global trade in exotic pets 2006–2012. Conserv. Biol. 2014, 28, 663–676. [Google Scholar] [CrossRef]
- D’Cruze, N.; Harrington, L.A.; Assou, D.; Green, J.; Macdonald, D.W.; Ronfot, D.; Segniagbeto, G.H.; Auliya, M. Betting the farm: A review of Ball Python and other reptile trade from Togo, West Africa. Nat. Conserv. 2020, 40, 65. [Google Scholar] [CrossRef]
- Feber, R.E.; Raebel, E.M.; D’Cruze, N.; Macdonald, D.W.; Baker, S.E. Some animals are more equal than others: Wild animal welfare in the media. Bioscience 2017, 67, 62–72. [Google Scholar] [CrossRef]
- Martin, A.; Coolsaet, B.; Corbera, E.; Dawson, N.M.; Fraser, J.A.; Lehmann, I.; Rodriguez, I. Justice and conservation: The need to incorporate recognition. Biol. Conserv. 2016, 197, 254–261. [Google Scholar] [CrossRef]
- Ruano-Chamorro, C.; Gurney, G.G.; Cinner, J.E. Advancing procedural justice in conservation. Conserv. Lett. 2022, 15, e12861. [Google Scholar] [CrossRef]
- Santiago-Ávila, F.J.; Lynn, W.S. Bridging compassion and justice in conservation ethics. Biol. Conserv. 2020, 248, 108648. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Humphries, B.D.; Hill, T.R.; Downs, C.T. Landowners’ perspectives of black-backed jackals (Canis mesomelas) on farmlands in KwaZulu-Natal, South Africa. Afr. J. Ecol. 2015, 53, 540–549. [Google Scholar] [CrossRef]
- Flores, D. Coyote America: A Natural and Supernatural History; Basic Books: New York, NY, USA, 2016. [Google Scholar]
- Baker, S.E.; Singleton, G.; Smith, R.H. The nature of the beast: Using biological processes in vertebrate pest management. In Key Topics in Conservation Biology; Macdonald, D.W., Service, K., Eds.; Blackwell Scientific: Oxford, UK, 2007; pp. 173–185. [Google Scholar]
- Cox, R.; Baker, S.E.; Macdonald, D.W.; Berdoy, M. Protecting egg prey from carrion crows: The potential of adversive conditioning. Appl. Anim. Behav. Sci. 2004, 87, 325–342. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Baker, S.E. Non-lethal control of fox predation: The potential of generalised aversion. Anim. Welf. 2004, 13, 77–85. [Google Scholar] [CrossRef]
- Baker, S.E.; Ellwood, S.A.; Watkins, R.W.; Macdonald, D.W. Non-lethal control of wildlife: Using chemical repellents as feeding deterrents for the European badger. J. Appl. Ecol. 2005, 42, 921–931. [Google Scholar] [CrossRef]
- Baker, S.E.; Ellwood, S.A.; Slater, D.; Watkins, R.; Macdonald, D.W. Food aversion plus odor cue protects crop from wild mammals. J. Wildl. Manag. 2008, 72, 785–791. [Google Scholar] [CrossRef]
- Baker, S.E.; Macdonald, D.W. Managing wildlife humanely with learned food aversions. In Wildlife Conservation on Farmland Volume 2: Conflict in the Countryside; Macdonald, D.W., Feber, R.E., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 260–275. [Google Scholar]
- Marker, L.L.; Dickman, A.J.; Macdonald, D.W. Perceived effectiveness of livestock-guarding dogs placed on Namibian farms. Rangel. Ecol. Manag. 2005, 58, 329–336. [Google Scholar] [CrossRef]
- Marker, L.; Dickman, A.J.; Mills, M.G.L.; Macdonald, D.W. Cheetahs and ranchers in Namibia: A case study. In The Biology and Conservation of Wild Felids; Macdonald, D.W., Loveridge, A.J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 353–372. [Google Scholar]
