Influence of Molybdenum and Organic Sources of Copper and Sulfur on the Performance, Carcass Traits, Blood Mineral Concentration, and Ceruloplasmin Activity in Lambs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Facilities, and Treatments
2.2. Performance, Blood Collection, and Analysis
2.3. Slaughter and Carcass Evaluation
2.4. Statistical Analysis
3. Results
3.1. Animals and Experimental Diet
3.2. Blood Mineral Concentration
3.3. Ceruloplasmin Activity
4. Discussion
4.1. Animals and Experimental Diet
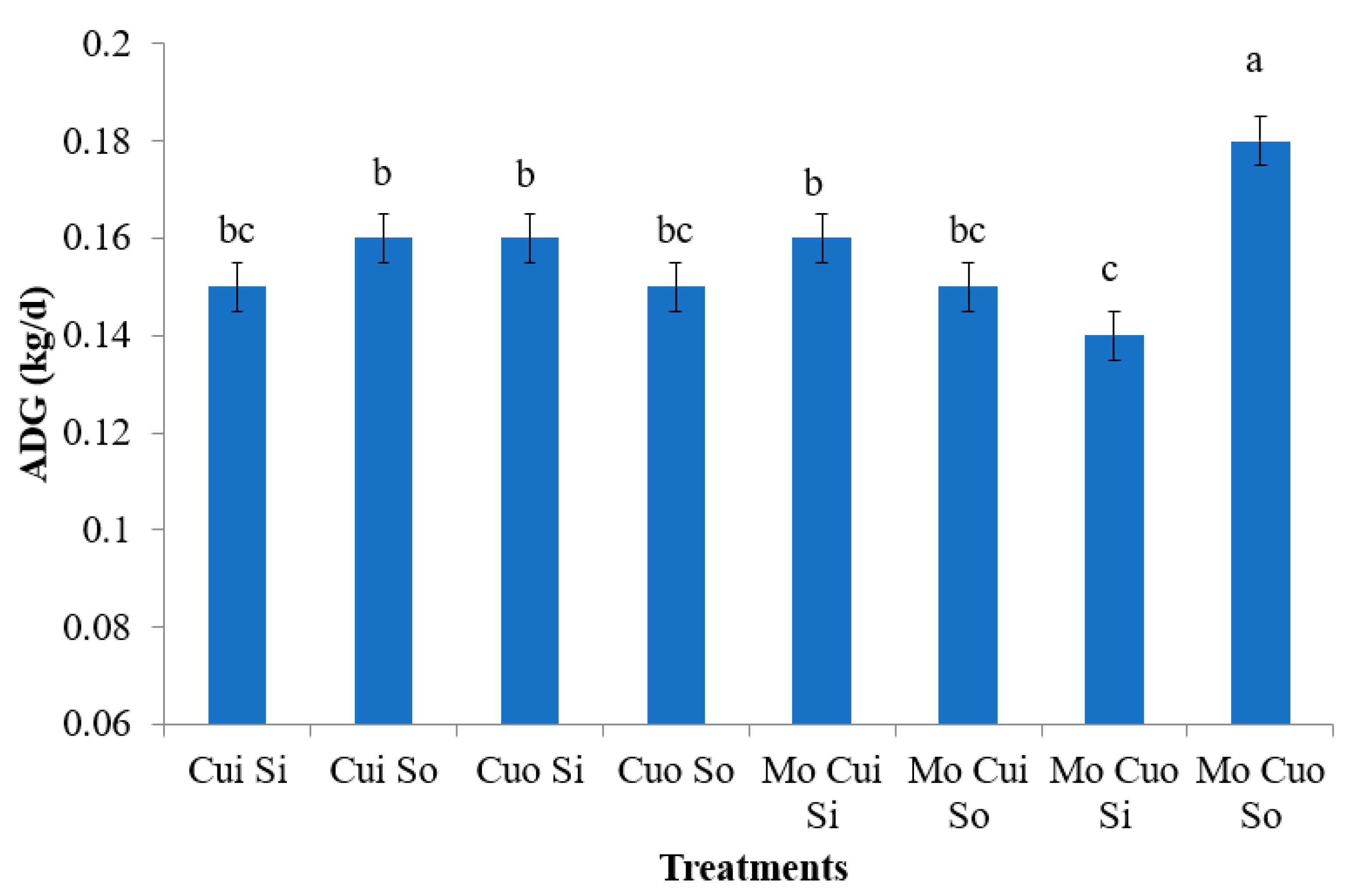
4.2. Performance and Carcass Traits
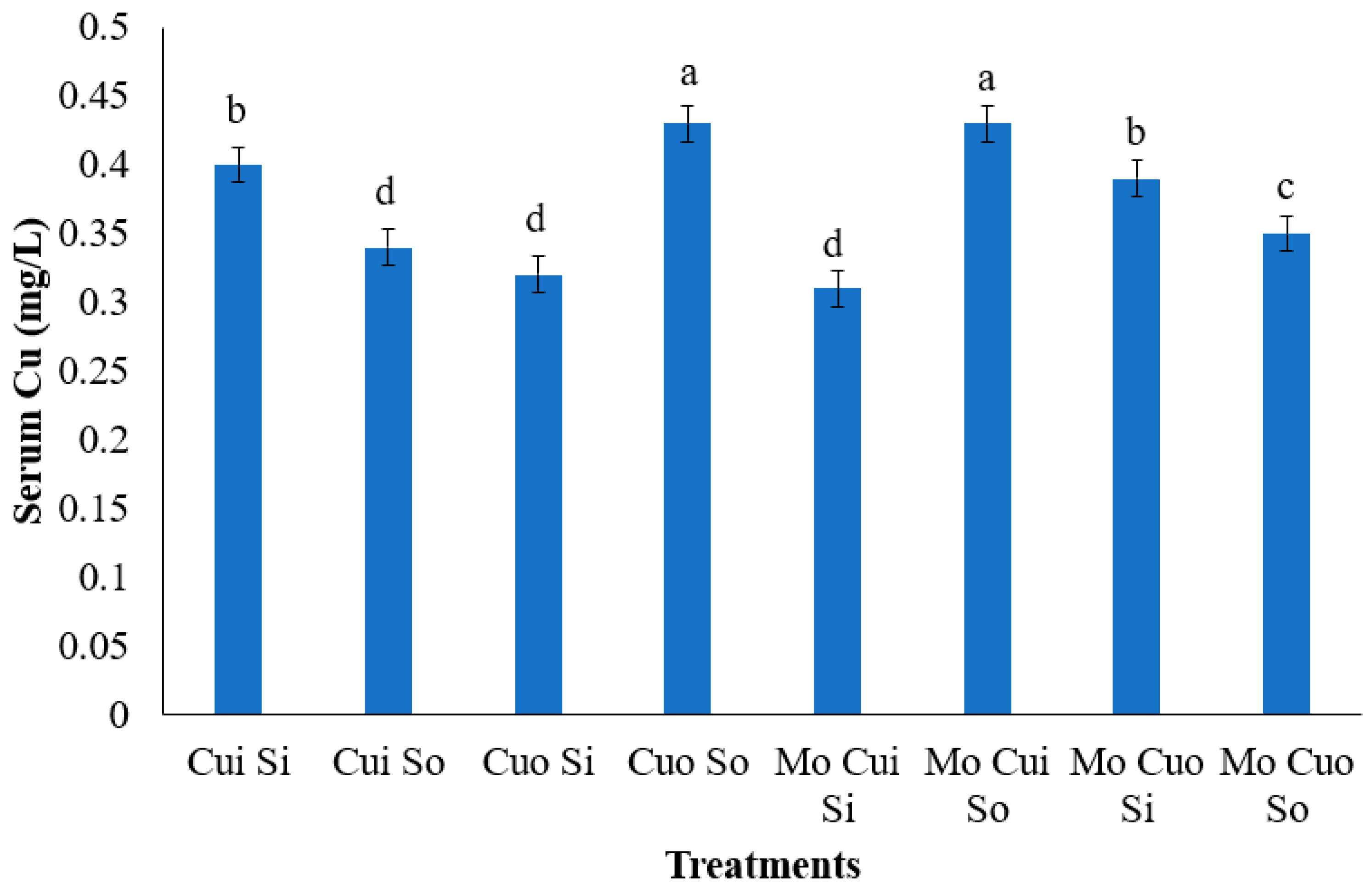
4.3. Blood Mineral Concentration
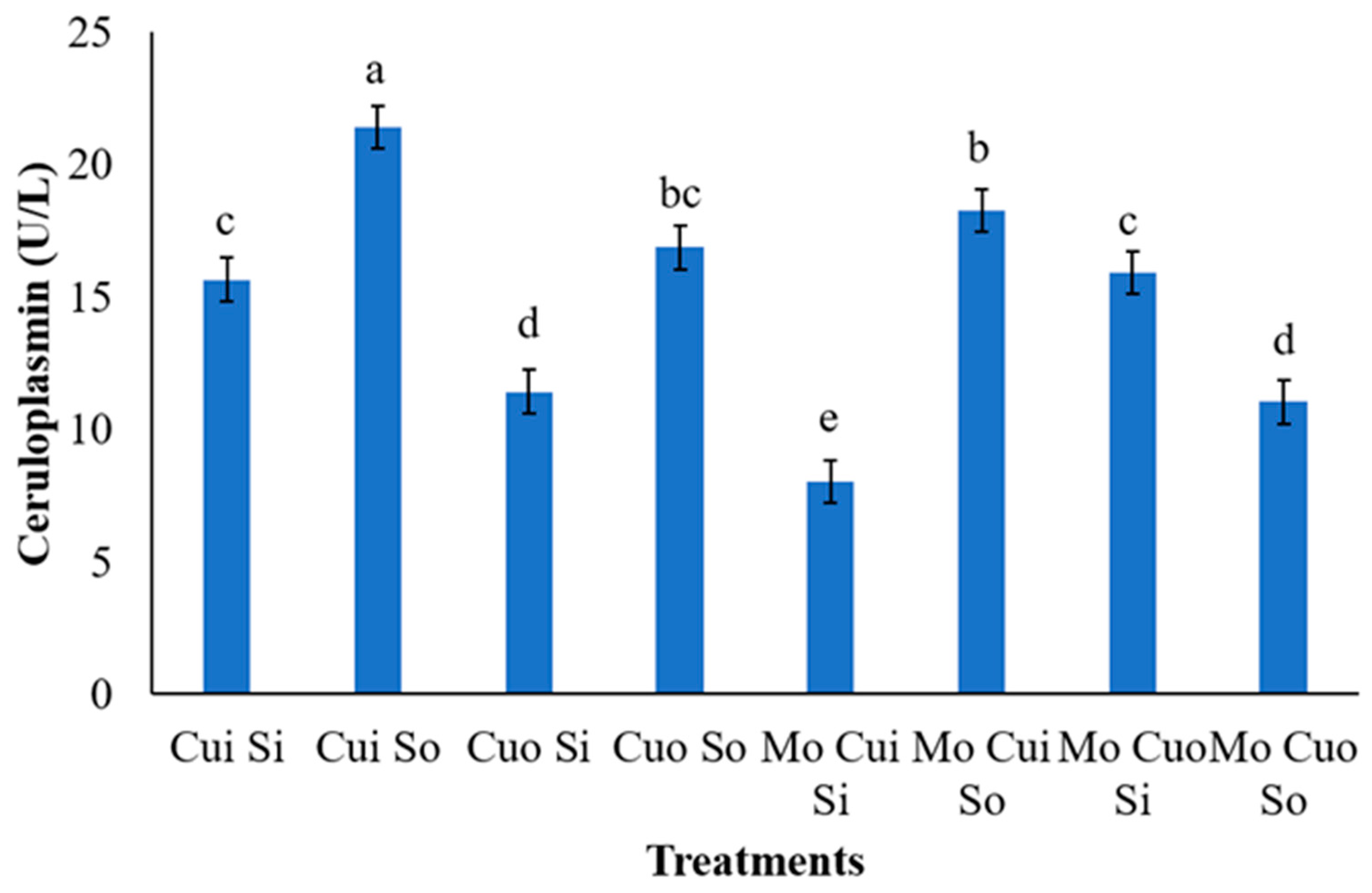
4.4. Ceruloplasmin Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zigo, F.; Farkašóvá, Z.; Elečko, J.; Lapin, M.; Chripková, M.; Czerski, A. Effect of parenteral administration of Selenium and vitamin e on health status of mammary gland and on selected antioxidant indexes in blood of dairy cows. Pol. J. Vet. Sci. 2014, 17, 217–223. [Google Scholar] [CrossRef]
- Vasiľ, M.; Zigo, F.; Lacková, Z.; Ondrašovičová, S. Comparison of the effectiveness of selenium and vitamin E supplementation on the health of the mammary gland of sheep. Acta Fytotech. Zootech. 2021, 24, 75–79. [Google Scholar] [CrossRef]
- Spears, J.W.; Brandao, V.L.N.; Heldt, J. Invited Review: Assessing trace mineral status in ruminants, and factors that affect measurements of trace mineral status. Appl. Anim. Sci. 2022, 38, 252–267. [Google Scholar] [CrossRef]
- Henry, P.; Miles, R. Interactions among the trace minerals. Ciência Anim. Bras. 2000, 1, 95–105. [Google Scholar]
- Clarkson, A.H.; Paine, S.; Martín-Tereso, J.; Kendall, N.R. Copper physiology in ruminants: Trafficking of systemic copper, adaptations to variation in nutritional supply and thiomolybdate challenge. Nutr. Res. Rev. 2020, 33, 43–49. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, M.; Miranda, M. Copper supplementation, a challenge in cattle. Animals 2020, 10, 1890. [Google Scholar] [CrossRef]
