Growth Rate Distribution and Potential Non-Linear Relationship between Body Weight and Walking Ability in Turkeys
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggrey, S.E.; González-Cerón, F.; Rekaya, R. Genes Associated with Functional Traits in Poultry: Implications for Sustainable Genetic Improvement. In Achieving Sustainable Production of Poultry Meat; Breeding and Nutrition; Applegate, T., Dodd, B., Eds.; Science Publishing Ltd.: Cambridge, UK, 2017; Volume 2, pp. 4–24. [Google Scholar]
- Bradshaw, R.; Kirkden, R.; Broom, D. A Review of the Aetiology and Pathology of Leg Weakness in Broilers in Relation to Welfare. Avian Poult. Biol. Rev. 2002, 13, 45–103. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Józefiak, D.; Światkiewicz, S. Infectious and non-infectious factors associated with leg disorders in poultry—A review. Ann. Anim. Sci. 2017, 17, 645–669. [Google Scholar] [CrossRef]
- Santos, M.N.; Widowski, T.M.; Kiarie, E.G.; Guerin, M.T.; Edwards, A.M.; Torrey, S. In pursuit of a better broiler: Walking ability and incidence of contact dermatitis in conventional and slower growing strains of broiler chickens. Poult. Sci. 2022, 101, 101768. [Google Scholar] [CrossRef]
- Edwards, H.M.; Veltmann, J.R. The role of Calcium and phosphorus in the etiology of tibial dyschondroplasia in young chicks. J. Nutr. 1983, 113, 1568–1575. [Google Scholar] [CrossRef]
- Shim, M.Y.; Karnuah, A.B.; Anthony, N.B.; Pesti, G.M.; Aggrey, S.E. The effects of broiler chicken growth rate on valgus, varus, and tibial dyschondroplasia. Poult. Sci. 2012, 91, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Vasdal, G.; Moe, R.O.; de Jong, I.C.; Granquist, E.G. The relationship between measures of fear of humans and lameness in broiler chicken flocks. Animal 2018, 12, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Rayner, A.C.; Newberry, R.C.; Vas, J.; Mullan, S. Slow-growing broilers are healthier and express more behavioural indicators of positive welfare. Sci. Rep. 2020, 10, 15151. [Google Scholar] [CrossRef]
- Quinton, C.; Wood, B.; Miller, S. Genetic analysis of survival and fitness in turkeys with multiple-trait animal models. Poult. Sci. 2011, 90, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Havenstein, G.B.; Nestor, K.E.; Toelle, V.D.; Bacon, W.L. Estimates of genetic parameters in Turkeys. Poult. Sci. 1988, 67, 1378–1387. [Google Scholar] [CrossRef]
- Nestor, K.E.; Anderson, J.W.; Velleman, S.G. Genetic variation in pure lines and crosses of large-bodied turkey lines. 1. body weight, walking ability, and body measurements of live birds. Poult. Sci. 2001, 80, 1087–1092. [Google Scholar] [CrossRef]