- McManus, J.S.; Dickman, A.J.; Gaynor, D.; Smuts, B.H.; Macdonald, D.W. Dead or alive? Comparing costs and benefits of lethal and non-lethal human-wildlife conflict mitigation on livestock farms. Oryx 2015, 49, 687–695. [Google Scholar] [CrossRef]
- Baker, S.E.; Macdonald, D.W. Non-lethal predator control: Exploring the options. In Advances in Vertebrate Pest Management; Cowan, P.D., Feare, C.J., Eds.; Filander Verlag: Furth, Germany, 1999; pp. 251–566. [Google Scholar]
- Petracca, L.S.; Frair, J.L.; Bastille-Rousseau, G.; Hunt, J.E.; Macdonald, D.W.; Sibanda, L.; Loveridge, A.J. The effectiveness of hazing African lions as a conflict mitigation tool: Implications for carnivore management. Ecosphere 2019, 10, e02967. [Google Scholar] [CrossRef]
- Baker, S.E.; Macdonald, D.W. Not so humane mole tube traps. Anim. Welf. 2012, 21, 613–615. [Google Scholar] [CrossRef]
- Baker, S.E.; Shaw, R.F.; Atkinson, R.P.D.; West, P.; Macdonald, D.W. Potential welfare impacts of kill-trapping European moles (Talpa europaea) using scissor traps and Duffus traps: A post-mortem examination study. Anim. Welf. 2015, 24, 1–14. [Google Scholar] [CrossRef]
- Allen, B.L.; Allen, L.R.; Ballard, G.; Drouilly, M.; Fleming, P.J.S.; Hampton, J.O.; Hayward, M.W.; Kerley, G.I.H.; Meek, P.D.; Minnie, L.; et al. Bringing objectivity to wildlife management: Welfare effects of guardian dogs. Biol. Conserv. Restor. Sustain. 2019, 236, 582. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Mathews, F.; Berdoy, M.L. The behaviour and ecology of Rattus norvegicus: From opportunism to kamikaze tendencies. In Ecologically-based Management of Rodent Pests. ACIAR Monograph No. 59; Singleton, G.R., Hinds, L.A., Leirs, H., Zhang, Z., Eds.; ACIAR: Canberra, Australia, 1999; pp. 49–80. [Google Scholar]
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef]
- Battersby, S.A. Rodents as carriers of disease. In Rodent Pests and their Control; Buckle, A.P., Smith, R.H., Eds.; CABI International: Oxfordshire, UK, 2015; pp. 81–100. [Google Scholar]
- Lambert, M.; Vial, F.; Pietravalle, S.; Cowan, D. Results of a 15-year systematic survey of commensal rodents in English dwellings. Sci. Rep. 2017, 7, 15882. [Google Scholar] [CrossRef]
- Pesticide Safety Directorate. Assessment of Humaneness of Vertebrate Control Agents—Evaluation of Fully Approved or Provisionally Approved Products, No. 171 (December 1997); Pesticides Safety Directorate: York, UK, 1997. [Google Scholar]
- Buckle, A.P.; Smith, R.H. Rodent Pests and their Control; CAB International: Wallingford, UK, 2015. [Google Scholar]
- Schlötelburg, A.; Geduhn, A.; Schmolz, E.; Friesen, A.; Baker, S.; Martenson, N.; Le Laidier, G.; Urzinger, M.; Klute, O.; Schröer, D.; et al. NoCheRo-Guidance for the evaluation of rodent traps. In Part A Break Back/Snap Traps; German Environment Agency: Dessau, Germany, 2021. [Google Scholar]
- Dubois, S.; Fenwick, N.; Ryan, E.A.; Baker, L.; Baker, S.E.; Beausoleil, N.J.; Carter, S.; Cartwright, B.; Costa, F.; Draper, C.; et al. Consensus principles for ethical wildlife control. Conserv. Biol. 2017, 31, 753–760. [Google Scholar] [CrossRef]
- Frantz, S.C.; Padula, C.M. A laboratory test method for evaluating the efficacy of glueboards for trapping house mice. In Vertebrate Pest Control and Management Materials: 4th Symposium; Kaukeinen, D.E., Ed.; American Society for Testing and Materials: Philadelphia, PA, USA, 1983; pp. 209–225. [Google Scholar]