- Saran Netto, A.; Zanetti, M.A.; Correa, L.B.; Claro GRDel Salles, M.S.V.; Vilela, F.G. Effects of dietary selenium, sulphur and copper levels on selenium concentration in the serum and liver of lamb. Asian-Australas. J. Anim. Sci. 2014, 27, 1082–1087. [Google Scholar] [CrossRef]
- Spears, J.W. Comparative Trace Element Nutrition: Trace Mineral Bioavailability in Ruminants. J. Nutr. 2003, 133, 1506–1509. [Google Scholar] [CrossRef]
- Humphries, W.R.; Phillippo, M.; Young, B.W.; Bremner, I. The influence of dietary iron and molybdenum on copper metabolism in calves. Br. J. Nutr. 1983, 49, 77–86. [Google Scholar] [CrossRef]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; CAB International: Oxfordshire, UK, 2010. [Google Scholar]
- Gooneratne, S.R.; Buckley, W.T.; Christensen, D.A. Review of Copper Deficiency and Metabolism in Ruminants. Can. J. Anim. Sci. 1989, 69, 819–845. [Google Scholar] [CrossRef]
- Cullen, J.M.; Stalker, M.J. Liver and Biliary System. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 258–352.e1. [Google Scholar]
- Marcilese, N.A.; Ammerman, C.B.; Valsecchi, R.M.; Dunavant, B.G.; Davis, G.K. Effect of dietary molybdenum and sulfate upon urinary excretion of copper in sheep. J. Nutr. 1970, 100, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.; Tipton, K. Ceruloplasmin and what it might do. J. Neural Transm. 2007, 114, 777–781. [Google Scholar] [PubMed]
- Barboza, P.S.; Blake, J.E. Ceruloplasmin as an indicator of copper reserves in wild ruminants at high latitudes. J. Wildl. Dis. 2001, 37, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W. Organic trace minerals in ruminant nutrition. Anim. Feed Sci. Technol. 1996, 58, 151–163. [Google Scholar] [CrossRef]