- Updike, M.; Zerby, H.; Sawdy, J.; Lilburn, M.; Kaletunc, G.; Wick, M. Turkey breast meat functionality differences among turkeys selected for body weight and/or breast yield. Meat Sci. 2005, 71, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, H.M.; Leishman, E.M.; Vanderhout, R.J.; Adams, S.M.; Mohr, J.; Wood, B.J.; Baes, C.F.; Barbut, S. Describing the relationships among meat quality traits in domestic turkey (Meleagris gallopavo) populations. Poult. Sci. 2022, 101, 102055. [Google Scholar] [CrossRef] [PubMed]
- Kapell, D.N.R.G.; Hocking, P.M.; Glover, P.K.; Kremer, V.D.; Avendano, S. Genetic basis of leg health and its re-lationship with body weight in purebred turkey lines. Poult. Sci. 2017, 96, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Leishman, E.M.; Vanderhout, R.J.; Abdalla, E.A.; van Staaveren, N.; Naim, A.; Barbut, S.; Wood, B.J.; Harlander-Matauschek, A.; Baes, C.F. Genetic parameters of feather corticosterone and fault bars and correlations with production traits in turkeys (Meleagris gallopavo). Sci. Rep. 2023, 13, 38. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Rekaya, R.; Sapp, R.; Wing, T.; Aggrey, S. Genetic evaluation for growth, body composition, feed efficiency, and leg soundness. Poult. Sci. 2013, 92, 923–929. [Google Scholar] [CrossRef]
- González-Cerón, F.; Rekaya, R.; Anthony, N.B.; Aggrey, S.E. Genetic analysis of leg problems and growth in a random mating broiler population. Poult. Sci. 2015, 94, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.J.; Grimes, J.L.; Oviedo-Rondón, E.O.; Barasch, I.; Evans, C.; Dalmagro, M.; Nixon, J. Footpad dermatitis severity on turkey flocks and correlations with locomotion, litter conditions, and body weight at market age. J. Appl. Poult. Res. 2014, 23, 268–279. [Google Scholar] [CrossRef]
- Dong, Y.; Fraley, G.S.; Siegford, J.M.; Zhu, F.; Erasmus, M.A. Comparing different environmental enrichments for improving the welfare and walking ability of male turkeys. PLoS ONE 2023, 18, e0285347. [Google Scholar] [CrossRef]
- Rasmussen, S.N.; Erasmus, M.; Riber, A.B. The relationships between age, fear responses, and walking ability of broiler chickens. Appl. Anim. Behav. Sci. 2022, 254, 105713. [Google Scholar] [CrossRef]
- Taskin, A.; Karadavut, U.; Çayan, H. Behavioural responses of white and bronze turkeys (Meleagris gallopavo) to tonic immobility, gait score and open field tests in free-range system. J. Appl. Anim. Res. 2018, 18, 1253–1259. [Google Scholar] [CrossRef]
- Emmerson, D.A.; Anthony, N.B.; Nestor, K.E.; Saif, Y.M. Genetic association of selection for increased leg muscle and increased shank diameter with body composition and walking ability. Poult. Sci. 1991, 70, 739–745. [Google Scholar] [CrossRef]
- Ye, X.; Anderson, J.W.; Noble, D.O.; Zhu, J.; Nestor, K.E. Influence of crossing a line selected for increased shank width and a commercial sire line on performance and walking ability of turkeys. Poult. Sci. 1997, 76, 1327–1331. [Google Scholar] [CrossRef]
