Potential of the Red Macroalga Bonnemaisonia hamifera in Reducing Methane Emissions from Ruminants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
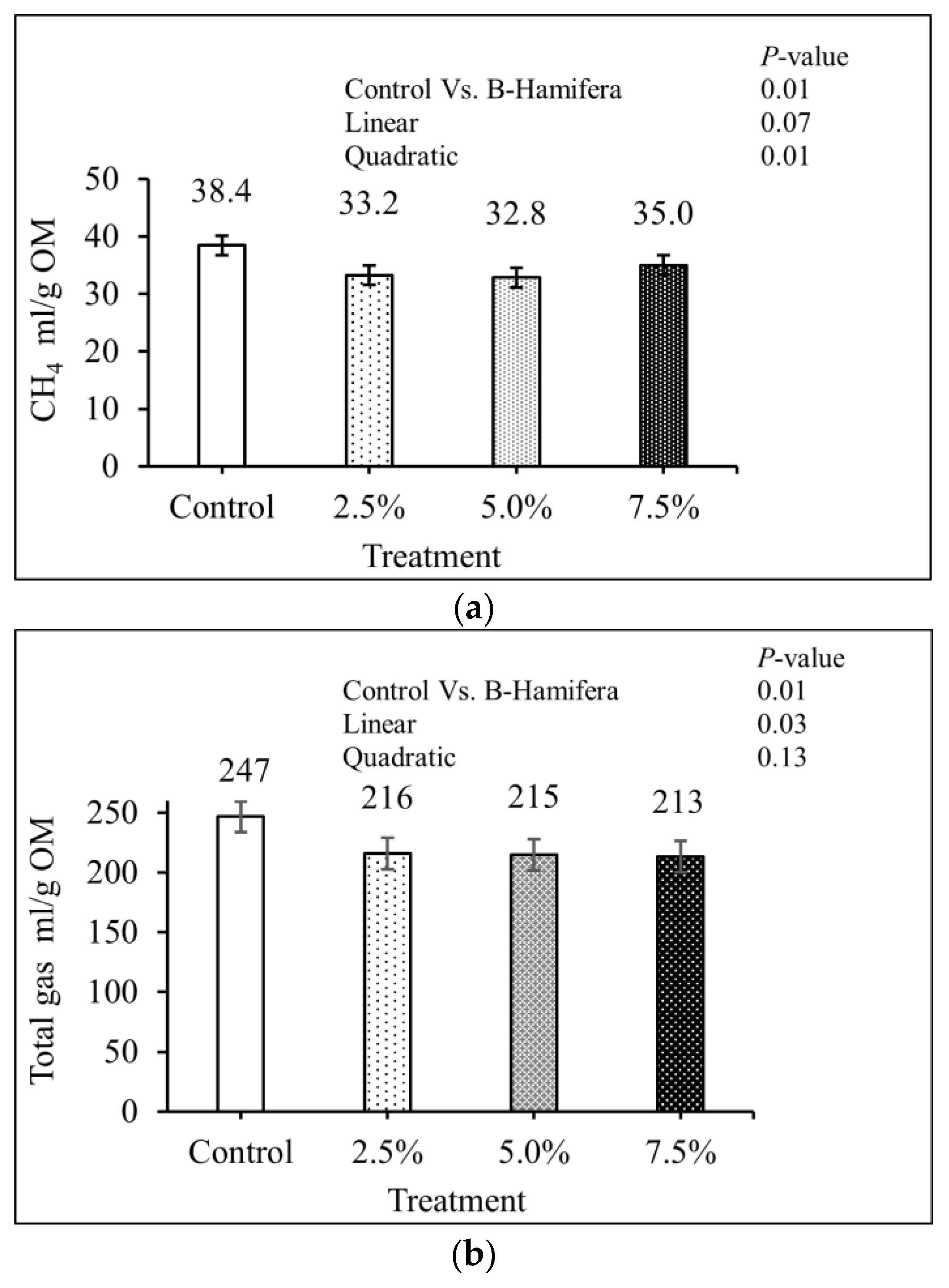
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNEP. Partnership and United Nations Environment Programme. Reducing Consumer Food Waste Using Green and Digital Technologies. 2021. Available online: https://www.unep.org/resources/publication/reducing-consumer-food-waste-using-green-and-digital-technologies (accessed on 14 July 2023).