- Sinclair, L.A.; Hart, K.J.; Johnson, D.; Mackenzie, A.M. Effect of inorganic or organic copper fed without or with added sulfur and molybdenum on the performance, indicators of copper status, and hepatic mRNA in dairy cows. J. Dairy Sci. 2013, 96, 4355–4367. [Google Scholar] [CrossRef]
- Ward, J.D.; Spears, J.W.; Kegley, E.B. Bioavailability of Copper Proteinate and Copper Carbonate Relative to Copper Sulfate in Cattle. J. Dairy Sci. 1996, 79, 127–132. [Google Scholar]
- Zhou, J.; Ren, Y.; Wen, X.; Yue, S.; Wang, Z.; Wang, L.; Peng, Q.; Hu, R.; Zou, H.; Jiang, Y.; et al. Comparison of coated and uncoated trace elements on growth performance, apparent digestibility, intestinal development and microbial diversity in growing sheep. Front. Microbiol. 2022, 13, 1080182. [Google Scholar]
- NRC. National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- AOAC. Official Methods od Analysis-Animal Feed. In Official Methods of Analysis, 17th ed.; The Association of Official Analytical Cheimists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Mason, V.C. The influence of the Maillard reaction upon the nutritive value of fibrous feeds. Anim. Feed Sci. Technol. 1991, 32, 45–53. [Google Scholar]
- Schosinsky, K.H.; Lehmann, H.P.; Beeler, M.F. Measurement of ceruloplasmin from its oxidase activity in serum by use of o dianisidine dihydrochloride. Clin. Chem. 1974, 20, 1556–1563. [Google Scholar]
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa 3, de 17 de Janeiro de 2000. Aprova o Regulamento Técnico de Métodos de Insensibilização para o Abate Humanitário de Animais de Açougue. Brazilian Law. Diário Oficial da União. 2000; pp. 3–10. Available online: http://www.agricultura.gov.br/assuntos/sustentabilidade/bem-estar-animal/arquivos/arquivos-legislacao/in-03-de-2000.pdf (accessed on 1 May 2023).
