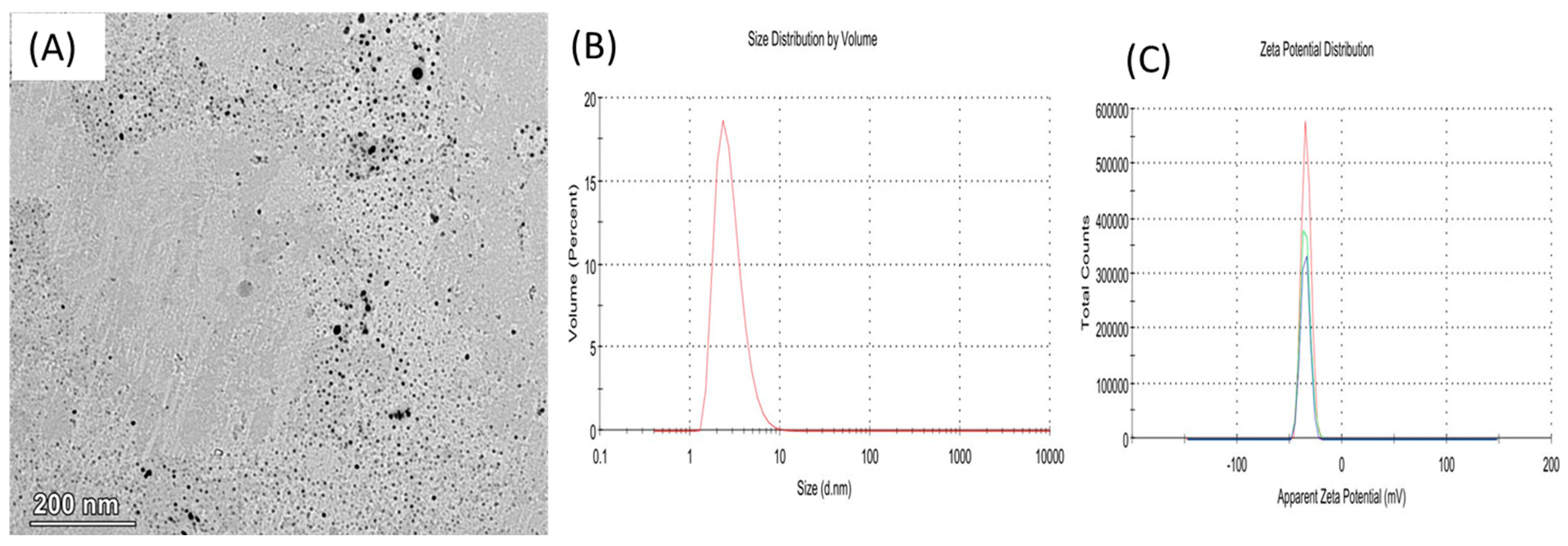
1. Introduction
Heat stress (HS) is a condition where rabbits cannot balance their heat production and emissions [
1]. During the summer in Egypt, the elevated ambient temperature can easily induce HS in rabbits, which causes a series of unfavorable impacts on rabbit productivity [
2]. As is known, HS has multiple adverse impacts on rabbits’ health statuses and their production performance. It has been revealed that HS is responsible for a 9–12% increase in mortality rate, 20–25% reductions in average daily weight gain, and 8–15% decreases in feed conversion ratio, as well as negatively affecting the carcass traits and quality of meat in growing rabbits [
3,
4]. Thus, HS poses a significant challenge for the rabbit industry, made worse by recent global warming [
1]. Several previous studies have investigated prospective mitigation strategies, and nutritional manipulation has proven to be a dynamic mitigation approach [
4,
5]. The dietary supplementation of animal diets with herbs or spices and their derivatives has provided appropriate evidence as a potential alternative to antibiotics for alleviating the negative effects of HS during summer. These products have gained acceptability among consumers as natural feed additives [
6,
7,
8]. Previous studies have revealed significant effects of adding medical herbs and spices (phytogenic additives) to growing rabbits’ diets on growth indices and general health status [
9,
10]. Cardamom, as a common spice, is widely used for flavoring and culinary purposes worldwide. Cardamom consists of seeds of the dried fruits of Elettaria cardamomum. Moreover, cardamom fruit contains lipids, phytosterols, sterols, phenolic acids, and essential oils. Cardamom essential oil is rich in limonene, α-terpinyl acetate, α-pinene, myrcene, α-terpineol, sabinene, terpinolene, linalyl acetate, linalool, and phellandrene [
11]. Generally, multiple published reports have clarified that the cardamom essential oil (CEO) isolated from fruits has antispasmodic, anti-inflammatory, and antimicrobial activities [
12,
13]. The anti-inflammatory and antioxidant effects of cardamom essential oil have been confirmed in several investigations [
14,
15]. Despite these beneficial effects of cardamom essential oil, its low solubility, permeability, storage instability, and bioavailability have limited its use in some pharmaceutical uses. Nanoemulsions are a nanotechnology method for improving the previous features of essential oils. In this sense, recent studies have confirmed that converting phytochemicals into nano-form can endow them with several biological activities, making them more convenient for dealings under industrial conditions and more mobile in biological systems [
16,
17]. Based on the anti-inflammatory, anti-microbial, and antioxidant properties of cardamom essential oil, we theorized that the nano-form of cardamom essential oil (NCEO) could potentially reduce the adverse effects of HS in growing rabbits. Therefore, the current study aims to examine the effect of dietary NCEO supplementation on the growth indices, carcass traits, physiological responses, hemato-biochemical variables, redox balance, inflammatory responses, and immunity statuses of V-line growing rabbits exposed to a high ambient temperature.
