β-Hydroxybutyrate Effects on Bovine Caruncular Epithelial Cells: A Model for Investigating the Peri-Implantation Period Disruption in Ketotic Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Stimulation of BCECs with BHBA
2.3. Cell Metabolism (MTT Assay)
2.4. Cell Motility (Live-Cell Imaging)
2.5. RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
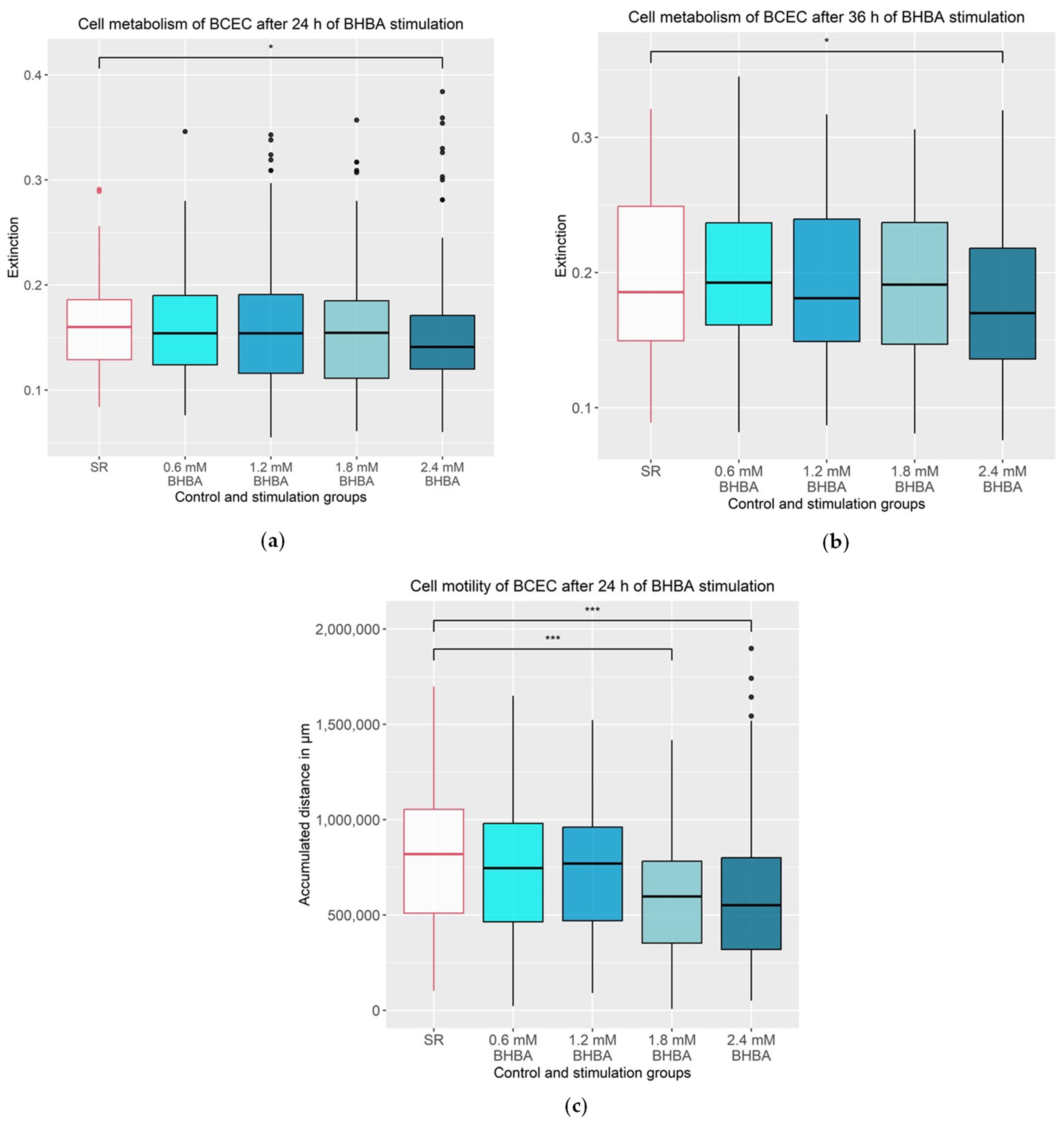
3.1. Effects of BHBA on the Metabolism and Motility of the BCEC
3.2. Effects of BHBA on the Gene Expression of IL6, RELA and TNF
3.3. Effects of BHBA on the Gene Expression of PTGER2 and PTGES2
3.4. Effects of BHBA on the Gene Expression of the Integrin Subunits ITGAV, ITGA6, ITGB1 and ITGB3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grummer, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Anim. Sci. 1995, 73, 2820–2833. [Google Scholar] [CrossRef]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy. Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K. Advances in transition cow biology: New frontiers in production diseases. In Production Diseases in Farm Animals, Proceedings of the 12th International Conference on Production Diseases in Farm Animals, East Lansing, MI, USA, 19–22 July 2004; Joshi, N.P., Herdt, T.H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006. [Google Scholar]
- Gulinski, P. Ketone bodies—Causes and effects of their increased presence in cows’ body fluids: A review. Vet. World 2021, 14, 1492–1503. [Google Scholar] [CrossRef]
- Duffield, T. Subclinical Ketosis in Lactating Dairy Cattle. Vet. Clin. North. Am. Food Anim. 2000, 16, 231–253. [Google Scholar] [CrossRef]
- Iwersen, M.; Falkenberg, U.; Voigtsberger, R.; Forderung, D.; Heuwieser, W. Evaluation of an electronic cowside test to detect subclinical ketosis in dairy cows. J. Dairy. Sci. 2009, 92, 2618–2624. [Google Scholar] [CrossRef]
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- Rossi, F.; Righi, F.; Romanelli, S.; Quarantelli, A. Reproductive efficiency of dairy cows under negative energy balance conditions. Ann. Della Fac. Di Med. Vet. Univ. Di Parma 2008, 28, 173–180. [Google Scholar]
- Benedet, A.; Manuelian, C.L.; Zidi, A.; Penasa, M.; De Marchi, M. Invited review: Beta-hydroxybutyrate concentration in blood and milk and its associations with cow performance. Animal 2019, 13, 1676–1689. [Google Scholar] [CrossRef]
- Walsh, R.B.; Walton, J.S.; Kelton, D.F.; LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J. Dairy. Sci. 2007, 90, 2788–2796. [Google Scholar] [CrossRef]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy. Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy. Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef]
- Berge, A.C.; Vertenten, G. A field study to determine the prevalence, dairy herd management systems, and fresh cow clinical conditions associated with ketosis in western European dairy herds. J. Dairy. Sci. 2014, 97, 2145–2154. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Mierziak, J.; Burgberger, M.; Wojtasik, W. 3-Hydroxybutyrate as a Metabolite and a Signal Molecule Regulating Processes of Living Organisms. Biomolecules 2021, 11, 402. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Tsirigos, A.; Whitaker-Menezes, D.; Pavlides, S.; Pestell, R.G.; Chiavarina, B.; Frank, P.G.; Flomenberg, N.; Howell, A.; Martinez-Outschoorn, U.E.; et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010, 9, 3506–3514. [Google Scholar] [CrossRef]