- Dias, R.S.; López, S.; Montanholi, Y.R.; Smith, B.; Haas, L.S.; Miller, S.P.; France, J. A meta-analysis of the effects of dietary copper, molybdenum, and sulfur on plasma and liver copper, weight gain, and feed conversion in growing-finishing cattle. J. Anim. Sci. 2013, 91, 5714–5723. [Google Scholar]
- Dick, A.T.; Dewey, D.W.; Gawthorne, J.M. Short note: Thiomolybdates and the copper-molybdenum-sulphur interaction in ruminant nutrition. J. Agric. Sci. 1975, 85, 567–568. [Google Scholar] [CrossRef]
- Suttle, N.F. The role of organic sulphur in the copper-molybdenum-S interrelationship in ruminant nutrition. Br. J. Nutr. 1975, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Dezfoulian, A.H.; Aliarabi, H.; Tabatabaei, M.M.; Zamani, P.; Alipour, D.; Bahari, A.; Fadayifar, A. Influence of different levels and sources of copper supplementation on performance, some blood parameters, nutrient digestibility and mineral balance in lambs. Livest. Sci. 2012, 147, 9–19. [Google Scholar] [CrossRef]
- Garrine, C.; Fernandes, T.; Yoshikawa, C.; Correa, L.; Bell, V.; Netto, A.; Zanetti, M.A. Effects of Copper Supplementation on Lipid Oxidation and Meat Quality of Merino X Texel Lambs. J. Food Nutr. Res. 2021, 9, 539–549. [Google Scholar]
- Monin, G.; Sellier, P. Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: The case of the Hampshire breed. Meat Sci. 1985, 13, 49–63. [Google Scholar] [CrossRef]
- Suttle, N.F. Effects of organic and inorganic sulphur on the availability of dietary copper to sheep. Br. J. Nutr. 1974, 32, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Suttle, N.F. Sulphur in Ruminant Nutrition. Annu. Rev. Nutr. 1991, 11, 121–140. [Google Scholar]
- Pott, E.B.; Henry, P.R.; Zanetti, M.A.; Rao, P.V.; Hinderberger, E.J.; Ammerman, C.B. Effects of high dietary molybdenum concentration and duration of feeding time on molybdenum and copper metabolism in sheep. Anim. Feed Sci. Technol. 1999, 79, 93–105. [Google Scholar] [CrossRef]
- Galbraith, H.; Chigwada, W.; Scaife, J.R.; Humphries, W.R. The effect of dietary molybdenum supplementation on tissue copper concentrations, mohair fibre and carcass characteristics of growing Angora goats. Anim. Feed Sci. Technol. 1997, 67, 83–90. [Google Scholar] [CrossRef]
- McDowell, L.R. Copper and Molybdenum. In Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 68–79. [Google Scholar]
| Item | Basal Diet | |
|---|---|---|
| Ingredients | ||
| Corn meal | 553.0 | |
| Wheat middlings | 10.0 | |
| Cottonseed hulls | 250.0 | |
| Soybean meal | 125.0 | |
| Soybean oil | 10.0 | |
| Limestone | 12.0 | |
| Dicalcium phosphate | 20.0 | |
| Urea | 10.0 | |
| Vitamin premix a | 05.0 | |
| Mineral premix b | 05.0 | |
| Chemical composition | ||
| Dry matter (g/kg OM c) | 906.5 | |
| aNDF | 282.0 | |
| aADF | 166.4 | |
| Crude protein | 157.3 | |
| Ether extract | 42.8 | |
| Ash | 57.0 | |
| Calcium | 11.8 | |
| Phosphorus | 5.8 | |
| Treatment | Molybdenum | Copper | Sulfur |
|---|---|---|---|
| T0 | 1.02 | 9.96 | 2561.88 |
| T1 | 9.54 | 8.42 | 2369.52 |
| T2 | 1.05 | 15.70 | 4340.04 |
| T3 | 0.97 | 15.72 | 5178.19 |
| T4 | 1.18 | 17.22 | 4829.88 |
| T5 | 0.90 | 17.61 | 5209.69 |
| T6 | 11.60 | 15.74 | 4645.46 |
| T7 | 10.21 | 19.91 | 5288.51 |
| T8 | 11.92 | 16.67 | 5190.82 |
| T9 | 11.72 | 17.16 | 4906.29 |
| Mineral Inclusion | Time (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mo | Cu | S | Treatment | 0 | 28 | 56 | 84 | Mean |
| Without | Without | Without | T0 | 23.87 | 27.71 | 32.91 | 37.21 | 30.42 |
| With | Without | Without | T1 | 21.53 | 24.31 | 29.54 | 34.28 | 27.41 |
| Without | Inorganic | Inorganic | T2 | 21.98 | 24.68 | 30.28 | 33.85 | 27.69 |
| Organic | T3 | 22.38 | 25.65 | 31.89 | 35.85 | 28.94 | ||
| Organic | Inorganic | T4 | 22.61 | 25.83 | 31.23 | 36.01 | 28.92 | |