- Norse, E.A.; Rosenbaum, K.L.; Wilcove, D.S.; Wilcox, B.A. Conserving Biological Diversity in our National Forests; The Wilderness Society: Washington, DC, USA, 1986. [Google Scholar]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Pimm, S.L. What is biodiversity conservation? This article belongs to Ambio’s 50th Anniversary Collection. Theme: Biodiversity Conservation. Ambio 2021, 50, 976–980. [Google Scholar] [CrossRef]
- Bonesi, L.; Rushton, S.P.; Macdonald, D.W. Trapping for mink control and water vole survival: Identifying key criteria using a spatially explicit individual based model. Biol. Conserv. 2007, 136, 636–650. [Google Scholar] [CrossRef]
- Harrington, L.A.; Harrington, A.L.; Moorhouse, T.; Gelling, M.; Bonesi, L.; Macdonald, D.W. American mink control on inland rivers in southern England: An experimental test of a model strategy. Biol. Conserv. 2009, 142, 839–849. [Google Scholar] [CrossRef]
- Department of Conservation. Predator Free 2050; Practical Guide to Trapping. Department of Conservation, New Zealand Government. 2021. Available online: https://www.doc.govt.nz/nature/pests-and-threats/predator-free-2050/ (accessed on 1 August 2023).
- Mathews, F. Conservation and animal welfare: Consensus statement and guiding principles Conservation and Animal Welfare Science Workshop. Anim. Welf. 2010, 19, 191–192. [Google Scholar]
- Woodroffe, R.; Bourne, F.J.; Cox, D.R.; Donnelly, C.A.; Gettinby, G.; McInerney, J.P.; Morrison, W.I. Ilfare of badgers (Meles meles) subjected to culling: Patterns of trap-related injury. Anim. Ilfare 2005, 14, 11–17. [Google Scholar] [CrossRef]
- Gelling, M.; Johnson, P.J.; Moorhouse, T.P.; Macdonald, D.W. Measuring Animal Welfare within a Reintroduction: An Assessment of Different Indices of Stress in Water Voles Arvicola amphibius. PLoS ONE 2012, 7, e41081. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Gelling, M.; McLaren, G.W.; Mian, R.; Macdonald, D.W. Physiological consequences of captive conditions in water voles (Arvicola terrestris). J. Zool. 2007, 271, 19–26. [Google Scholar] [CrossRef]
- Gelling, M.; McLaren, G.W.; Mathews, F.; Mian, R.; Macdonald, D.W. Impact of trapping and handling on Leukocyte Coping Capacity in bank voles (Clethrionomys glareolus) and wood mice (Apodemus sylvaticus). Anim. Welf. 2009, 18, 1–7. [Google Scholar] [CrossRef]
- Narayan, E.J. Non-invasive reproductive and stress endocrinology in amphibian conservation physiology. Conserv. Physiol. 2013, 1, cot011. [Google Scholar] [CrossRef] [PubMed]
- McLaren, G.W.; Macdonald, D.W.; Georgiou, C.; Mathews, F.; Newman Mian, C.R. Leukocyte coping capacity: A novel technique for measuring the stress response in vertebrates. Exp. Physiol. 2003, 88, 541–546. [Google Scholar] [CrossRef]
- Huber, N.; Marasco, V.; Painer, J.; Vetter, S.G.; Göritz, F.; Kaczensky, P.; Walzer, C. Leukocyte coping capacity: An integrative parameter for wildlife welfare within conservation interventions. Front Vet. Sci. 2019, 6, 105. [Google Scholar] [CrossRef]
- Fisher, P.; Warburton, B.; Morgan, D.; Cowan, P.; Duckworth, J. Animal welfare in vertebrate pest management and research in New Zealand. In Proceedings of the Blue Sky to Deep Water: The Reality and the Promise, Proceedings of the ANZCCART Conference, Auckland, New Zealand, 29 June–1 July 2008; pp. 89–94. [Google Scholar]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations. Version 1.0.; IUCN Species Survival Commission: Gland, Switzerland, 2013. [Google Scholar]