4. Discussion
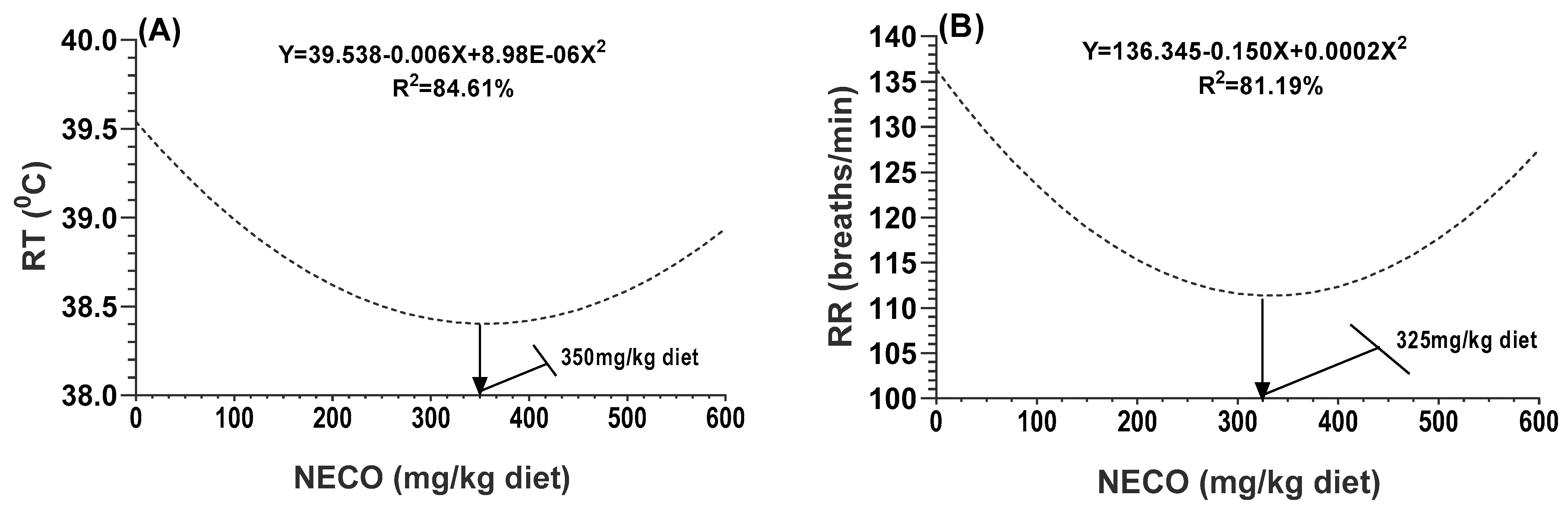
The profitable production of rabbit meat is negatively affected by heat stress (HS), as rabbits’ growth requires a convenient climate for the expression of the good-quality traits embedded in the genetic components of rabbits [
33,
34]. Rabbits have few numbers of sweat glands, mainly under their feet and on their lips. These sweat glands are not effective at regulating exceeded body temperature, particularly in hot conditions, which makes them more sensitive to HS, which has adverse effects on their welfare, adaptation, feed efficiency, growth performance, redox status, inflammatory response, and health status [
7,
35,
36], and also disrupts the fertility traits in rabbit does [
7] and bucks [
37]. The temperature–humidity index (THI) can be useful for assessing the risk of HS on rabbits induced by hot environments [
38]. Even though the values of the THI in this study indicated that the growing rabbits were subjected to severe HS conditions [
20], the NCEO-supplemented rabbits had a better capacity for heat tolerance, growth performance, redox balance, and health status. The fortification of the rabbit diets with NCEO resulted in a significant decrease in body temperature. This indicates that the bioactive components presented in NCEO, such as flavones and flavonoids, have thermoregulatory effects [
6,
17]. To achieve thermoregulation, there must be a balance between heat gain and heat loss in the animal body. Therefore, under severe HS conditions, rabbits begin to increase their panting rate (respiratory rate) to dissipate the heat load via the respiratory tract using evaporation, which explains the observed increment in breathing rate in the control group compared to the other treated groups. The thermoregulatory actions of some herbs have been confirmed in animals by Abd El-Hack et al. [
39], due to their robust antioxidant properties.