| Organic | T5 | 22.64 | 25.88 | 30.94 | 35.25 | 28.68 | ||
| With | Inorganic | Inorganic | T6 | 22.31 | 25.63 | 30.56 | 35.4 | 28.48 |
| Organic | T7 | 21.66 | 25.45 | 30.11 | 34.8 | 28.01 | ||
| Organic | Inorganic | T8 | 20.91 | 23.98 | 28.79 | 32.86 | 26.63 | |
| Organic | T9 | 21.6 | 25.26 | 31.45 | 36.75 | 28.77 | ||
| Principal effects | ||||||||
| Without | 22.4 | 25.51 | 31.08 | 35.24 | 28.56 | |||
| With | 21.62 | 25.08 | 30.23 | 34.95 | 27.97 | |||
| Inorganic | 22.08 | 25.35 | 30.71 | 34.98 | 28.28 | |||
| Organic | 21.94 | 25.23 | 30.6 | 35.22 | 28.25 | |||
| Inorganic | 21.95 | 25.03 | 30.21 | 34.53 | 27.93 | |||
| Organic | 22.07 | 25.56 | 31.1 | 35.67 | 28.6 | |||
| Average data | ||||||||
| Mean | 22.23 | 25.34 | 30.87 | 35.32 | 28.49 | |||
| SEM | 0.67 | 0.83 | 0.96 | 1.09 | 0.59 | |||
| Statistical probabilities of contrasts | ||||||||
| Control vs. others [(T0) vs. (T1 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9)] | NS | |||||||
| Mo vs. others (T1 vs. T0 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9) | NS | |||||||
| Mo presence (T0 vs. T1) | NS | |||||||
| Cu source [(T3 + T4) vs. (T5 + T6)] | NS | |||||||
| S source [(T3 vs. T5) vs. (T4 + T6)] | NS | |||||||
| Mo × Cu [(T2 + T4 + T7 + T9) vs. (T3 + T5 + T6 + T8)] | NS | |||||||
| Mo × S [(T2 + T3 + T8 + T9) vs. (T4 + T5 + T6 + T7)] | NS | |||||||
| Cu × S [(T3 + T4 + T7 + T8) vs. (T2 + T5 + T6 + T9)] | NS | |||||||
| Mo × Cu × S [(T3 + T4 + T6 + T9) vs. (T2 + T5 + T7 + T8)] | NS | |||||||
| Mineral Inclusion | Time (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mo | Cu | S | Treatment | 0 | 28 | 56 | 84 | Mean |
| Without | Without | Without | T0 | 0.14 | 0.19 | 0.15 | 0.16 | |
| With | Without | Without | T1 | 0.1 | 0.19 | 0.17 | 0.15 | |
| Without | Inorganic | Inorganic | T2 | 0.1 | 0.2 | 0.14 | 0.15 | |
| Organic | T3 | 0.12 | 0.22 | 0.14 | 0.16 | |||
| Organic | Inorganic | T4 | 0.11 | 0.19 | 0.17 | 0.16 | ||
| Organic | T5 | 0.12 | 0.18 | 0.15 | 0.15 | |||
| With | Inorganic | Inorganic | T6 | 0.12 | 0.18 | 0.17 | 0.16 | |
| Organic | T7 | 0.12 | 0.17 | 0.17 | 0.15 | |||
| Organic | Inorganic | T8 | 0.1 | 0.17 | 0.15 | 0.14 | ||
| Organic | T9 | 0.13 | 0.22 | 0.19 | 0.18 | |||
| Principal effects | ||||||||
| Without | 0.11 | 0.2 | 0.15 | 0.15 | ||||
| With | 0.12 | 0.18 | 0.17 | 0.16 | ||||
| Inorganic | 0.11 | 0.19 | 0.16 | 0.15 | ||||
| Organic | 0.11 | 0.19 | 0.16 | 0.16 | ||||
| Inorganic | 0.11 | 0.19 | 0.16 | 0.15 | ||||
| Organic | 0.12 | 0.2 | 0.16 | 0.16 | ||||
| Average data | ||||||||
| Mean | 0.12 | 0.19 | 0.16 | 0.16 | ||||
| SEM | 0.007 | 0.007 | 0.007 | 0.005 | ||||
| Statistical probabilities of contrasts | ||||||||
| Control vs. others [(T0) vs. (T1 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9)] | NS | |||||||
| Mo vs. others (T1 vs. T0 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9) | NS | |||||||
| Mo presence (T0 vs. T1) | NS | |||||||
| Cu source [(T3 + T4) vs. (T5 + T6)] | NS | |||||||
| S source [(T3 vs. T5) vs. (T4 + T6)] | NS | |||||||
| Mo × Cu [(T2 + T4 + T7 + T9) vs. (T3 + T5 + T6 + T8)] | NS | |||||||
| Mo × S [(T2 + T3 + T8 + T9) vs. (T4 + T5 + T6 + T7)] | NS | |||||||
| Cu × S [(T3 + T4 + T7 + T8) vs. (T2 + T5 + T6 + T9)] | NS | |||||||
| Mo × Cu × S [(T3 + T4 + T6 + T9) vs. (T2 + T5 + T7 + T8)] | 0.0283 | |||||||
| Mineral Inclusion | Variable | ||||||
|---|---|---|---|---|---|---|---|
| Mo | Cu | S | Treatment | pH 0 h | pH 24 h | Hot Carcass Weight (kg) | Cold Carcass Weight (kg) |
| Without | Without | Without | T0 | 6.17 | 5.62 | 19.65 | 19.04 |
| With | Without | Without | T1 | 6.15 | 5.63 | 17.5 | 16.8 |
| Without | Inorganic | Inorganic | T2 | 6.48 | 5.77 | 17.3 | 16.7 |
| Organic | T3 | 6.46 | 5.6 | 18.5 | 17.9 | ||