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Kinley, R.D.; Magnusson, M.; de Nys, R.; Tomkins, N.W. The potential of macroalgae for beef production systems in Northern Australia. J. Appl. Phycol. 2015, 27, 2001–2005. [Google Scholar] [CrossRef]
- Kinley, R.D.; de Nys, R.; Vucko, M.J.; Machado, L.; Tomkins, N.W. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim. Prod. Sci. 2016, 56, 282–289. [Google Scholar] [CrossRef]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Stefenoni, H.A.; Räisänen, S.E.; Cueva, S.F.; Wasson, D.E.; Lage, C.F.A.; Melgar, A.; Fetter, M.E.; Smith, P.; Hennessy, M.; Vecchiarelli, B.; et al. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J. Dairy Sci. 2021, 104, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Krizsan, S.J.; Ramin, M.; Chagas, J.C.; Halmemies-Beauchet-Filleau, A.; Singh, A.; Schnürer, A.; Danielsson, R. Effects on rumen microbiome and milk quality of dairy cows fed a grass silage-based diet supplemented with the macroalga Asparagopsis taxiformis. Front. Anim. Sci. 2023, 4, 1112969. [Google Scholar] [CrossRef]
- Nilsson, J.; Martin, M. Exploratory environmental assessment of large-scale cultivation of seaweed used to reduce enteric methane emissions. Sustain. Prod. Consum. 2022, 30, 413–423. [Google Scholar] [CrossRef]
- Mihaila, A.A.; Glasson, C.R.; Lawton, R.; Muetzel, S.; Molano, G.; Magnusson, M. New temperate seaweed targets for mitigation of ruminant methane emissions: An in vitro assessment. Appl. Phycol. 2022, 3, 274–284. [Google Scholar] [CrossRef]
- Krizsan, S.J.; Hayes, M.; Gröndahl, F.; Ramin, M.; O’Hara, P.; Kenny, O. Characterization and in vitro assessment of seaweed bioactives with potential to reduce methane production. Front. Anim. Sci. 2022, 3, 1062324. [Google Scholar] [CrossRef]
- Ramin, M.; Huhtanen, P. Development of an in vitromethod for determination of methane production kinetics usinga fully automated in vitro gas system—A modelling approach. Anim. Feed Sci. Technol. 2012, 174, 190–200. [Google Scholar] [CrossRef]
- AOAC International. AOAC International Official Methods of Analysis, 9th ed.; AOAC Int.: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Abbott, D.W.; Aasen, I.M.; Beauchemin, K.A.; Gröndahl, F.; Gruninger, R.; Hayes, M.; Huws, S.; Kenny, D.A.; Kirwan, S.; Krizsan, S.J.; et al. Seaweed and seaweed bioactives for mitigation of enteric methane: Challenges and opportunities. Animals 2020, 10, 2432. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Puhakka, L.; Jaakkola, S.; Simpura, I.; Kokkonen, T.; Vanhatalo, A. Effects of replacing rapeseed meal with fava bean at two concentrate crude protein levels on feed intake, nutrient digestion, and milk production in cows fed grass silage-based diets. J. Dairy Sci. 2016, 99, 7993–8006. [Google Scholar] [CrossRef] [PubMed]
- Muizelaar, W.; Groot, M.; van Duinkerken, G.; Peters, R.; Dijkstra, J. Safety and transfer study: Transfer of bromoform present in Asparagopsis taxiformis to milk and urine of lactating dairy cows. Foods 2021, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Enge, S.; Nylund, G.M.; Harder, T.; Pavia, H. An exotic chemical weapon explains low herbivore damage in an invasive alga. Ecology 2012, 93, 2736–2745. [Google Scholar] [CrossRef]
- Alvarez-Hess, P.S.; Jacobs, J.L.; Kinley, R.D.; Roque, B.M.; Neachtain, A.S.O.; Chandra, S.; Williams, S.R.O. Twice daily feeding of canola oil steeped with Asparagopsis armata reduced methane emissions of lactating dairy cows. Anim. Feed Sci. Technol. 2023, 297, 115579. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Effects of rumen-protected folic acid and branched-chain volatile fatty acids supplementation on lactation performance, ruminal fermentation, nutrient digestion and blood metabolites in dairy cows. Anim. Feed Sci. Technol. 2019, 247, 157–165. [Google Scholar] [CrossRef]
| Item | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Control | B. hamifera Inclusion Level (% OM) | |||||||
| 2.5% | 5.0% | 7.5% | Control vs. B. hamifera | Linear | Quadratic | |||
| Total VFA, mM | 148 | 162 | 159 | 155 | 6.6 | 0.10 | 0.44 | 0.08 |
| VFA molar proportions, mmol/mol | ||||||||
| Acetate | 575 | 574 | 575 | 577 | 2.4 | 0.94 | 0.39 | 0.42 |
| Propionate | 241 | 246 | 244 | 245 | 2.2 | 0.03 | 0.11 | 0.13 |
| Butyrate | 98.4 | 96.7 | 96.9 | 97.5 | 0.90 | 0.10 | 0.39 | 0.09 |
| Isobutyrate | 14.9 | 14.2 | 14.2 | 14.6 | 0.34 | 0.06 | 0.38 | 0.04 |
| 2-Methylbutyrate | 11.6 | 10.9 | 11.0 | 11.2 | 0.31 | 0.06 | 0.36 | 0.05 |
| Isovalerate | 13.8 | 12.8 | 13.0 | 13.3 | 0.39 | 0.04 | 0.33 | 0.03 |
| Valerate | 24.5 | 23.8 | 23.8 | 19.9 | 3.49 | 0.52 | 0.24 | 0.53 |
| Caproate | 21.2 | 21.9 | 21.3 | 21.6 | 0.40 | 0.29 | 0.62 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guinguina, A.; Hayes, M.; Gröndahl, F.; Krizsan, S.J. Potential of the Red Macroalga Bonnemaisonia hamifera in Reducing Methane Emissions from Ruminants. Animals 2023, 13, 2925. https://doi.org/10.3390/ani13182925
Guinguina A, Hayes M, Gröndahl F, Krizsan SJ. Potential of the Red Macroalga Bonnemaisonia hamifera in Reducing Methane Emissions from Ruminants. Animals. 2023; 13(18):2925. https://doi.org/10.3390/ani13182925
Chicago/Turabian StyleGuinguina, Abdulai, Maria Hayes, Fredrik Gröndahl, and Sophie Julie Krizsan. 2023. "Potential of the Red Macroalga Bonnemaisonia hamifera in Reducing Methane Emissions from Ruminants" Animals 13, no. 18: 2925. https://doi.org/10.3390/ani13182925
APA StyleGuinguina, A., Hayes, M., Gröndahl, F., & Krizsan, S. J. (2023). Potential of the Red Macroalga Bonnemaisonia hamifera in Reducing Methane Emissions from Ruminants. Animals, 13(18), 2925. https://doi.org/10.3390/ani13182925