- Gouirand, V.; Gicquel, T.; Lien, E.C.; Jaune-Pons, E.; Da Costa, Q.; Finetti, P.; Metay, E.; Duluc, C.; Mayers, J.R.; Audebert, S.; et al. Ketogenic HMG-CoA lyase and its product beta-hydroxybutyrate promote pancreatic cancer progression. EMBO J. 2022, 41, e110466. [Google Scholar] [CrossRef]
- Huang, D.; Li, T.; Wang, L.; Zhang, L.; Yan, R.; Li, K.; Xing, S.; Wu, G.; Hu, L.; Jia, W.; et al. Hepatocellular carcinoma redirects to ketolysis for progression under nutrition deprivation stress. Cell Res. 2016, 26, 1112–1130. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef]
- Lupton, J.R. Microbial degradation products influence colon cancer risk: The butyrate controversy. J. Nutr. 2004, 134, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, L.; Zhang, C.; Cheng, X.; Zhang, Y.; Guo, Y.; Long, M.; Yang, S.; He, J. Palmitic Acid and beta-Hydroxybutyrate Induce Inflammatory Responses in Bovine Endometrial Cells by Activating Oxidative Stress-Mediated NF-kappaB Signaling. Molecules 2019, 24, 2421. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Li, D.; Li, Y.; Song, Y.; Deng, Q.; Wang, J.; Zhang, Y.; Ding, H.; Yin, L.; et al. beta-Hydroxybutyrate activates the NF-kappaB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell Physiol. Biochem. 2014, 33, 920–932. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, H.; Zhao, Z.; Peng, Z.; Wang, Z.; Liu, G.; Li, X. Non-Esterified Fatty Acids Over-Activate the TLR2/4-NF-Kappab Signaling Pathway to Increase Inflammatory Cytokine Synthesis in Neutrophils from Ketotic Cows. Cell Physiol. Biochem. 2018, 48, 827–837. [Google Scholar] [CrossRef]
- Abuajamieh, M.; Kvidera, S.K.; Fernandez, M.V.; Nayeri, A.; Upah, N.C.; Nolan, E.A.; Lei, S.M.; DeFrain, J.M.; Green, H.B.; Schoenberg, K.M.; et al. Inflammatory biomarkers are associated with ketosis in periparturient Holstein cows. Res. Vet. Sci. 2016, 109, 81–85. [Google Scholar] [CrossRef]
- El-Deeb, W.M.; El-Bahr, S.M. Biomarkers of ketosis in dairy cows at postparturient period: Acute phase proteins and pro-inflammatory cytokines. Vet. Arh. 2017, 87, 431–440. [Google Scholar] [CrossRef]
- Shen, T.; Li, X.; Loor, J.J.; Zhu, Y.; Du, X.; Wang, X.; Xing, D.; Shi, Z.; Fang, Z.; Li, X.; et al. Hepatic nuclear factor kappa B signaling pathway and NLR family pyrin domain containing 3 inflammasome is over-activated in ketotic dairy cows. J. Dairy. Sci. 2019, 102, 10554–10563. [Google Scholar] [CrossRef]
- Diskin, M.G.; Parr, M.H.; Morris, D.G. Embryo death in cattle: An update. Reprod. Fertil. Dev. 2012, 24, 244–251. [Google Scholar] [CrossRef]
- Lonergan, P.; Forde, N. Maternal-embryo interaction leading up to the initiation of implantation of pregnancy in cattle. Animal 2014, 8 (Suppl. S1), 64–69. [Google Scholar] [CrossRef]
