First Serologic Survey of Erysipelothrix rhusiopathiae in Wild Boars Hunted for Private Consumption in Portugal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
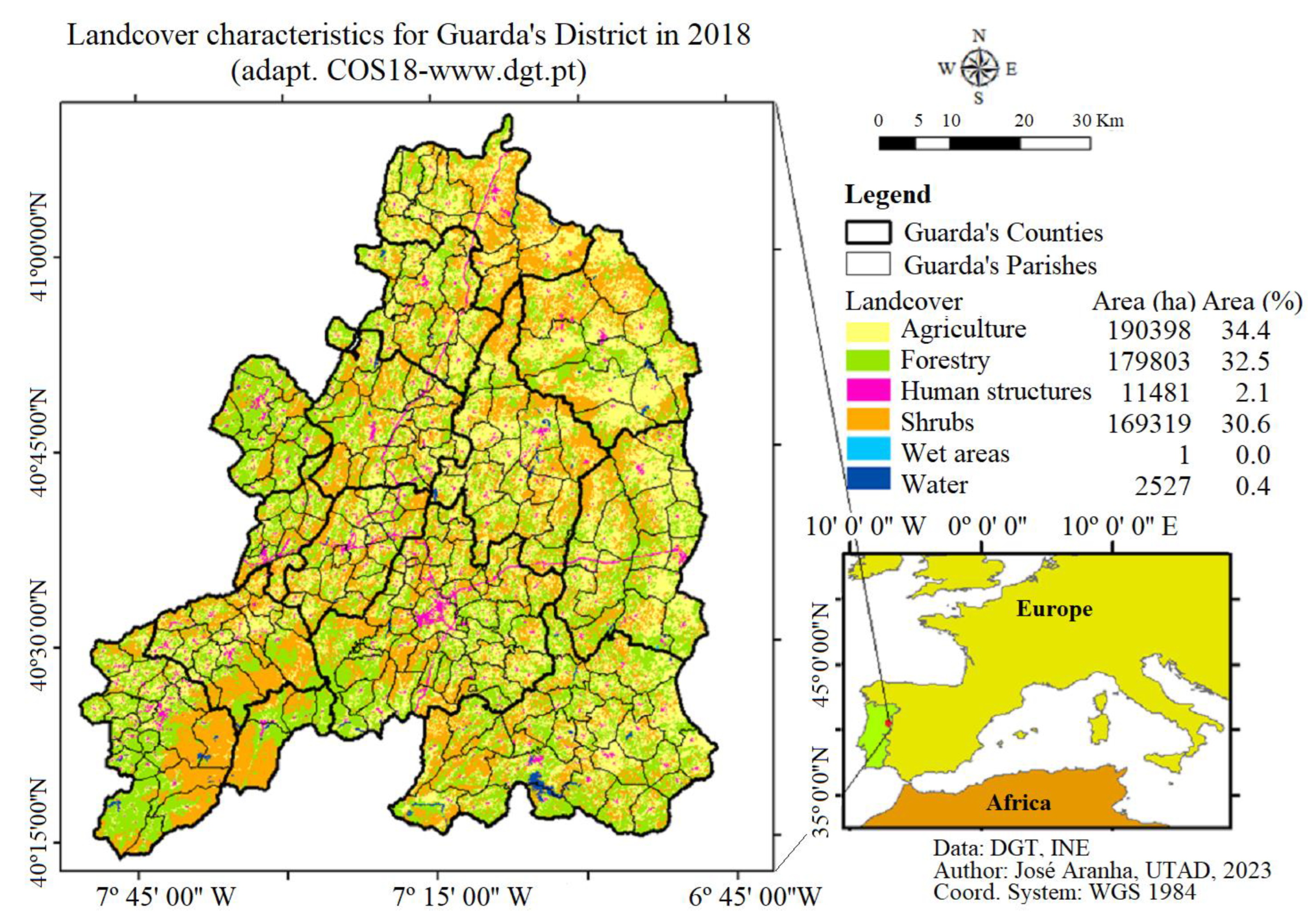
2.1. Area of Study
2.2. Sampling and Laboratory Analysis
2.3. Statistical Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, N.S.; Massei, G.; Wint, W. The European Distribution of Sus Scrofa. Model Outputs from the Project Described within the Poster—Where are All the Boars? An Attempt to Gain a Continental Perspective. Open Health Data 2016, 4, e1. [Google Scholar] [CrossRef]
- Fencaça. 2023. Available online: https://www.fencaca.pt/noticias/sobreabundante-populacao-cerca-300-mil-javalis-portugal-animais-estao-atacar-todo-o-lado (accessed on 25 August 2023).
