Evaluation of the Effect of Induced Endotoxemia on ROTEM S® and Platelet Parameters in Beagle Dogs Anaesthetized with Sevoflurane
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Standpoint
2.2. Animals
2.3. Preanaesthetic Preparation, Anaesthesia and Instrumentation
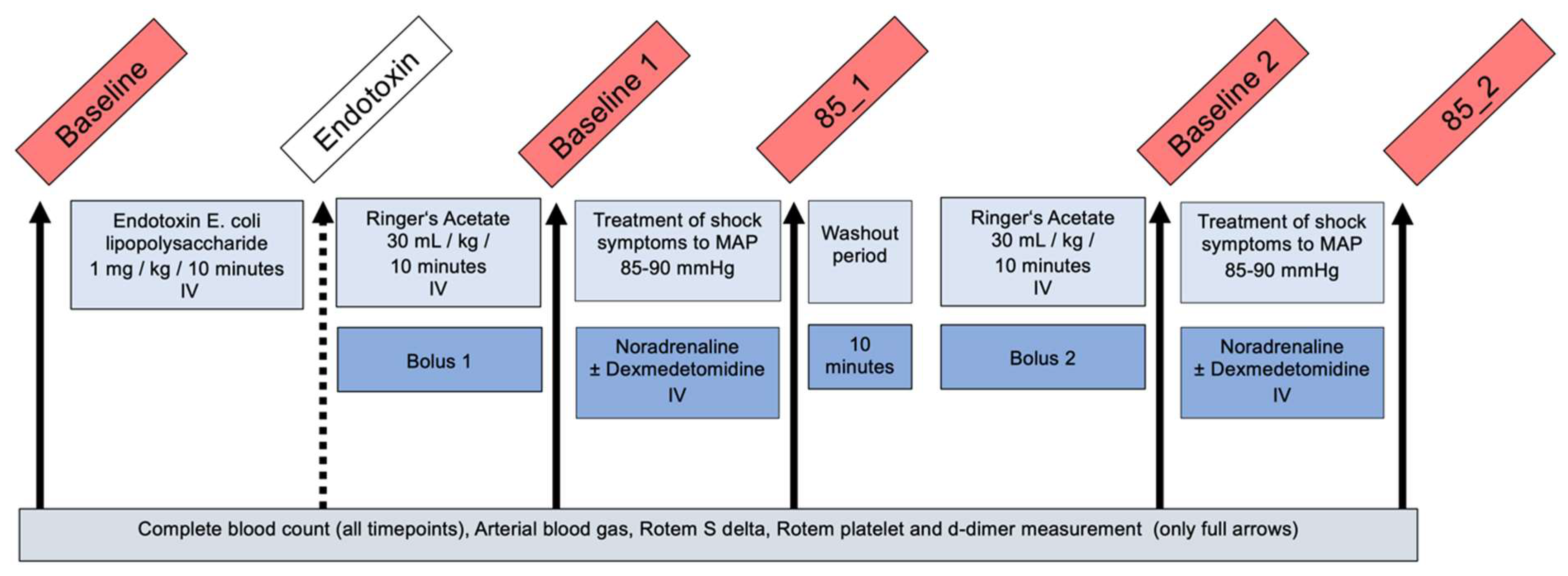
2.4. Experimental Design
2.5. Blood Collection and Analysis
2.6. Statistical Analysis
3. Results
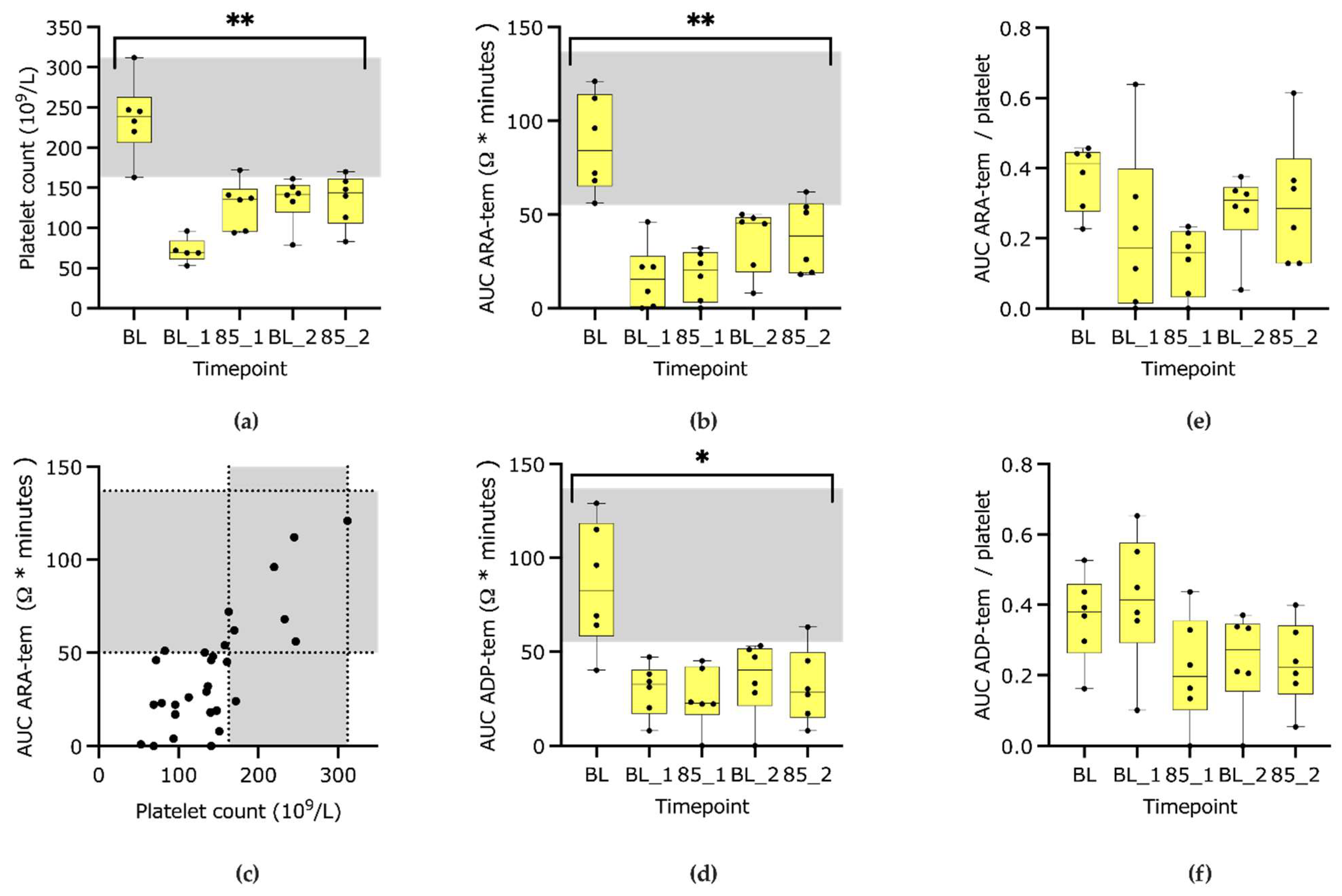
3.1. Platelet Count and Function
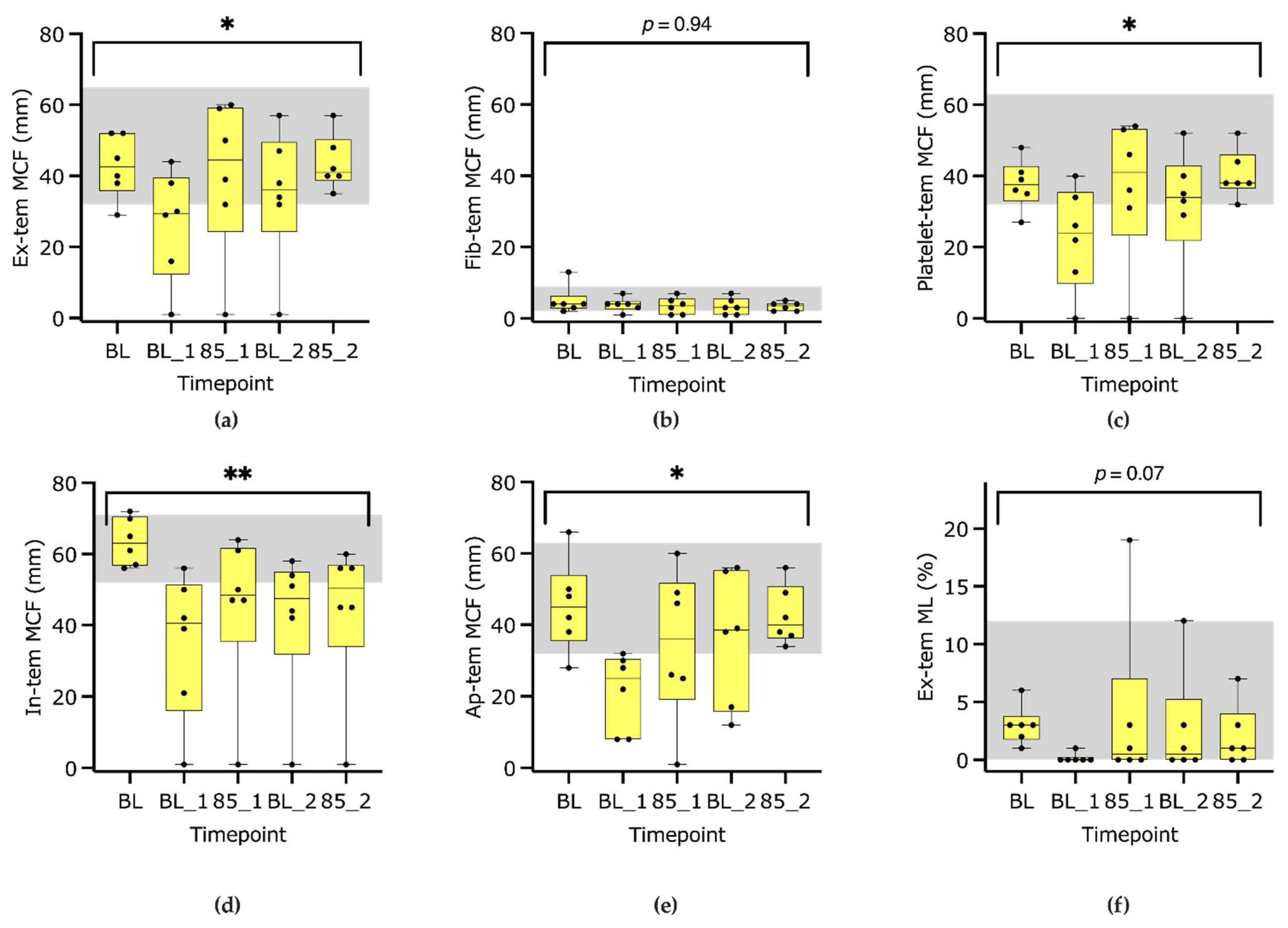
3.2. ROTEM S Parameters
3.3. D-Dimer
4. Discussion
4.1. Platelet Count and Function
4.2. ROTEM S Delta
4.3. D-Dimers and Maximum Lysis
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Duration from start of endotoxin to timepoint | ||||
| Timepoint | Median | Min | Max | |
| Baseline | −14 | −29 | −12 | |