- Spencer, T.E. Early pregnancy: Concepts, challenges, and potential solutions. Anim. Front. 2013, 3, 48–55. [Google Scholar] [CrossRef]
- Bridges, G.A.; Day, M.L.; Geary, T.W.; Cruppe, L.H. Triennial Reproduction Symposium: Deficiencies in the uterine environment and failure to support embryonic development. J. Anim. Sci. 2013, 91, 3002–3013. [Google Scholar] [CrossRef]
- Diskin, M.G.; Morris, D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 260–267. [Google Scholar] [CrossRef]
- Forde, N.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. ‘Conceptualizing’ the Endometrium: Identification of Conceptus-Derived Proteins During Early Pregnancy in Cattle. Biol. Reprod. 2015, 92, 156. [Google Scholar] [CrossRef]
- Atkinson, B.A.; King, G.J.; Amoroso, E.C. Development of the caruncular and intercaruncular regions in the bovine endometrium. Biol. Reprod. 1984, 30, 763–774. [Google Scholar] [CrossRef]
- Roberts, R.M. Interferon-tau, a Type 1 interferon involved in maternal recognition of pregnancy. Cytokine Growth Factor Rev. 2007, 18, 403–408. [Google Scholar] [CrossRef]
- Griffith, O.W.; Chavan, A.R.; Protopapas, S.; Maziarz, J.; Romero, R.; Wagner, G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 2017, 114, E6566–E6575. [Google Scholar] [CrossRef]
- Ristimaki, A.; Jaatinen, R.; Ritvos, O. Regulation of prostaglandin F2 alpha receptor expression in cultured human granulosa-luteal cells. Endocrinology 1997, 138, 191–195. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Campbell, W.B. An increase in hypothalamic capacity to synthesize prostaglandin E2 precedes the first preovulatory surge of gonadotropins. Endocrinology 1982, 111, 1031–1037. [Google Scholar] [CrossRef]
- Arosh, J.A.; Banu, S.K.; McCracken, J.A. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants. J. Dairy. Sci. 2016, 99, 5926–5940. [Google Scholar] [CrossRef]
- Chan, S.Y. Effects of prostaglandin E2 and F2α on peri-implantation development of mouse embryos in vitro. Prostaglandins 1991, 42, 321–336. [Google Scholar] [CrossRef]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Longhurst, C.M.; Jennings, L.K. Integrin-mediated signal transduction. Cell Mol. Life Sci. 1998, 54, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Bazer, F.W.; Jaeger, L.A.; Ka, H.; Garlow, J.E.; Pfarrer, C.; Spencer, T.E.; Burghardt, R.C. Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol. Reprod. 2001, 65, 820–828. [Google Scholar] [CrossRef]
- Bowen, J.A.; Bazer, F.W.; Burghardt, R.C. Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol. Reprod. 1996, 55, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, A.E.; Calarco, P.G.; Damsky, C.H. Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development 1993, 119, 1175–1186. [Google Scholar] [CrossRef]
- Erikson, D.W.; Burghardt, R.C.; Bayless, K.J.; Johnson, G.A. Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alpha v beta 6 on porcine trophectoderm cells and integrin alpha v beta 3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration. Biol. Reprod. 2009, 81, 814–825. [Google Scholar] [CrossRef]