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Massei, G.; Roy, S.; Bunting, R. Too Many Hogs? A Review of Methods to Mitigate Impact by Wild Boar and Feral Hogs. Hum. –Wildl. Interact. 2011, 5, 79–99. [Google Scholar] [CrossRef]
- Gortazar, C.; Diez-Delgado, I.; Barasona, J.A.; Vicente, J.; De La Fuente, J.; Boadella, M. The wild side of disease control at the wildlife-livestock-human interface: A review. Front. Vet. Sci. 2015, 1, 27. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Segalez, J.; Gortazar, C. A review of viral diseases of the European wild boar: Effects of population dynamics and reservoir role. Vet. J. 2008, 176, 158–169. [Google Scholar] [CrossRef]
- Abrantes, A.C.; Vieira-Pinto, M. 15 years overview of European zoonotic surveys in wild boar and red deer: A systematic review. One Health 2023, 16, 100519. [Google Scholar] [CrossRef]
- Vieira-Pinto, M.; Vinhas, B.; Coelho, C. Initial Examination of Wild Large Game on the Spot—Importance and Rules. J. Nutr. Ecol. Food Res. 2014, 1, 1–3. [Google Scholar] [CrossRef]
- ICNF. 2019. Principais Indicadores Do Sector Cinegético 2018. Available online: https://www.icnf.pt/api/file/doc/f34c10ecb41c9bc8. (accessed on 10 November 2019).
- EC. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law. Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 10 November 2019).
- EC. Commission Implementing Regulation (EU) 2019/627 Laying down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and Amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls. 2019. Available online: https://eur-lex.europa.eu/eli/reg_impl/2019/62 (accessed on 10 November 2019).
- Khan, S.; Atanasova, K.R.; Krueger, W.S.; Ramirez, A.; Gray, G. Epidemiology, geographical distribution, and economic consequences of swine zoonoses: A narrative review. Emerg. Microbes Infect. 2013, 2, 1–11. [Google Scholar] [CrossRef]
- Ugochukwu, I.C.; Samuel, F.; Orakpoghenor, O.; Nwobi, O.C.; Anyaoha, C.O.; Majesty-Alukagberie, L.O.; Ugochukwu, M.O.; Ugochukwu, E.I. Erysipelas, the opportunistic zoonotic disease: History, epidemiology, pathology, and diagnosis—A review. Comp. Clin. Pathol. 2018, 28, 853–859. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, B.J.; Riley, T.V. Erysipelothrix rhusiopathiae. Vet. Microbiol. 2010, 140, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Muramatsu, M.; Suzuki, S.; Tamura, Y.; Sawada, T.; Takahashi, T. Evaluation on the pathogenicity of Erysipelothrix tonsillarum for pigs by immunosuppression with cyclophosphamide or dexamethasone. Res. Vet. Sci. 2011, 90, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Risco, D.; Llario, P.F.; Velarde, R.; García, W.L.; Benítez, J.M.; García, A.; Bermejo, F.; Cortés, M.; Rey, J.; de Mendoza, J.H.; et al. Outbreak of Swine Erysipelas in a Semi-Intensive Wild Boar Farm in Spain. Transbound. Emerg. Dis. 2011, 58, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Coutinho, T.A. Erysipelas. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Formenti, N.; Calo, S.; Vitale, N.; Eriksson, H.; Giovannini, S.; Salogni, C.; D’Incau, M.; Pacciarini, M.L.; Zanoni, M.; Alborali, G.L.; et al. Influence of Anthropic Environmental-Related Factors on Erysipelas in Wild Boar. EcoHealth 2021, 18, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Ruiz, S.; Laguna, E.; Vicente, J.; García-Bocanegra, I.; Martínez-Guijosa, J.; Cano-Terriza, D.; Risalde, M.A.; Acevedo, P. Characterization and management of interaction risks between livestock and wild ungulates on outdoor pig farms in Spain. Porc. Health Manag. 2022, 8, 1–14. [Google Scholar] [CrossRef]
- Triguero-Ocaña, R.; Laguna, E.; Jiménez-Ruiz, S.; Fernández-López, J.; García-Bocanegra, I.; Barasona, J.A.; Risalde, M.A.; Montoro, V.; Vicente, J.; Acevedo, P. The wildlife-livestock interface on extensive free-ranging pig farms in central Spain during the “montanera” period. Transbound. Emerg. Dis. 2021, 68, 2066–2078. [Google Scholar] [CrossRef]
- EC. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for Food of Animal Origin. 2004. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32004R0853E (accessed on 10 November 2019).
- DGT (Direcção Geral do Território), Portugal. 2023. Available online: www.dgt.pt (accessed on 25 August 2023).
- ICNF (Instituto da Conservação da Natureza e das Florestas), Portugal. 2023. Available online: www.icnf.pt (accessed on 25 August 2023).
- Schley, L.; Roper, T.J. Diet of wild boar Sus scrofa in Western Europe, with particular reference to the consumption of agricultural crops. Mammal Rev. 2003, 33, 43–56. [Google Scholar] [CrossRef]
- INE (Instituto Nacional de Estatistica), Portugal. 2023. Available online: www.ine.pt (accessed on 25 August 2023).
- Arenas-Montes, A.; García-Bocanegra, I.; Paniagua, J.; Franco, J.J.; Miró, F.; Fernández-Morente, M.; Carbonero, A.; Arenas, A. Blood Sampling by Puncture in the Cavernous Sinus from Hunted Wild Boar. Eur. J. Wildl. Res. 2013, 59, 299–303. [Google Scholar] [CrossRef]
- Daniel, W.W.; Cross, C.L. Biostatistics: A Foundation for Analysis in the Health Sciences, 11th ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Paiva, R.; Reis, P.; Coelho, I.S. Valor Económico da Caça em Portugal; Tipografia Lousanense: Lisboa, Portugal, 2017; ISBN 978-972-579-044-1. [Google Scholar]
- Vicente, J.; León-Vizcaíno, L.; Gortázar, C.; Cubero, M.J.; González, M.; Martín-Atance, P. Antibodies to selected viral and bacterial pathogens in European wild boars from southcentral Spain. J. Wildl. Dis. 2002, 38, 649–652. [Google Scholar] [CrossRef]
- Boadella, M.; Ruiz-Fons, J.F.; Vicente, J.; Martín, M.; Segalés, J.; Gortazar, C. Seroprevalence evolution of selected pathogens in Iberian wild boar. Transbound. Emerg. Dis. 2012, 59, 395–404. [Google Scholar] [CrossRef]
- Cano-Manuel, F.J.; López-Olvera, J.; Fandos, P.; Soriguer, R.C.; Pérez, J.M.; Granados, J.E. Long-term monitoring of 10 selected pathogens in wild boar (Sus scrofa) in Sierra Nevada National Park, southern Spain. Vet. Microbiol. 2014, 174, 148–154. [Google Scholar] [CrossRef]
- Shimizu, T.; Okamoto, C.; Aoki, H.; Harada, K.; Kataoka, Y.; Ono, F.; Mutsuyo, K.; Takai, S. Serological surveillance for antibodies against Erysipelothrix species in wild boar and deer in Japan. Jpn. J. Vet. Res. 2016, 64, 91–94. [Google Scholar]