| BL1 | 58 | 49 | 65 | |
| 85_1 | 165 | 136 | 197 | |
| BL2 | 228 | 184 | 255 | |
| 85_2 | 276 | 222 | 370 | |
| Resting time from collection to analysis of blood sample | ||||
| Parameter | Timepoint | Median | Min | Max |
| In-tem (minutes) | Baseline | 17 | 8 | 39 |
| BL1 | 23 | 8 | 28 | |
| 85_1 | 11 | 6 | 28 | |
| BL2 | 29 | 25 | 56 | |
| 85_2 | 41 | 25 | 93 | |
| Ex-tem (minutes) | Baseline | 18 | 7 | 39 |
| BL1 | 17 | 7 | 28 | |
| 85_1 | 11 | 5 | 23 | |
| BL2 | 28 | 23 | 58 | |
| 85_2 | 41 | 24 | 92 | |
| Fib-tem (minutes) | Baseline | 17 | 9 | 40 |
| BL1 | 18 | 8 | 29 | |
| 85_1 | 11 | 7 | 25 | |
| BL2 | 31 | 25 | 57 | |
| 85_2 | 42 | 26 | 96 | |
| Ap-tem (minutes) | Baseline | 18 | 9 | 41 |
| BL1 | 19 | 10 | 31 | |
| 85_1 | 13 | 8 | 26 | |
| BL2 | 32 | 26 | 58 | |
| 85_2 | 43 | 26 | 94 | |