- Swaisgood, R.R. The conservation-welfare nexus in reintroduction programmes: A role for sensory ecology. Anim. Welf. 2010, 19, 125–137. [Google Scholar] [CrossRef]
- Montes, I.; McLaren, G.W.; Macdonald, D.W.; Mian, R. The effect of transport stress on neutrophil activation in wild badgers (Meles meles). Anim. Welf. 2004, 13, 355–359. [Google Scholar] [CrossRef]
- Proulx, G.; Cattet, M.; Serfass, T.L.; Baker, S.E. Updating the AIHTS Trapping Standards to Improve Animal Welfare and Capture Efficiency and Selectivity. Animals 2020, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Virgos, E.; Lozano, J.; Cabezas-Diaz, S.; Macdonald, D.W.; Zalewski, A.; Atienza, J.C.; Proulx, G.; Ripple, W.J.; Rosalino, L.M.; Santos-Reis, M.; et al. A poor international standard for trap selectivity threatens carnivore conservation. Biodivers. Conserv. 2016, 25, 1409–1419. [Google Scholar] [CrossRef]
- Teixeira, C.P.; De Azevedo, C.S.; Mendl, M.; Cipreste, C.F.; Young, R.J. Revisiting translocation and reintroduction programmes: The importance of considering stress. Anim. Behav. 2007, 73, 1–13. [Google Scholar] [CrossRef]
- Gelling, M.; Montes, I.; Moorhouse, T.P.; Macdonald, D.W. Captive housing during water vole (Arvicola terrestris) reintroduction: Does short-term social stress impact on animal welfare? PLoS ONE 2010, 5, e9791. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, T.P.; Gelling, M.; Macdonald, D.W. Water vole restoration in the Upper Thames. In Wildlife Conservation on Farmland Volume 1: Managing for Nature on Lowland Farms; Macdonald, D.W., Feber, R.E., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 255–268. [Google Scholar]
- Maran, T.; Põdra, M.; Põlma, M.; Macdonald, D.W. The survival of captive-born animals in restoration programmes–Case study of the endangered European mink Mustela lutreola. Biol. Conserv. 2009, 142, 1685–1692. [Google Scholar] [CrossRef]
- Dawkins, M.S.; Roberts, S.J.; Cain, R.J.; Nickson, T.; Donnelly, C.A. Early warning of footpad dermatitis and hockburn in broiler chicken flocks using optical flow, bodyweight and water consumption. Vet. Rec. 2017, 180, 499. [Google Scholar] [CrossRef]
- Maran, T.; Põdra, M.; Harrington, L.A.; Macdonald, D.W. European mink: Restoration attempts for a species on the brink of extinction. In Biology and Conservation of Musteloids; Macdonald, D.W., Newman, C., Harrington, L.A., Eds.; Oxford University Press: Oxford, UK, 2017; pp. 370–388. [Google Scholar]
- Hampton, J.O.; Hyndman, T.H.; Allen, B.L.; Fischer, B. Animal Harms and Food Production: Informing Ethical Choices. Animals 2021, 11, 1225. [Google Scholar] [CrossRef]
- Hawkins, P. Guidelines on wildlife research that aim to cover a broad range of issues and provide comprehensive guidance; A collection of existing guidelines presented at the meeting: Harmonisation of the Care and Use of Animals in Field Research. In Harmonisation of the Care and Use of Animals in Field Research; Norecopa: Gardermoen, Norway, 2008; Available online: https://norecopa.no/meetings/wildlife-2008 (accessed on 1 August 2023).