- Pfarrer, C.; Hirsch, P.; Guillomot, M.; Leiser, R. Interaction of Integrin Receptors with Extracellular Matrix is Involved in Trophoblast Giant Cell Migration in Bovine Placentomes. Placenta 2003, 24, 588–597. [Google Scholar] [CrossRef]
- Kimmins, S.; MacLaren, L.A. Cyclic modulation of integrin expression in bovine endometrium. Biol. Reprod. 1999, 61, 1267–1274. [Google Scholar] [CrossRef]
- MacIntyre, D.M.; Lim, H.C.; Ryan, K.; Kimmins, S.; Small, J.A.; MacLaren, L.A. Implantation-associated changes in bovine uterine expression of integrins and extracellular matrix. Biol. Reprod. 2002, 66, 1430–1436. [Google Scholar] [CrossRef]
- Zeiler, M.; Leiser, R.; Johnson, G.A.; Tinneberg, H.R.; Pfarrer, C. Development of an in vitro model for bovine placentation: A comparison of the in vivo and in vitro expression of integrins and components of extracellular matrix in bovine placental cells. Cells Tissues Organs 2007, 186, 229–242. [Google Scholar] [CrossRef]
- Bridger, P.S.; Menge, C.; Leiser, R.; Tinneberg, H.R.; Pfarrer, C.D. Bovine caruncular epithelial cell line (BCEC-1) isolated from the placenta forms a functional epithelial barrier in a polarised cell culture model. Placenta 2007, 28, 1110–1117. [Google Scholar] [CrossRef]
- Mossman, H.W. Classics revisited: Comparative morphogenesis of the fetal membranes and accessory uterine structures. Placenta 1991, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dilly, M.; Hambruch, N.; Haeger, J.D.; Pfarrer, C. Epidermal growth factor (EGF) induces motility and upregulates MMP-9 and TIMP-1 in bovine trophoblast cells. Mol. Reprod. Dev. 2010, 77, 622–629. [Google Scholar] [CrossRef]
- Wilfinger, W.W.; Mackey, K.; Chomczynski, P. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques 1997, 22, 474–476, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Filor, V.; Seeger, B.; de Buhr, N.; von Kockritz-Blickwede, M.; Kietzmann, M.; Oltmanns, H.; Meissner, J. Investigation of the pathophysiology of bacterial mastitis using precision-cut bovine udder slices. J. Dairy. Sci. 2022, 105, 7705–7718. [Google Scholar] [CrossRef] [PubMed]
- Piechotta, M.; Kedves, K.; Araujo, M.G.; Hoeflich, A.; Metzger, F.; Heppelmann, M.; Muscher-Banse, A.; Wrenzycki, C.; Pfarrer, C.; Schuberth, H.J.; et al. Hepatic mRNA expression of acid labile subunit and deiodinase 1 differs between cows selected for high versus low concentrations of insulin-like growth factor 1 in late pregnancy. J. Dairy. Sci. 2013, 96, 3737–3749. [Google Scholar] [CrossRef]
- Burgers, E.E.A.; Goselink, R.M.A.; Bruckmaier, R.M.; Gross, J.J.; Jorritsma, R.; Kemp, B.; Kok, A.; van Knegsel, A.T. Effect of voluntary waiting period on metabolism of dairy cows during different phases of the lactation. J. Anim. Sci. 2023, 101, skad194. [Google Scholar] [CrossRef]
- Liboreiro, D.N.; Machado, K.S.; Silva, P.R.; Maturana, M.M.; Nishimura, T.K.; Brandao, A.P.; Endres, M.I.; Chebel, R.C. Characterization of peripartum rumination and activity of cows diagnosed with metabolic and uterine diseases. J. Dairy. Sci. 2015, 98, 6812–6827. [Google Scholar] [CrossRef]
