Glutamine Regulates Gene Expression Profiles to Increase the Proliferation of Porcine Intestinal Epithelial Cells and the Expansion of Intestinal Stem Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture and Treatment of IPEC-J2 Cells
2.2. Culture and Treatment of Intestinal Organoids
2.3. RNA Extraction and cDNA Library Construction
2.4. Transcriptomics Analysis
2.5. Protein–Protein Interaction (PPI) Analysis
2.6. Statistical Analysis
3. Results
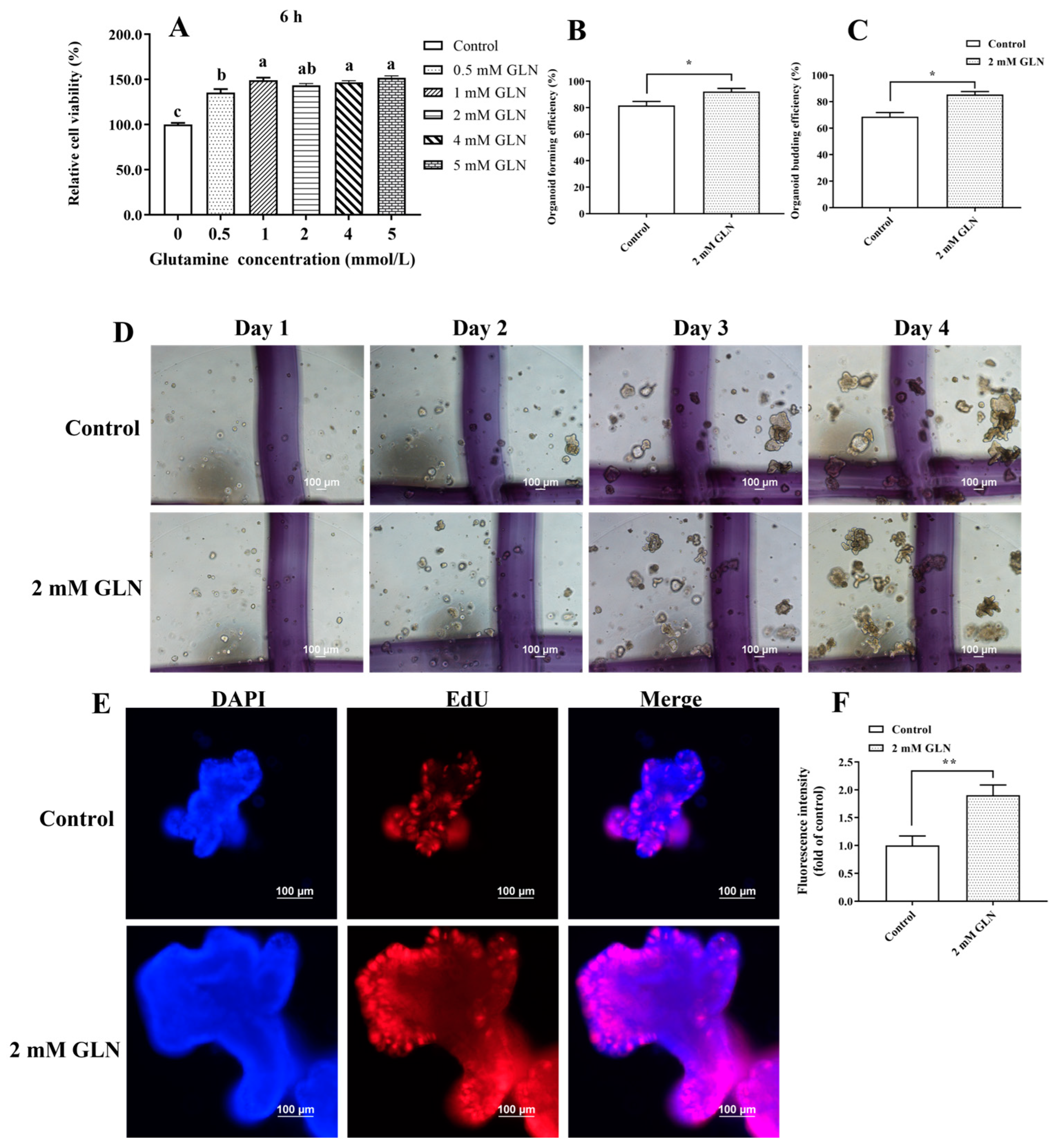
3.1. Glutamine Accelerates Porcine Intestinal Epithelial Cell Proliferation and Stem Cell Expansion
3.2. Glutamine Regulates Gene Expression Profiles in the Porcine Intestinal Epithelial Cells
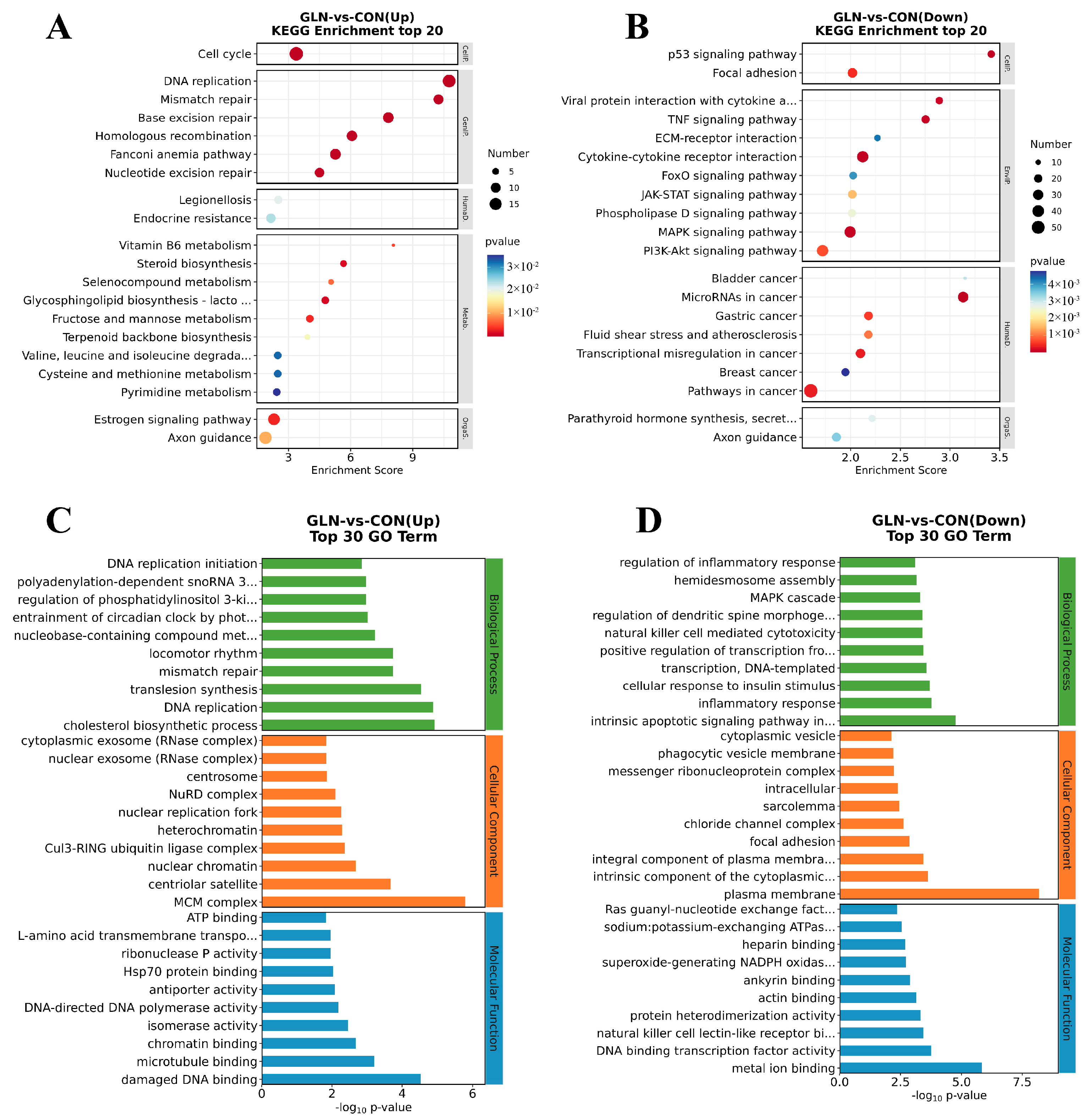
3.3. Identification of DEGs and Functional Enrichment Analysis between the Control and Glutamine Supplementation Groups
3.4. Unveiling the Vital Pathway and Genes Involved in Cell Proliferation and Anti-Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pentinmikko, N.; Iqbal, S.; Mana, M.; Andersson, S.; Cognetta, A.B., 3rd; Suciu, R.M.; Roper, J.; Luopajärvi, K.; Markelin, E.; Gopalakrishnan, S.; et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 2019, 571, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.; Zhang, J.; Zhao, S.; Li, X.G. Gut-on-a-chip for disease models. J. Tissue Eng. 2023, 14, 20417314221149882. [Google Scholar] [CrossRef] [PubMed]
- Gehart, H.; Clevers, H. Tales from the crypt: New insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Lu, T.; Wu, J.; Fan, D.; Liu, B.; Zhu, X.; Guo, H.; Du, Y.; Liu, F.; Tian, Y.; et al. Gut microbiota drives macrophage-dependent self-renewal of intestinal stem cells via niche enteric serotonergic neurons. Cell Res. 2022, 32, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Procopio, J.; Lima, M.M.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef]
- Sakai, R.; Ooba, Y.; Watanabe, A.; Nakamura, H.; Kawamata, Y.; Shimada, T.; Takumi, A.; van Goudoever, J.B.; Narita, T. Glutamate metabolism in a human intestinal epithelial cell layer model. Amino Acids 2020, 52, 1505–1519. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Bao, X.; Yang, F.; Tang, X.; Jiang, Q.; Yang, C.; Yin, Y.; Yao, K. Glutamine boosts intestinal stem cell-mediated small intestinal epithelial development during early weaning: Involvement of WNT signaling. Stem Cell Rep. 2023, 18, 1451–1467. [Google Scholar] [CrossRef]