- Shimoji, Y.; Osaki, M.; Ogawa, Y.; Shiraiwa, K.; Nishikawa, S.; Eguchi, M.; Yamamoto, T.; Tsutsui, T. Wild boars: A potential source of Erysipelothrix rhusiopathiae infection in Japan. Microbiol. Immunol. 2019, 63, 465–468. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kijima, M.; Takahashi, T.; Yoshimura, H.; Tani, O.; Kojyou, T.; Yamawaki, Y.; Animoto, T. Serovar, pathogenicity and antimicrobial susceptibility of Erysipelothrix rhusiopathiae isolates from farmed wild boars (Sus scrofa) affected with septicemic erysipelas in Japan. Res. Vet. Sci. 1999, 67, 301–303. [Google Scholar] [CrossRef]
- Bosch, J.; Iglesias, I.; Muñoz, M.J.; De la Torre, A. A Cartographic Tool for Managing African Swine Fever in Eurasia: Mapping Wild Boar Distribution Based on the Quality of Available Habitats. Transbound. Emerg. Dis. 2017, 64, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Dovrat, G.; Perevolotsky, A.; Neeman, G. Wild boars as seed dispersal agents of exotic plants from agricultural lands to conservation areas. J. Arid. Environ. 2012, 78, 49–54. [Google Scholar] [CrossRef]
- Vieira-Pinto, M.; Coelho, C.; Vinhas, B.; Proença, J.S. Game meat hygiene and safety in Portugal. In Game Meat Hygiene in Focus, 1st ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 223–240. [Google Scholar]
- Duran, C.O.; Render, J.A. Porcine dermatitis and nephropathy syndrome: A new condition to include in the differential diagnosis list for skin discoloration in swine. J. Swine Health Prod. 1997, 5, 241–244. [Google Scholar]
- Abrantes, A.C.; Serejo, J.; Vieira-Pinto, M. Risk Practices for Occupational Zoonotic Exposure to Tuberculosis in a High-Risk Population in Portugal. Trop. Med. Infect. Dis. 2023, 8, 167. [Google Scholar] [CrossRef]
- Wang, T.; Khan, D.; Mobarakai, N. Erysipelothrix rhusiopathiae endocarditis. IDCases 2020, 22, e00958. [Google Scholar] [CrossRef]
- Andrychowski, J.; Jasielski, P.; Netczuk, T.; Czernicki, Z. Empyema in spinal canal in thoracic region, abscesses in paravertebral space, spondylitis: In clinical course of zoonosis Erysipelothrix rhusiopathiae. Eur. Spine J. 2012, 21 (Suppl. S4), 557–563. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canotilho, J.; Abrantes, A.C.; Risco, D.; Fernández-Llario, P.; Aranha, J.; Vieira-Pinto, M. First Serologic Survey of Erysipelothrix rhusiopathiae in Wild Boars Hunted for Private Consumption in Portugal. Animals 2023, 13, 2936. https://doi.org/10.3390/ani13182936
Canotilho J, Abrantes AC, Risco D, Fernández-Llario P, Aranha J, Vieira-Pinto M. First Serologic Survey of Erysipelothrix rhusiopathiae in Wild Boars Hunted for Private Consumption in Portugal. Animals. 2023; 13(18):2936. https://doi.org/10.3390/ani13182936
Chicago/Turabian StyleCanotilho, João, Ana Carolina Abrantes, David Risco, Pedro Fernández-Llario, José Aranha, and Madalena Vieira-Pinto. 2023. "First Serologic Survey of Erysipelothrix rhusiopathiae in Wild Boars Hunted for Private Consumption in Portugal" Animals 13, no. 18: 2936. https://doi.org/10.3390/ani13182936