- Zhou, Z.M.; Zhou, Y.B.; Newman, C.; Macdonald, D.W. Scaling up pangolin protection in China. Front. Ecol. Environ. 2014, 12, 97–98. [Google Scholar] [CrossRef]
- Fraser, D. Toward a synthesis of conservation and animal welfare science. Anim. Welf. 2010, 19, 121–124. [Google Scholar] [CrossRef]
- Fischer, J.; Brosi, B.; Daily, G.C.; Ehrlich, P.R.; Goldman, R.; Goldstein, J.; Lindenmayer, D.B.; Manning, A.D.; Mooney, H.A.; Pejchar, L.; et al. Should agricultural policies encourage land sparing or wildlife-friendly farming? Front. Ecol. Environ. 2008, 6, 380–385. [Google Scholar] [CrossRef]
- Pywell, R.F.; Heard, M.S.; Woodcock, B.A.; Hinsley, S.; Ridding, L.; Nowakowski, M.; Bullock, J.M. Wildlife-friendly farming increases crop yield: Evidence for ecological intensification. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151740. [Google Scholar] [CrossRef] [PubMed]
- Barber-Meyer, S.M. Dealing with the clandestine nature of wildlife-trade market surveys. Conserv. Biol. 2010, 24, 918–923. [Google Scholar] [CrossRef]
- D’Cruze, N.; Singh, B.; Mookerjee, A.; Harrington, L.A.; Macdonald, D.W. A socio-economic survey of pangolin hunting in Assam, Northeast India. Nat. Conserv. 2018, 30, 83–105. [Google Scholar] [CrossRef]
- Harrington, L.A.; D’Cruze, N.; Macdonald, D.W. Rise to fame: Events, media activity and public interest in pangolins and pangolin trade, 2005–2016. Nat. Conserv. 2018, 30, 107–133. [Google Scholar] [CrossRef]
- D’Cruze, N.; Singh, B.; Morrison, T.; Schmidt-Burbach, J.; Macdonald, D.W.; Mookerjee, A. A star attraction: The illegal trade in Indian Star Tortoises. Nat. Conserv. 2015, 13, 1–19. [Google Scholar] [CrossRef]
- Bauer, H.; Nowell, K.; Sillero-Zubiri, C.; Macdonald, D.W. Lions in the modern arena of CITES. Conserv. Lett. 2018, 11, e12444. [Google Scholar] [CrossRef]
- Williams, V.L.; Newton, D.J.; Loveridge, A.J.; Macdonald, D.W. Bones of Contention: An Assessment of the South African Trade in African Lion Panthera Leo Bones and Other Body Parts; TRAFFIC: Cambridge, UK; WildCRU: Oxford, UK, 2015. [Google Scholar]
- Moorhouse, T.P.; Elwin, A.; Ye, Y.C.; Zhou, Z.M.; Cruze, N.C.; Macdonald, D.W. Beyond the Pharmacopoeia: To what extent is trade for “TCM” limited to official TCM taxa? Glob. Ecol. Conserv. 2021, 32, e01906. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; D’Cruze, N.C.; Sun, E.; Elwin, A.; Macdonald, D.W. What are TCM doctors’ attitudes towards replacing animal-origin medicinal materials with plant-origin alternatives? Glob. Ecol. Conserv. 2022, 34, e02045. [Google Scholar] [CrossRef]
- Can, Ö.E.; D’Cruze, N.; Macdonald, D.W. 2019. Dealing in deadly pathogens: Taking stock of the legal trade in live wildlife and potential risks to human health. Glob. Ecol. Conserv. 2019, 17, e00515. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Macdonald, D.W. COVID-19, health, conservation, and shared wellbeing: Details matter. Trends Ecol. Evol. 2020, 35, 748–750. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Johnson, R.N.; Newman, C.; Buesching, C.D.; Macdonald, D.W.; Zhou, Y. Private possession drives illegal wildlife trade in China. Front. Ecol. Environ. 2015, 13, 353–354. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Balaskas, M.; D’Cruze, N.; Macdonald, D.W. Information Could Reduce Consumer Demand for Exotic Pets. Conserv. Lett. 2017, 10, 337–345. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Laurenson, M.K. Infectious disease: Inextricable linkages between human and ecosystem health. Biol. Conserv. 2006, 131, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, T.P.; D’Cruze, N.C.; Macdonald, D.W. Unethical use of wildlife in tourism: What’s the problem, who is responsible, and what can be done? J. Sustain. Tour. 2017, 25, 505–516. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; D’Cruze, N.C.; Macdonald, D.W. The effect of priming, nationality and greenwashing on preferences for wildlife tourist attractions. Glob. Ecol. Conserv. 2017, 12, 188–203. [Google Scholar] [CrossRef]
- Partecke, J.; Schwabl, I.; Gwinner, E. Stress and the city: Urbanization and its effects on the stress physiology in European blackbirds. Ecology 2006, 87, 1945–1952. [Google Scholar] [CrossRef]
- Dubois, S.; Fraser, D. Rating harms to wildlife: A survey showing convergence between conservation and animal welfare views. Anim. Welf. 2013, 22, 49–55. [Google Scholar] [CrossRef]
- Thomas, B.; Taylor, R.; Dunlevy, P.; Mouritsen, K.; Kemp, J. The Ka Mate reverse-bait snap trap—A promising new development. In Island Invasives: Eradication and Management; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 233–238. [Google Scholar]
- Bull, J.W.; Ejrnæs, R.; Macdonald, D.W.; Svenning, J.C.; Sandom, C.J. Fences can support restoration in human-dominated ecosystems when rewilding with large predators. Restor. Ecol. 2019, 27, 198–209. [Google Scholar] [CrossRef]
- Blumstein, D.T. Conservation and animal welfare issues arising from forestry practices. Anim. Welf. 2010, 19, 151–157. [Google Scholar] [CrossRef]
- Hampton, J.O.; Teh-White, K. Animal welfare, social license, and wildlife use industries. J. Wildl. Manag. 2019, 83, 12–21. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Boitani, L. Carnivore management: A plea for an ecological ethic. In Animal Rights; Patterson, W., Ryder, R., Eds.; Centaur Press: New York, NY, USA, 1979; pp. 165–177. [Google Scholar]
- Macdonald, D.; Dawkins, M. Ethology—The science and the tool. In Animals in Research: New Perspectives in Animal Experiments; Sperlinger, D., Ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1981; pp. 203–223. [Google Scholar]
- Macdonald, D.W. Postscript: Science, compromise and tough choices. In Carnivore Conservation; Gittleman, J.L., Funk, S.M., Macdonald, D.W., Wayne, R.K., Eds.; Cambridge University Press: Cambridge, UK, 2001; Volume 5, pp. 524–538. [Google Scholar]
- Macdonald, D.W.; Collins, N.M.; Wrangham, R. Principles, practice and priorities: The quest for “alignment”. In Key Topics in Conservation Biology; Macdonald, D.W., Service, K., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 273–292. [Google Scholar]
- Macdonald, D.W.; Willis, K.J. Elephants in the room: Tough choices for a maturing discipline. In Key Topics in Conservation Biology 2; Macdonald, D.W., Willis, K.J., Eds.; Wiley-Blackwell: Oxford, UK, 2013; pp. 469–494. [Google Scholar]
- Vucetich, J.A.; Burnham, D.; Macdonald, E.A.; Bruskotter, J.T.; Marchini, S.; Zimmermann, A.; Macdonald, D.W. Just conservation: What is it and should we pursue it? Biol. Conserv. 2018, 221, 23–33. [Google Scholar] [CrossRef]