The above results indicate that the better growth performance of the NCEO-supplemented rabbits was achieved by enhancing the defeasibility of nutrients, which provide available energy for growth and heat dissipation from the body. In this study, the NCEO300 and NCEO600 groups had a higher FBW, ADG, SGR, and PI and a lower FCR than the control and other treated groups, which is in general agreement with the findings of former studies [
36,
40]. They reported that the dietary supplementation of rabbit diets with nano-emulsified essential oils enhanced their growth performance and feed utilization.
The improvement in growth performance (higher LBW and ADG) in the NCEO-supplemented rabbits may be attributed to the presence of some bioactive constituents (saponins, tannins, and flavonoids), which promote the digestion of nutrients and their absorption, like other essential oils, by increasing the secretion of digestive enzymes and destroying infectious bacteria [
39]. Another factor demonstrating the benefits of using NCEO was its stimulating effect on the animals’ digestive systems by increasing gastric acid secretion and representing a robust antimicrobial agent [
41,
42]. In the present study, the improvement in feed utilization (lower FCR) was triggered by larger increases in FBW rather than FI, which suggested that the NCEO-supplemented rabbits could more worthily utilize the diet supplemented with the NCEO [
43]. In parallel, Omidi et al. [
13] suggested that adding 50 mg of cardamom essential oils/kg diet can improve the ADG and FCR of broilers during the grower period (11–28 days).
Hematological attributes are considered to be good indicators for rabbits’ general health status, immune capacity, infectious diseases, and/or other environmental issues such as HS. High temperatures can disrupt the hematological variables, particularly the leucocyte and erythrocyte counts, making animals more sensitive to infection or disease. In this sense, Hashem et al. [
44] clarified that HS can lessen the leucocyte count levels, resulting in immune dysfunction through the altitude of WBC counts in the blood of rabbits. The addition of NCEO led to a significant increase in erythrocyte count, including PLT and RBC counts, as well as the concentration of HGB. Moreover, the dietary NCEO supplementation significantly decreased the WBC count, being in the normal range that reflected an improved health status of the growing rabbits exposed to a high ambient temperature.
In the current study, the relative weight of the liver was significantly higher in both the NECO300 and NECO600 groups than that in the control and NECO150 groups, reflecting the improved health status of the growing rabbits, as the liver has a crucial role in the synthesis of the enzymes related to heat tolerance, as well as blood protein synthesis [
7,
10,
33]. In this context, despite the rapid metabolization of essential oils, they can damage the liver and thus increase the blood content of their enzymes [
45]. Still, the lower serum contents from the liver enzymes (AST and ALT) and decreased urea and creatinine concentrations in our study showed that the administration of NCEO oil was safe and helpful for expressing liver and renal functions. In correspondence with this study, Traesel et al. [
46] reported that the extended use of high concentrations of essential oils could not cause renal and/or nephritis failure.
It is well-known that blood biochemical parameters can reflect the physiological responses of rabbits to their external and internal environments. Even though the TP of serum and its fraction usually decrease during HS [
20], in this study, there was a significant increase in the blood TP and albumin of the NCEO-supplemented rabbits, reflecting the good nutritional status of the treated groups. Concerning the blood glucose concentration, it increased markedly with a high ambient temperature [
47], and this may have been due to the depression of feed intake and subsequent reduction in metabolic rate [
37,
48]. In addition, Kiwull-Schöne et al. [
49] demonstrated that increased blood glucose levels were directly correlated with HS inducing the release of glucocorticoids to the blood. Herein, the decreasing blood glucose concentrations shown in the NCEO300 group could be explained by the greater activity of blood insulin [
50]. Our data are in harmony with Khalid et al. [
50], who reported that adding natural phytochemicals to heat-stressed growing rabbits’ diets significantly decreased the blood serum glucose.