- Vanholder, T.; Papen, J.; Bemers, R.; Vertenten, G.; Berge, A.C. Risk factors for subclinical and clinical ketosis and association with production parameters in dairy cows in the Netherlands. J. Dairy. Sci. 2015, 98, 880–888. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Wong, A.C.; Lundgren, P.; Golos, A.M.; Descamps, H.C.; Dohnalova, L.; Cramer, Z.; Tian, Y.; Yueh, B.; Eskiocak, O.; et al. beta-Hydroxybutyrate suppresses colorectal cancer. Nature 2022, 605, 160–165. [Google Scholar] [CrossRef]
- Shukla, S.K.; Gebregiworgis, T.; Purohit, V.; Chaika, N.V.; Gunda, V.; Radhakrishnan, P.; Mehla, K.; Pipinos, I.I.; Powers, R.; Yu, F.; et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab. 2014, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.; Trujillo, A.; Ma, X.; Beierle, E.A. Ketone bodies inhibit the viability of human neuroblastoma cells. J. Pediatr. Surg. 2009, 44, 212–216, 212–216; discussion 216. [Google Scholar] [CrossRef] [PubMed]
- Suriyasathaporn, W.; Daemen, A.J.; Noordhuizen-Stassen, E.N.; Dieleman, S.J.; Nielen, M.; Schukken, Y.H. Beta-hydroxybutyrate levels in peripheral blood and ketone bodies supplemented in culture media affect the in vitro chemotaxis of bovine leukocytes. Vet. Immunol. Immunopathol. 1999, 68, 177–186. [Google Scholar] [CrossRef]
- Shang, S.; Wang, L.; Zhang, Y.; Lu, H.; Lu, X. The Beta-Hydroxybutyrate Suppresses the Migration of Glioma Cells by Inhibition of NLRP3 Inflammasome. Cell Mol. Neurobiol. 2018, 38, 1479–1489. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, S.; Xu, C.; Li, L.; Zhang, Y.; Guo, Y.; Zhang, C.; Li, P.; Long, M.; He, J. Proanthocyanidins Protect against beta-Hydroxybutyrate-Induced Oxidative Damage in Bovine Endometrial Cells. Molecules 2019, 24, 400. [Google Scholar] [CrossRef]
- Guh, J.Y.; Chuang, T.D.; Chen, H.C.; Hung, W.C.; Lai, Y.H.; Shin, S.J.; Chuang, L.Y. Beta-hydroxybutyrate-induced growth inhibition and collagen production in HK-2 cells are dependent on TGF-beta and Smad3. Kidney Int. 2003, 64, 2041–2051. [Google Scholar] [CrossRef]
- Klement, R.J.; Kammerer, U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr. Metab. 2011, 8, 75. [Google Scholar] [CrossRef]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Takahashi, K.; Sawasaki, Y.; Hata, J.; Mukai, K.; Goto, T. Spontaneous transformation and immortalization of human endothelial cells. Vitr. Cell. Dev. Biol. 1990, 26, 265–274. [Google Scholar] [CrossRef]
- Rubin, A.L.; Yao, A.; Rubin, H. Relation of spontaneous transformation in cell culture to adaptive growth and clonal heterogeneity. Proc. Natl. Acad. Sci. USA 1990, 87, 482–486. [Google Scholar] [CrossRef]
- Walen, K.H. Spontaneous cell transformation: Karyoplasts derived from multinucleated cells produce new cell growth in senescent human epithelial cell cultures. Vitr. Cell. Dev. Biol. Anim. 2004, 40, 150–158. [Google Scholar] [CrossRef]