- Tran, T.Q.; Hanse, E.A.; Habowski, A.N.; Li, H.; Ishak Gabra, M.B.; Yang, Y.; Lowman, X.H.; Ooi, A.M.; Liao, S.Y.; Edwards, R.A.; et al. alpha-Ketoglutarate attenuates Wnt signaling and drives differentiation in colorectal cancer. Nat. Cancer 2020, 1, 345–358. [Google Scholar] [CrossRef]
- Qin, Y.C.; Zhou, J.Y.; Zhu, M.; Zan, G.X.; Gao, C.Q.; Yan, H.C.; Li, X.G.; Wang, X.Q. L-glutamate requires β-catenin signalling through Frizzled7 to stimulate porcine intestinal stem cell expansion. Cell Mol. Life Sci. 2022, 79, 523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Qin, Y.C.; Gao, C.Q.; Yan, H.C.; Li, X.G.; Wang, X.Q. Extracellular Glutamate-Induced mTORC1 Activation via the IR/IRS/PI3K/Akt Pathway Enhances the Expansion of Porcine Intestinal Stem Cells. J. Agric. Food Chem. 2019, 67, 9510–9521. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Sui, W.G.; Gao, C.Q.; Yan, H.C.; Yin, Y.L.; Li, H.C.; Wang, X.Q. L-Glutamate deficiency can trigger proliferation inhibition via down regulation of the mTOR/S6K1 pathway in pig intestinal epithelial cells. J. Anim. Sci. 2016, 94, 1541–1549. [Google Scholar] [CrossRef]
- Ye, J.L.; Gao, C.Q.; Li, X.G.; Jin, C.L.; Wang, D.; Shu, G.; Wang, W.C.; Kong, X.F.; Yao, K.; Yan, H.C.; et al. EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget 2016, 7, 38681–38692. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Zhu, M.; Chen, M.X.; Fan, H.B.; Fu, H.L.; Zhou, J.Y.; Zhai, Z.Y.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. Acute exposure to deoxynivalenol inhibits porcine enteroid activity via suppression of the Wnt/β-catenin pathway. Toxicol. Lett. 2019, 305, 19–31. [Google Scholar] [CrossRef]
- Li, X.G.; Chen, M.X.; Zhao, S.Q.; Wang, X.Q. Intestinal Models for Personalized Medicine: From Conventional Models to Microfluidic Primary Intestine-on-a-chip. Stem Cell Rev. Rep. 2022, 18, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Huang, D.G.; Zhu, M.; Gao, C.Q.; Yan, H.C.; Li, X.G.; Wang, X.Q. Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress. J. Cell Physiol. 2020, 235, 5613–5627. [Google Scholar] [CrossRef]