- Soyalp, S.; Hartono, E.; Willems, O.W.; Wood, B.J.; Aggrey, S.E.; Rekaya, R. Research Note: Analysis of body weight and walking ability in turkeys and the prediction of categorical responses across systematic effect classes using a linear threshold model. Poult. Sci. 2023, 102, 102993. [Google Scholar] [CrossRef]
- Nestor, K.E.; Anderson, J.W. Effect of crossing a line selected for increased shank width with two commercial sire lines on performance and walking ability of turkeys. Poult. Sci. 1998, 77, 1601–1607. [Google Scholar] [CrossRef][Green Version]
- Aslam, M.L.; Bastiaansen, J.W.; Crooijmans, R.P.; Ducro, B.J.; Vereijken, A.; Groenen, M.A. Genetic variances, heritabilities and maternal effects on body weight, breast meat yield, meat quality traits and the shape of the growth curve in turkey birds. BMC Genet. 2011, 12, 14. [Google Scholar] [CrossRef]
- Abdalla, E.E.A.; Schenkel, F.S.; Begli, H.E.; Willems, O.W.; van As, P.; Vanderhout, R.; Wood, B.J.; Baes, C.F. Single-Step Methodology for Genomic Evaluation in Turkeys (Meleagris gallopavo). Front. Genet. 2019, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Buchner, D.M.; Larson, E.B.; Wagner, E.H.; Koepsell, T.D.; DE Lateur, B.J. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing 1996, 25, 386–391. [Google Scholar] [CrossRef]
- Weeks, C.; Danbury, T.; Davies, H.; Hunt, P.; Kestin, S. The behaviour of broiler chickens and its modification by lameness. Appl. Anim. Behav. Sci. 2000, 67, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Kestin, S.C.; Gordon, S.; Su, G.; Sørensen, P. Relationships in broiler chickens between lameness, liveweight, growth rate and age. Vet. Rec. 2001, 148, 195–197. [Google Scholar] [CrossRef]
- Bassler, A.W.; Arnould, C.; Butterworth, A.; Colin, L.; de Jong, I.C.; Ferrante, V.; Ferrari, P.; Haslam, S.; Wemelsfelder, F.; Blokhuis, H.J. Potential risk factors associated with contact dermatitis, lameness, negative emotional state, and fear of humans in broiler chicken flocks. Poult. Sci. 2013, 92, 2811–2826. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.; Su, G.; Kestin, S.C. Effects of Age and Stocking Density on Leg Weakness in Broiler Chickens. Poult. Sci. 2000, 79, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Sanotra, G.S.; Berg, C.; Lund, J.D. A Comparison Between Leg Problems in Danish and Swedish Broiler Production. Anim. Welf. 2003, 12, 677–683. [Google Scholar] [CrossRef]
- Thomas, D.; Ravindran, V.; Thomas, D.; Camden, B.; Cottam, Y.; Morel, P.; Cook, C. Influence of stocking density on the performance, carcass characteristics and selected welfare indicators of broiler chickens. N. Z. Vet. J. 2004, 52, 76–81. [Google Scholar] [CrossRef] [PubMed]

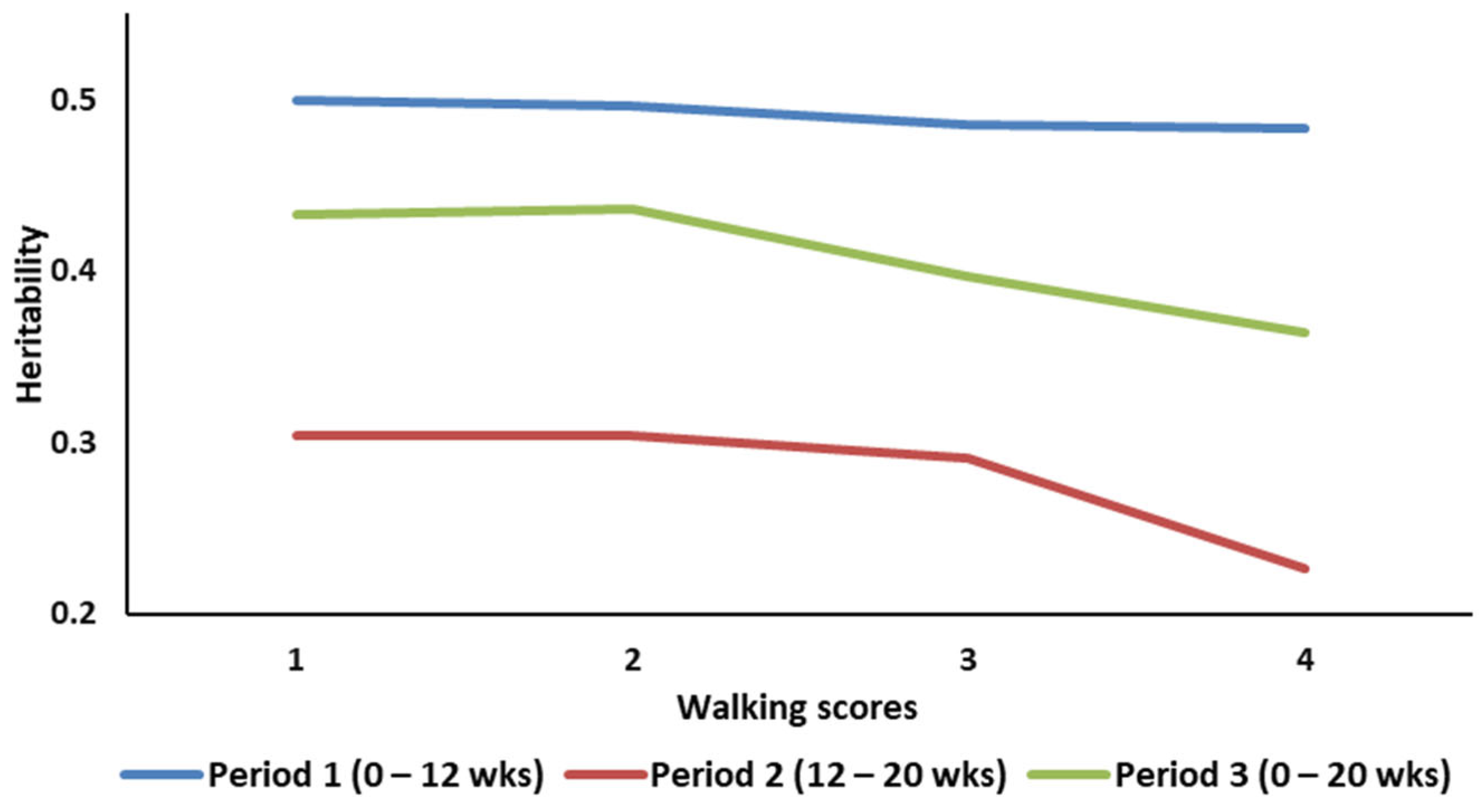

| Quartile | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Period 1 (0 to 12 weeks) | |||||
| 1 | 18,690 | 0.080 | 0.003 | 0.036 | 0.085 |
| 2 | 18,820 | 0.087 | 0.001 | 0.085 | 0.090 |
| 3 | 18,802 | 0.092 | 0.001 | 0.090 | 0.095 |