- Qin, X.; Yang, S.; Zhang, Y.; Li, L.; Li, P.; Long, M.; Guo, Y. Effects of non-esterified fatty acids on relative abundance of prostaglandin E(2) and F(2alpha) synthesis-related mRNA transcripts and protein in endometrial cells of cattle in vitro. Anim. Reprod. Sci. 2020, 221, 106549. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.; Wang, X.; Liu, L.; Huang, D.; Zhang, R.; Guo, L.; Wang, Z.; Li, X.; Liu, G.; et al. High levels of acetoacetate and glucose increase expression of cytokines in bovine hepatocytes, through activation of the NF-kappaB signalling pathway. J. Dairy. Res. 2016, 83, 51–57. [Google Scholar] [CrossRef]
- Song, Y.; Li, N.; Gu, J.; Fu, S.; Peng, Z.; Zhao, C.; Zhang, Y.; Li, X.; Wang, Z.; Li, X.; et al. beta-Hydroxybutyrate induces bovine hepatocyte apoptosis via an ROS-p38 signaling pathway. J. Dairy. Sci. 2016, 99, 9184–9198. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Xiong, J.; Goeddel, D.V. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 1995, 81, 495–504. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Dingman, A.L.; Palmer, T.D. Placental TNF-alpha signaling in illness-induced complications of pregnancy. Am. J. Pathol. 2011, 178, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, D.W.; Adams, K.M.; Gravett, M.G.; Witkin, S.S.; Novy, M.J. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am. J. Obs. Gynecol. 2006, 195, 1578–1589. [Google Scholar] [CrossRef]
- Thaxton, J.E.; Nevers, T.; Lippe, E.O.; Blois, S.M.; Saito, S.; Sharma, S. NKG2D blockade inhibits poly(I:C)-triggered fetal loss in wild type but not in IL-10-/- mice. J. Immunol. 2013, 190, 3639–3647. [Google Scholar] [CrossRef]
- Jackson, L.R.; Farin, C.E.; Whisnant, S. Tumor necrosis factor alpha inhibits in vitro bovine embryo development through a prostaglandin mediated mechanism. J. Anim. Sci. Biotechnol. 2012, 3, 7. [Google Scholar] [CrossRef]
- Skarzynski, D.J.; Woclawek-Potocka, I.; Korzekwa, A.; Bah, M.M.; Piotrowska, K.; Barszczewska, B.; Okuda, K. Infusion of exogenous tumor necrosis factor dose dependently alters the length of the luteal phase in cattle: Differential responses to treatment with indomethacin and L-NAME, a nitric oxide synthase inhibitor. Biol. Reprod. 2007, 76, 619–627. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Mathew, D.J.; Passaro, C.; Fair, T.; Lonergan, P. Embryonic maternal interaction in cattle and its relationship with fertility. Reprod. Domest. Anim. 2018, 53 (Suppl. S2), 20–27. [Google Scholar] [CrossRef]
- Skarzynski, D.J.; Miyamoto, Y.; Okuda, K. Production of prostaglandin f(2alpha) by cultured bovine endometrial cells in response to tumor necrosis factor alpha: Cell type specificity and intracellular mechanisms. Biol. Reprod. 2000, 62, 1116–1120. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Skarzynski, D.J.; Okuda, K. Is tumor necrosis factor alpha a trigger for the initiation of endometrial prostaglandin F(2alpha) release at luteolysis in cattle? Biol. Reprod. 2000, 62, 1109–1115. [Google Scholar] [CrossRef]
- Tanabe, T.; Tohnai, N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002, 68–69, 95–114. [Google Scholar] [CrossRef]
- Wathes, D.C.; Abayasekara, D.R.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef]