- Wong, C.C.; Xu, J.; Bian, X.; Wu, J.L.; Kang, W.; Qian, Y.; Li, W.; Chen, H.; Gou, H.; Liu, D.; et al. In Colorectal Cancer Cells with Mutant KRAS, SLC25A22-Mediated Glutaminolysis Reduces DNA Demethylation to Increase WNT Signaling, Stemness, and Drug Resistance. Gastroenterology 2020, 159, 2163–2180.e2166. [Google Scholar] [CrossRef]
- Najumudeen, A.K.; Ceteci, F.; Fey, S.K.; Hamm, G.; Steven, R.T.; Hall, H.; Nikula, C.J.; Dexter, A.; Murta, T.; Race, A.M.; et al. The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer. Nat. Genet. 2021, 53, 16–26. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, P.Y.; Zhang, Y.J.; Fan, S.J.; Wei, Y.; Yang, Z.F.; Wang, F.C.; Peng, X. Potential Effect of Glutamine in the Improvement of Intestinal Stem Cell Proliferation and the Alleviation of Burn-Induced Intestinal Injury via Activating YAP: A Preliminary Study. Nutrients 2023, 15, 18. [Google Scholar] [CrossRef]
- Holthausen, J.S.; Schregel, J.; Sciascia, Q.L.; Li, Z.Y.; Tuchscherer, A.; Vahjen, W.; Metges, C.C.; Zentek, J. Effects of Oral Glutamine Supplementation, Birthweight and Age on Colonic Morphology and Microbiome Development in Male Suckling Piglets. Microorganisms 2022, 10, 20. [Google Scholar] [CrossRef]
- Yu, X.T.; Zeng, T.; Li, G.J. Integrative enrichment analysis: A new computational method to detect dysregulated pathways in heterogeneous samples. BMC Genom. 2015, 16, 19. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Chen, H.R.; Zhang, J.L.; Cheng, A.S.L.; Yu, J.; To, K.F.; Kang, W. MCM family in gastrointestinal cancer and other malignancies: From functional characterization to clinical implication. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 12. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wang, S.; Liu, J.; Yan, W.; Han, P.; Tian, D. Identification of MCM family as potential therapeutic and prognostic targets for hepatocellular carcinoma based on bioinformatics and experiments. Life Sci. 2021, 272, 119227. [Google Scholar] [CrossRef] [PubMed]