Regarding the lipid profile, previous investigations have revealed favorable effects of CEO on cholesterol metabolism [
13]. There is a lack of research investigating CEO’s impacts on rabbits’ lipid profiles. Still, in parallel, the dietary supplementation of broilers’ diets with spice additives has been observed to have a hypocholesterolemic effect, as there were significant decreases in the concentrations of triglycerides, cholesterol, and low-density lipoproteins. Meanwhile, there was a significant increase in the concentration of high-density lipoprotein. Our results revealed that the dietary addition of 300 or 600 mg of NCEO/kg diet showed a potent biological effect on decreasing the concentrations of serum triglycerides and cholesterol compared to those in the untreated group. The present results may be explained by the fact that spice additives can regulate the production of cholesterol and exert a hypocholesterolemic effect throughout the inhibition of the 3-hydroxy-3-methyl-glutaryl-CoA reductase [
51].
It is worth noting that there was overproduction from reactive oxygen species (ROS) under HS conditions, which induced oxidative stress and impaired the function and structure of important molecules such as proteins and nucleic acids [
52]. The production of ROS is balanced by the antioxidant defense system, comprising enzymes such as SOD and GSH. Several former scientific researchers have observed that a high ambient temperature ordinarily increases oxidative stress by increasing lipid oxidation and/or proteins (protein carbonyl) and disrupting antioxidant enzyme production [
53,
54]. In the present study, the dietary administration of NCEO improved the indices of anti-oxidative stress; the NCEO300 group showed higher concentrations of SOD and GSH, and lower concentrations of MDA and PC, than those in the control. Moreover, the TAC increased by 67.65 and 102.67% in the NECO300 and NECO600 groups compared to that in the control. The present study agreed with numerous previous studies that deduced that essential oils could alleviate the negative effects of HS and achieve oxidative stability by inhibiting the diffusion of oxidation reactions and effectually deferring the oxidation of lipids and/or proteins and other nutrients [
7,
55].
The upper levels of pro-inflammatory cytokines, such as IFN-γ and IL-4, are induced in the blood serum along with an elevated ambient temperature, increasing the intestinal permeability to pathogens [
39]. Our study exhibited noteworthy decreases in the concentrations of pro-inflammatory cytokines (IFN-γ and IL-4) in the NCEO-supplemented rabbits compared to the control, indicating the potent anti-inflammatory activities of NCEO. On the other hand, the dietary treatment resulted in a significant increase in the concentration of NO, which is a small molecule that plays an indispensable role under physiological concentrations in defense against pathogens and thermos-tolerance during heat stress, as it has a decisive role in the vasodilation of the blood vessels of the skin [
48,
56,
57]. Moreover, mounting evidence suggests that NO plays other roles in neurotransmission and immune regulation [
7,
57]. The anti-inflammatory effect of NCEO is attributed to the presence of 1,8-cineole and α-terpinyl acetate [
14]. It was interesting to obtain an increased concentration of NO in the blood serum of the NCEO-supplemented rabbits, which is in line with the increased concentrations of the antioxidant indices (GSH, SOD, and TAC), indicating that the NO concentration was in the normal physiological range, as it is classified as a free radical as well.
Concerning general health status, HS is involved in shrinking newly weaned rabbits’ immune capacity, due to its ability to promote the synthesis of immunological variables such as IgM, IgA, and IgG [
36]. Further, the impairment of the immune response could make growing rabbits more sensitive to pathogens [
58,
59]. This immune imbalance caused by HS certainly delays the growth of growing rabbits [
10]. In the current study, the NCEO supplementation could promote the synthesis of immunological variables, including IgG and IgM, effectively overcoming the detrimental impacts of HS on newly weaned rabbits during summer. The affirmative effects of NCEO could be attributed to its antibacterial, antioxidant, and anti-inflammatory properties [
13]. These phytogenic additives can minimize bacteria colonization and hinder the growth of pathogenic and/or non-pathogenic strains of bacteria in the gut of rabbits [
42]; also, they can bring the ecosystems of microbes in the rabbit gut to equilibrium, leading to a better feed efficiency and metabolism [
52]. Studying the pathological alteration of the main organs in the body such as liver and kidney behind the blood chemistry may provide more insights into the potential of using NCEO for improving the functionality of renal/hepato cells in growing rabbits. As clarified in this research, NECO successfully re-established and recovered the near-normal renal–hepatic morphology induced by HS. In our previous work, we observed that some natural pigments (prodigiosin) enhanced the structure of the hepatic morphology in stressed growing rabbits [
58] by reducing the TNF-α in hepatic tissues. Recently, the renal-protective effect of cardamom essential oil was evidenced by [
15]. The potential of CNEO to sustain the renal histological profile could explain its protective action against HS. Transcriptomics and proteomics explorations should be performed during HS to provide insights into suitable strategies for mitigating HS in animals.