- Cheng, Z.; Wylie, A.; Ferris, C.; Ingvartsen, K.L.; Wathes, D.C.; Gplus, E.C. Effect of diet and nonesterified fatty acid levels on global transcriptomic profiles in circulating peripheral blood mononuclear cells in early lactation dairy cows. J. Dairy. Sci. 2021, 104, 10059–10075. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E298–E306. [Google Scholar] [CrossRef]



| Master Mix | Mix 1 in µL | Mix 2 in µL | Mix 3 in µL |
|---|---|---|---|
| SYBR Green | 10 | 10 | 10 |
| Nuclease-free H2O | 4 | 3 | 0 |
| Primer forward | 1.5 | 1.5 | 1.5 |
| Primer reverse | 0.5 | 1.5 | 4.5 |
| cDNA (5 ng/µL) | 4 | 4 | 4 |
| Total | 20 | 20 | 20 |
| Gene | Sequence (5′-3′) Forward Reverse | Mix | Product Length | Accession No. | References |
|---|---|---|---|---|---|
| ACTB | GATCAAGATCATCGCGCCCC ACAGTCCGCCTAGAAGCATT | 1 | 160 bp | NM_173979.3 | [56] |
| GAPDH | CAACATCAAGTGGGGTGATG GGCATTGCTGACAATCTTGA | 2 | 202 bp | NM_001034034.2 | [57] |
| TNF | GGTTCAAACACTCAGGTCCTCT CGGAGAGTTGATGTCGGCTA | 2 | 79 bp | NM_173966.3 | [56] |
| IL6 | AAGCGCATGGTCGACAAAAT AAGCAAATCGCCTGATTGAACC | 3 | 164 bp | NM_173923.2 | [56] |
| PTGES2 | CTATCTGGTGTCAGGGCAACC GGTGTACCAACCAGTCGTCC | 2 | 212 bp | NM_001166554.1 | PrimerBlast (NCBI) |
| PTGER2 | CCTTGCCTTTCACGATTTTTGC CTCAGGATGGCAAAGACCCA | 3 | 127 bp | NM_174588.2 | PrimerBlast (NCBI) |
| ITGA6 | TGCCACATATCACAAGGCTGA CTTACAGCGTGGTATCGGGG | 2 | 151 bp | NM_001109981.2 | PrimerBlast (NCBI) |
| ITGAV | AGCGCGTCTTCGATGTTTC TGTTGCCTGTGGCATCAAAC | 3 | 145 bp | NM_174367.1 | PrimerBlast (NCBI) |
| ITGB3 | GAAGCAGAGTGTGTCACGGA ATGGGTCTTGGCATCAGTGG | 2 | 142 bp | NM_001206490 | PrimerBlast (NCBI) |
| ITGB1 | TAGAGACTCCAGAGTGCCCC CCGTGTCCCATTTGGCATTC | 3 | 180 bp | NM_174368.3 | PrimerBlast (NCBI) |
| RELA (NF-kb p65) | TTTCAATGGACCCACCGACC TGATGGTGCTGAGAGATGGC | 1 | 125 bp | NM_001080242.2 | PrimerBlast (NCBI) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hildebrand, C.; Hollenbach, J.; Seeger, B.; Pfarrer, C. β-Hydroxybutyrate Effects on Bovine Caruncular Epithelial Cells: A Model for Investigating the Peri-Implantation Period Disruption in Ketotic Dairy Cows. Animals 2023, 13, 2950. https://doi.org/10.3390/ani13182950
Hildebrand C, Hollenbach J, Seeger B, Pfarrer C. β-Hydroxybutyrate Effects on Bovine Caruncular Epithelial Cells: A Model for Investigating the Peri-Implantation Period Disruption in Ketotic Dairy Cows. Animals. 2023; 13(18):2950. https://doi.org/10.3390/ani13182950
Chicago/Turabian StyleHildebrand, Carolin, Julia Hollenbach, Bettina Seeger, and Christiane Pfarrer. 2023. "β-Hydroxybutyrate Effects on Bovine Caruncular Epithelial Cells: A Model for Investigating the Peri-Implantation Period Disruption in Ketotic Dairy Cows" Animals 13, no. 18: 2950. https://doi.org/10.3390/ani13182950
APA StyleHildebrand, C., Hollenbach, J., Seeger, B., & Pfarrer, C. (2023). β-Hydroxybutyrate Effects on Bovine Caruncular Epithelial Cells: A Model for Investigating the Peri-Implantation Period Disruption in Ketotic Dairy Cows. Animals, 13(18), 2950. https://doi.org/10.3390/ani13182950