- Dequeker, B.J.H.; Scherr, M.J.; Brandão, H.B.; Gassler, J.; Powell, S.; Gaspar, I.; Flyamer, I.M.; Lalic, A.; Tang, W.; Stocsits, R.; et al. MCM complexes are barriers that restrict cohesin-mediated loop extrusion. Nature 2022, 606, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Santosa, V.; Ishiguro, K.; Kanemaki, M.T. MCMBP promotes the assembly of the MCM2-7 hetero-hexamer to ensure robust DNA replication in human cells. eLife 2022, 11, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Liu, W.; Yan, H.; Zhang, Y.; Cheung, A.H.K.; Zhang, J.; Chen, B.; Liang, L.; Zhou, Z.; et al. MCM6 is a critical transcriptional target of YAP to promote gastric tumorigenesis and serves as a therapeutic target. Theranostics 2022, 12, 6509–6526. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, S.; Ni, Y.; Zhou, L.; Yang, C.; Zhang, C.; Sun, X.; Xu, N.; Xu, S.; Wang, Y.; et al. Minichromosome maintenance protein family member 6 mediates hepatocellular carcinoma progression by recruiting UBE3A to induce P53 ubiquitination. Int. J. Biol. Macromol. 2023, 248, 125854. [Google Scholar] [CrossRef]
- Baxley, R.M.; Leung, W.; Schmit, M.M.; Matson, J.P.; Yin, L.; Oram, M.K.; Wang, L.; Taylor, J.; Hedberg, J.; Rogers, C.B.; et al. Bi-allelic MCM10 variants associated with immune dysfunction and cardiomyopathy cause telomere shortening. Nat. Commun. 2021, 12, 1626. [Google Scholar] [CrossRef]
- Cacialli, P.; Dogan, S.; Linnerz, T.; Pasche, C.; Bertrand, J.Y. Minichromosome maintenance protein 10 (mcm10) regulates hematopoietic stem cell emergence in the zebrafish embryo. Stem Cell Rep. 2023, 18, 1534–1546. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Lai, W.; Yao, L.; Xu, E.; Chen, X.; Zhang, Y.-y.; Li, X.-G. Glutamine Regulates Gene Expression Profiles to Increase the Proliferation of Porcine Intestinal Epithelial Cells and the Expansion of Intestinal Stem Cells. Animals 2023, 13, 2917. https://doi.org/10.3390/ani13182917
Zhu M, Lai W, Yao L, Xu E, Chen X, Zhang Y-y, Li X-G. Glutamine Regulates Gene Expression Profiles to Increase the Proliferation of Porcine Intestinal Epithelial Cells and the Expansion of Intestinal Stem Cells. Animals. 2023; 13(18):2917. https://doi.org/10.3390/ani13182917
Chicago/Turabian StyleZhu, Min, Weiming Lai, Lewen Yao, E Xu, Xiang Chen, Yi-yu Zhang, and Xiang-Guang Li. 2023. "Glutamine Regulates Gene Expression Profiles to Increase the Proliferation of Porcine Intestinal Epithelial Cells and the Expansion of Intestinal Stem Cells" Animals 13, no. 18: 2917. https://doi.org/10.3390/ani13182917