| Organic | Inorganic | T4 | 6.36 | 5.68 | 19.03 | 18.33 | |
| Organic | T5 | 6.16 | 5.66 | 19.05 | 18.43 | ||
| With | Inorganic | Inorganic | T6 | 6.6 | 5.71 | 18.15 | 17.5 |
| Organic | T7 | 6.45 | 5.85 | 18 | 17.43 | ||
| Organic | Inorganic | T8 | 6.51 | 5.7 | 16.8 | 16.18 | |
| Organic | T9 | 6.34 | 5.64 | 18.93 | 18.23 | ||
| Principal effects | |||||||
| Without | 6.36 | 5.68 | 18.47 | 17.84 | |||
| With | 6.47 | 5.72 | 17.97 | 17.33 | |||
| Inorganic | 6.5 | 5.73 | 17.99 | 17.38 | |||
| Organic | 6.34 | 5.67 | 18.45 | 17.79 | |||
| Inorganic | 6.49 | 5.72 | 18.82 | 17.18 | |||
| Organic | 6.35 | 5.69 | 18.62 | 17.99 | |||
| Average data | |||||||
| Mean | 6.36 | 5.68 | 18.35 | 17.72 | |||
| SEM | 0.052 | 0.024 | 0.608 | 0.598 | |||
| Statistical probabilities of contrasts | |||||||
| Control vs. others [(T0) vs. (T1 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9)] | NS | NS | NS | NS | |||
| Mo vs. others (T1 vs. T0 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9) | NS | NS | NS | NS | |||
| Mo presence (T0 vs. T1) | NS | NS | NS | NS | |||
| Cu source [(T3 + T4) vs. (T5 + T6)] | NS | NS | NS | NS | |||
| S source [(T3 vs. T5) vs. (T4 + T6)] | NS | NS | NS | NS | |||
| Mo × Cu [(T2 + T4 + T7 + T9) vs. (T3 + T5 + T6 + T8)] | NS | NS | NS | NS | |||
| Mo × S [(T2 + T3 + T8 + T9) vs. (T4 + T5 + T6 + T7)] | NS | NS | NS | NS | |||
| Cu × S [(T3 + T4 + T7 + T8) vs. (T2 + T5 + T6 + T9)] | NS | NS | NS | NS | |||
| Mo × Cu × S [(T3 + T4 + T6 + T9) vs. (T2 + T5 + T7 + T8)] | NS | NS | NS | NS | |||
| Mineral Inclusion | Treatment | Time (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mo | Cu | S | 0 | 28 | 56 | 84 | Mean | |
| Without | Without | Without | T0 | 0.45 | 0.43 | 0.46 | 0.45 | 0.45 |
| With | Without | Without | T1 | 0.35 | 0.36 | 0.35 | 0.25 | 0.33 |
| Without | Inorganic | Inorganic | T2 | 0.43 | 0.39 | 0.37 | 0.4 | 0.4 |
| Organic | T3 | 0.44 | 0.33 | 0.3 | 0.34 | 0.35 | ||
| Organic | Inorganic | T4 | 0.44 | 0.33 | 0.3 | 0.32 | 0.35 | |
| Organic | T5 | 0.35 | 0.4 | 0.38 | 0.43 | 0.39 | ||
| With | Inorganic | Inorganic | T6 | 0.3 | 0.33 | 0.32 | 0.31 | 0.31 |
| Organic | T7 | 0.45 | 0.36 | 0.41 | 0.43 | 0.41 | ||
| Organic | Inorganic | T8 | 0.41 | 0.36 | 0.37 | 0.39 | 0.39 | |
| Organic | T9 | 0.3 | 0.35 | 0.34 | 0.35 | 0.33 | ||
| Principal effects | ||||||||
| Without | 0.36 | 0.34 | 0.37 | 0.37 | ||||
| With | 0.35 | 0.36 | 0.37 | 0.36 | ||||
| Inorganic | 0.35 | 0.35 | 0.37 | 0.37 | ||||
| Organic | 0.36 | 0.35 | 0.37 | 0.36 | ||||
| Inorganic | 0.35 | 0.34 | 0.36 | 0.36 | ||||
| Organic | 0.36 | 0.36 | 0.39 | 0.37 | ||||
| Average data | ||||||||
| Mean | 0.4 | 0.37 | 0.36 | 0.37 | 0.37 | |||
| SEM | 0.015 | 0.013 | 0.016 | 0.013 | 0.007 | |||
| Statistical probabilities of contrasts | ||||||||
| Control vs. others [(T0) vs. (T1 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9)] | NS | NS | 0.0212 | 0.0088 | NS | |||
| Mo vs. others (T1 vs. T0 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9) | NS | NS | NS | 0.0024 | NS | |||
| Mo presence (T0 vs. T1) | NS | NS | NS | NS | NS | |||
| Cu source [(T3 + T4) vs. (T5 + T6)] | NS | NS | NS | NS | NS | |||
| S source [(T3 vs. T5) vs. (T4 + T6)] | NS | NS | NS | NS | NS | |||
| Mo × Cu [(T2 + T4 + T7 + T9) vs. (T3 + T5 + T6 + T8)] | NS | NS | NS | NS | NS | |||
| Mo × S [(T2 + T3 + T8 + T9) vs. (T4 + T5 + T6 + T7)] | NS | NS | NS | NS | NS | |||
| Cu × S [(T3 + T4 + T7 + T8) vs. (T2 + T5 + T6 + T9)] | NS | NS | NS | NS | NS | |||
| Mo × Cu × S [(T3 + T4 + T6 + T9) vs. (T2 + T5 + T7 + T8)] | NS | NS | NS | 0.0022 | NS | |||
| Mineral Inclusion | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mo | Cu | S | ||||||
| Without | Without | Without | T0 | 1227.11 | 1021.41 | 1159.69 | 1170.3 | 1144.63 |