| 4 | 18,782 | 0.099 | 0.003 | 0.095 | 0.134 |
| Period 2 (12 to 20 weeks) | |||||
| 1 | 18,714 | 0.131 | 0.011 | 0.034 | 0.145 |
| 2 | 18,662 | 0.152 | 0.004 | 0.145 | 0.158 |
| 3 | 18,886 | 0.164 | 0.003 | 0.158 | 0.170 |
| 4 | 18,832 | 0.181 | 0.009 | 0.170 | 0.550 |
| Period 3 (0 to 20 weeks) | |||||
| 1 | 18,734 | 0.102 | 0.004 | 0.075 | 0.107 |
| 2 | 18,807 | 0.110 | 0.0017 | 0.107 | 0.113 |
| 3 | 18,770 | 0.116 | 0.0016 | 0.113 | 0.119 |
| 4 | 18,783 | 0.124 | 0.004 | 0.119 | 0.228 |
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | |
|---|---|---|---|---|
| Period 1 (0 to 12 weeks) | ||||
| CLASS 1 | 32.4 | 35.2 | 37.5 | 42.6 |
| CLASS 2 | 41.2 | 41.7 | 41.8 | 41.0 |
| CLASS 3 | 23.3 | 20.5 | 18.6 | 14.9 |
| CLASS 4 | 2.9 | 2.5 | 1.9 | 1.3 |
| Period 2 (12 to 20 weeks) | ||||
| CLASS 1 | 34.9 | 35.4 | 36.2 | 41.1 |
| CLASS 2 | 39.1 | 41.3 | 43.0 | 42.3 |
| CLASS 3 | 22.7 | 20.8 | 18.7 | 15.2 |
| CLASS 4 | 3.2 | 2.3 | 1.9 | 1.1 |
| Period 3 (0 to 20 weeks) | ||||
| CLASS 1 | 32.6 | 34.2 | 37.3 | 43.5 |
| CLASS 2 | 39.3 | 42.0 | 42.6 | 41.8 |
| CLASS 3 | 24.3 | 21.3 | 18.3 | 13.4 |
| CLASS 4 | 3.6 | 2.3 | 1.5 | 1.0 |
| Growth Quartile | ||||
|---|---|---|---|---|
| Period | 1 | 2 | 3 | 4 |
| 1 | 0.537 | 0.502 | 0.486 | 0.439 |
| 2 | 0.558 | 0.503 | 0.483 | 0.439 |
| 3 | 0.578 | 0.508 | 0.475 | 0.419 |
| Period 1 | Period 2 | Period 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait 2 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| 1 | 0.18 | 0.89 | 0.87 | 0.84 | 0.19 | 0.91 | 0.90 | 0.88 | 0.22 | 0.90 | 0.88 | 0.86 |
| 2 | 0.20 | 0.94 | 0.86 | 0.22 | 0.93 | 0.91 | 0.22 | 0.91 | 0.89 | |||
| 3 | 0.19 | 0.94 | 0.19 | 0.94 | 0.26 | 0.94 | ||||||
| 4 | 0.22 | 0.20 | 0.26 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soyalp, S.; Hartono, E.; Willems, O.W.; Bai, X.; Wood, B.J.; Aggrey, S.E.; Rekaya, R. Growth Rate Distribution and Potential Non-Linear Relationship between Body Weight and Walking Ability in Turkeys. Animals 2023, 13, 2979. https://doi.org/10.3390/ani13182979
Soyalp S, Hartono E, Willems OW, Bai X, Wood BJ, Aggrey SE, Rekaya R. Growth Rate Distribution and Potential Non-Linear Relationship between Body Weight and Walking Ability in Turkeys. Animals. 2023; 13(18):2979. https://doi.org/10.3390/ani13182979
Chicago/Turabian StyleSoyalp, Samet, Evan Hartono, Owen W. Willems, Xuechun Bai, Benjamin J. Wood, Samuel E. Aggrey, and Romdhane Rekaya. 2023. "Growth Rate Distribution and Potential Non-Linear Relationship between Body Weight and Walking Ability in Turkeys" Animals 13, no. 18: 2979. https://doi.org/10.3390/ani13182979
APA StyleSoyalp, S., Hartono, E., Willems, O. W., Bai, X., Wood, B. J., Aggrey, S. E., & Rekaya, R. (2023). Growth Rate Distribution and Potential Non-Linear Relationship between Body Weight and Walking Ability in Turkeys. Animals, 13(18), 2979. https://doi.org/10.3390/ani13182979