- Batavia, C.; Nelson, M.P.; Bruskotter, J.T.; Jones, M.S.; Yanco, E.; Ramp, D.; Bekoff, M.; Wallach, A.D. Emotion as a source of moral understanding in conservation. Conserv. Biol. 2021, 35, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- 139Vucetich, J.A.; Nelson, M.P. What are 60 warblers worth? Killing in the name of conservation. Oikos 2007, 116, 1267–1278. [Google Scholar] [CrossRef]
- Mason, G.J. Invertebrate welfare: Where is the real evidence for conscious affective states? Trends Ecol. Evol. 2011, 26, 212–213. [Google Scholar] [CrossRef][Green Version]
- Pooley, S.; Redpath, S. Speaking up for collaboration in conservation: A response to Vucetich; et al. Just conservation: What is it and should we pursue it? Biol. Conserv. 2018, 223, 186–187. [Google Scholar] [CrossRef]
- Vucetich, A.; Burnham, D.; Macdonald, E.A.; Bruskotter, J.T.; Marchini, S.; Zimmermann, A.; Macdonald, D.W. Authority, cultural relativism and the principles of just conservation: Rejoinder to Pooley and Redpath. Biol. Conserv. 2018, 223, 184–185. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Tattersall, F.H. Britain’s Mammals: The Challenge for Conservation; People’s Trust for Endangered Species: London, UK, 2001. [Google Scholar]
- Colwell, M. Beak, Tooth and Claw: Living with Predators in Britain; HarperCollins: London, UK, 2021. [Google Scholar]
- Pooley, S.; Barua, M.; Beinart, W.; Dickman, A.; Holmes, G.; Lorimer, J.; Loveridge, A.J.; Macdonald, D.W.; Marvin, G.; Redpath, S.; et al. An interdisciplinary review of current and future approaches to improving human-predator relations. Conserv. Biol. 2017, 31, 513–523. [Google Scholar] [CrossRef]
- Baker, S.E.; Maw, S.A.; Johnson, P.J.; Macdonald, D.M. Not in my backyard: Public perceptions of wildlife and ‘pest control’ in and around UK homes, and local authority “pest control”. Animals 2020, 10, 222. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Cook, C.N.; Pressey, R.L.; Pullin, A.S.; Runge, M.C.; Salafsky, N.; Sutherland, W.J.; Williamson, M.A. Decision support frameworks and tools for conservation. Conserv. Lett. 2018, 11, e12385. [Google Scholar] [CrossRef]
- CRRU UK. CRRU UK Code of Best Practice; Best Practice Guidance for Rodent Control and the Safe Use of Rodenticides. Campaign for Responsible Rodenticide Use, UK. 2021. Available online: www.thinkwildlife.org/code-of-best-practice/ (accessed on 1 August 2023).
- Pierce, R.; Teroroko, T. Enhancing biosecurity at the Phoenix Islands Protected Area (PIPA), Kiribati. In Island Invasives: Eradication and Management; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 41–486. [Google Scholar]
- Macdonald, D.W. Bartering biodiversity: What are the options? In Economic Policy: Objectives, Instruments And Implementation; Macdonald, D.W., Sillero-Zubiri, C., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 142–171. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macdonald, D.W. Mitigating Human Impacts on Wild Animal Welfare. Animals 2023, 13, 2906. https://doi.org/10.3390/ani13182906
Macdonald DW. Mitigating Human Impacts on Wild Animal Welfare. Animals. 2023; 13(18):2906. https://doi.org/10.3390/ani13182906
Chicago/Turabian StyleMacdonald, David W. 2023. "Mitigating Human Impacts on Wild Animal Welfare" Animals 13, no. 18: 2906. https://doi.org/10.3390/ani13182906
APA StyleMacdonald, D. W. (2023). Mitigating Human Impacts on Wild Animal Welfare. Animals, 13(18), 2906. https://doi.org/10.3390/ani13182906