| With | Without | Without | T1 | 1437.58 | 1245.49 | 1559.48 | 1479.99 | 1430.63 |
| Without | Inorganic | Inorganic | T2 | 1318.22 | 1144.77 | 1488.13 | 1481.75 | 1358.22 |
| Organic | T3 | 1324.11 | 1121.26 | 1330.45 | 1351.19 | 1281.75 | ||
| Organic | Inorganic | T4 | 1311.83 | 1127.96 | 1200.98 | 1220.72 | 1215.37 | |
| Organic | T5 | 1401.44 | 1255.69 | 1503.38 | 1687.64 | 1462.03 | ||
| With | Inorganic | Inorganic | T6 | 1400.63 | 1224.19 | 1490.01 | 1506.15 | 1405.24 |
| Organic | T7 | 1348.67 | 1200.14 | 1422.88 | 1458.74 | 1357.6 | ||
| Organic | Inorganic | T8 | 1241.01 | 1021.26 | 1101.2 | 1067.52 | 1107.75 | |
| Organic | T9 | 1393.11 | 1234.41 | 1451.48 | 1517.24 | 1399.06 | ||
| Principal effects | ||||||||
| Without | 1162.42 | 1380.74 | 1435.32 | 1329.34 | ||||
| With | 1170 | 1366.39 | 1387.41 | 1317.41 | ||||
| Inorganic | 1172.59 | 1432.87 | 1449.46 | 1350.7 | ||||
| Organic | 1159.83 | 1314.26 | 1373.28 | 1296.05 | ||||
| Inorganic | 1129.54 | 1320.08 | 1319.04 | 1271.64 | ||||
| Organic | 1202.87 | 1427.05 | 1503.7 | 1375.11 | ||||
| Average data | ||||||||
| Mean | 1334.98 | 1153.07 | 1360.72 | 1383.46 | 1308.06 | |||
| SEM | 23.36 | 20.67 | 49.25 | 53.39 | 20.81 | |||
| Statistical probabilities of contrasts | ||||||||
| Control vs. others [(T0) vs. (T1 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9)] | NS | NS | NS | NS | NS | |||
| Mo vs. others (T1 vs. T0 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9) | NS | NS | NS | NS | NS | |||
| Mo presence (T0 vs. T1) | NS | NS | NS | NS | NS | |||
| Cu source [(T3 + T4) vs. (T5 + T6)] | NS | NS | NS | NS | NS | |||
| S source [(T3 vs. T5) vs. (T4 + T6)] | NS | NS | NS | NS | NS | |||
| Mo × Cu [(T2 + T4 + T7 + T9) vs. (T3 + T5 + T6 + T8)] | NS | NS | NS | NS | NS | |||
| Mo × S [(T2 + T3 + T8 + T9) vs. (T4 + T5 + T6 + T7)] | NS | NS | NS | NS | NS | |||
| Cu × S [(T3 + T4 + T7 + T8) vs. (T2 + T5 + T6 + T9)] | NS | NS | NS | NS | 0.0355 | |||
| Mo × Cu × S [(T3 + T4 + T6 + T9) vs. (T2 + T5 + T7 + T8)] | NS | NS | NS | NS | NS | |||
| Mineral Inclusion | Treatment | Time (days) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mo | Cu | S | 0 | 28 | 56 | 84 | Mean | |
| Without | Without | Without | T0 | 0.036 | 0.014 | 0.055 | 0.054 | 0.07 |
| With | Without | Without | T1 | 0.022 | 0.89 | 0.715 | 0.991 | 0.654 |
| Without | Inorganic | Inorganic | T2 | 0.032 | 0.075 | 0.212 | 0.032 | 0.087 |
| Organic | T3 | 0.028 | 0.072 | 0.027 | 0.027 | 0.039 | ||
| Organic | Inorganic | T4 | 0.024 | 0.068 | 0.031 | 0.027 | 0.037 | |
| Organic | T5 | 0.027 | 0.087 | 0.024 | 0.026 | 0.041 | ||
| With | Inorganic | Inorganic | T6 | 0.029 | 0.141 | 0.097 | 0.113 | 0.095 |
| Organic | T7 | 0.033 | 0.142 | 0.128 | 0.103 | 0.102 | ||
| Organic | Inorganic | T8 | 0.042 | 0.146 | 0.147 | 0.139 | 0.118 | |
| Organic | T9 | 0.027 | 0.134 | 0.064 | 0.097 | 0.081 | ||
| Principal effects | ||||||||
| Without | 0.075 | 0.073 | 0.028 | 0.051 | ||||
| With | 0.141 | 0.109 | 0.113 | 0.099 | ||||
| Inorganic | 0.108 | 0.116 | 0.069 | 0.081 | ||||
| Organic | 0.109 | 0.067 | 0.072 | 0.069 | ||||
| Inorganic | 0.107 | 0.122 | 0.078 | 0.085 | ||||
| Organic | 0.109 | 0.06 | 0.063 | 0.066 | ||||
| Average data | ||||||||
| Mean | 0.03 | 0.187 | 0.145 | 0.156 | 0.129 | |||
| SEM | 0.0029 | 0.0372 | 0.0372 | 0.0441 | 0.0176 | |||
| Statistical probabilities of contrasts | ||||||||
| Control vs. others [(T0) vs. (T1 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9)] | NS | NS | NS | 0.0021 | NS | |||
| Mo vs. others (T1 vs. T0 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9) | NS | 0.0001 | 0.0001 | 0.0001 | NS | |||
| Mo presence (T0 vs. T1) | NS | 0.0182 | NS | 0.0054 | NS | |||
| Cu source [(T3 + T4) vs. (T5 + T6)] | NS | NS | NS | NS | NS | |||
| S source [(T3 vs. T5) vs. (T4 + T6)] | NS | NS | NS | NS | NS | |||
| Mo × Cu [(T2 + T4 + T7 + T9) vs. (T3 + T5 + T6 + T8)] | NS | NS | NS | NS | NS | |||
| Mo × S [(T2 + T3 + T8 + T9) vs. (T4 + T5 + T6 + T7)] | NS | NS | NS | NS | NS | |||
| Cu × S [(T3 + T4 + T7 + T8) vs. (T2 + T5 + T6 + T9)] | NS | NS | NS | NS | NS | |||
| Mo × Cu × S [(T3 + T4 + T6 + T9) vs. (T2 + T5 + T7 + T8)] | NS | NS | NS | NS | NS | |||
| Mineral Inclusion | Treatments | Time (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mo | Cu | S | 0 | 28 | 56 | 84 | Mean | |
| Without | Without | Without | T0 | 21.58 | 17.28 | 23.37 | 20.15 | 20.59 |
| With | Without | Without | T1 | 13.32 | 14.82 | 17.06 | 11.44 | 14.16 |
| Without | Inorganic | Inorganic | T2 | 22.96 | 17.12 | 17.98 | 15.65 | 18.3 |
| Organic | T3 | 20.7 | 15.85 | 18.26 | 21.41 | 19.05 | ||
| Organic | Inorganic | T4 | 18.85 | 15.99 | 13.02 | 11.43 | 14.82 | |
| Organic | T5 | 17.33 | 15.76 | 16.62 | 16.88 | 16.65 | ||
| With | Inorganic | Inorganic | T6 | 13.04 | 14.47 | 12.73 | 8.02 | 12.06 |
| Organic | T7 | 19.63 | 15.24 | 16.59 | 18.26 | 17.43 | ||
| Organic | Inorganic | T8 | 17.73 | 17.04 | 17.86 | 15.92 | 17.14 | |
| Organic | T9 | 14.38 | 16.34 | 13.12 | 11.03 | 13.71 | ||
| Principal effects | ||||||||
| Without | 16.18 | 16.47 | 16.34 | 17.21 | ||||
| With | 15.77 | 15.07 | 13.31 | 15.09 | ||||
| Inorganic | 15.67 | 16.39 | 15.84 | 16.71 | ||||
| Organic | 16.28 | 15.15 | 13.81 | 15.58 | ||||
| inorganic | 16.15 | 15.4 | 12.75 | 15.58 | ||||
| Organic | 15.8 | 16.14 | 16.89 | 16.71 | ||||
| Average data | ||||||||
| Mean | 18.08 | 16.05 | 16.98 | 15.26 | 16.59 | |||
| SEM | 0.76 | 0.64 | 0.97 | 0.82 | 0.41 | |||
| Statistical Probabilities of Contrasts | ||||||||
| Control vs. others [(T0) vs. (T1 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9)] | 0.0345 | NS | NS | 0.0013 | 0.0053 | |||
| Mo vs. Others (T1 vs. T0 + T2 + T3 + T4 + T5 + T6 + T7 + T8 + T9) | 0.043 | NS | NS | NS | NS | |||
| Mo presence (T0 vs. T1) | 0.0196 | NS | NS | 0.0254 | NS | |||
| Cu source [(T3 + T4) vs. (T5 + T6)] | NS | NS | NS | NS | NS | |||
| S source [(T3 vs. T5) vs. (T4 + T6)] | NS | NS | NS | 0.031 | NS | |||
| Mo × Cu [(T2 + T4 + T7 + T9) vs. (T3 + T5 + T6 + T8)] | NS | NS | NS | NS | NS | |||
| Mo × S [(T2 + T3 + T8 + T9) vs. (T4 + T5 + T6 + T7)] | NS | NS | NS | NS | NS | |||
| Cu × S [(T3 + T4 + T7 + T8) vs. (T2 + T5 + T6 + T9)] | NS | NS | NS | 0.0055 | NS | |||
| Mo × Cu × S [(T3 + T4 + T6 + T9) vs. (T2 + T5 + T7 + T8)] | NS | NS | NS | 0.0074 | NS | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, R.M.C.; da Silva, T.H.; da Silva Brandão Guimarães, I.C.; Bezerra, H.V.A.; Saran Netto, A.; Rodrigues, P.H.M.; Pacheco, J.C.G.; Zanetti, M.A. Influence of Molybdenum and Organic Sources of Copper and Sulfur on the Performance, Carcass Traits, Blood Mineral Concentration, and Ceruloplasmin Activity in Lambs. Animals 2023, 13, 2945. https://doi.org/10.3390/ani13182945
Conti RMC, da Silva TH, da Silva Brandão Guimarães IC, Bezerra HVA, Saran Netto A, Rodrigues PHM, Pacheco JCG, Zanetti MA. Influence of Molybdenum and Organic Sources of Copper and Sulfur on the Performance, Carcass Traits, Blood Mineral Concentration, and Ceruloplasmin Activity in Lambs. Animals. 2023; 13(18):2945. https://doi.org/10.3390/ani13182945
Chicago/Turabian StyleConti, Renata Maria Consentino, Thiago Henrique da Silva, Iuli Caetano da Silva Brandão Guimarães, Helena Viel Alves Bezerra, Arlindo Saran Netto, Paulo Henrique Mazza Rodrigues, José Carlos Guilardi Pacheco, and Marcus Antonio Zanetti. 2023. "Influence of Molybdenum and Organic Sources of Copper and Sulfur on the Performance, Carcass Traits, Blood Mineral Concentration, and Ceruloplasmin Activity in Lambs" Animals 13, no. 18: 2945. https://doi.org/10.3390/ani13182945