| ARA-tem (minutes) | Baseline | 28 | 19 | 47 |
| BL1 | 21 | 16 | 32 | |
| 85_1 | 23 | 20 | 25 | |
| BL2 | 20 | 19 | 21 | |
| 85_2 | 21 | 19 | 23 | |
| ADP-tem (minutes) | Baseline | 33 | 20 | 60 |
| BL1 | 23 | 19 | 33 | |
| 85_1 | 24 | 21 | 42 | |
| BL2 | 22 | 21 | 46 | |
| 85_2 | 22 | 20 | 24 | |
| Test | Parameter | Timepoint | Median | Min | Max | Reference Interval |
|---|---|---|---|---|---|---|
| Ex-tem | CT (sec) | Baseline | 35 | 26 | 69 | 23–87 |
| BL1 | 53 | 23 | 3600 | |||
| 85_1 | 53 | 31 | 3600 | |||
| BL2 | 81 | 31 | 3600 | |||
| 85_2 | 65 | 33 | 77 | |||
| CFT (sec) | Baseline | 196 | 129 | 519 | 85–357 | |
| BL1 | 579 | 184 | 3600 | |||
| 85_1 | 175 | 78 | 3600 | |||
| BL2 | 308 | 103 | 3600 | |||
| 85_2 | 189 | 117 | 270 | |||
| A (°) | Baseline | 56 | 40 | 65 | 42–77 | |
| BL1 | 36 | 20 | 58 | |||
| 85_1 | 67 | 48 | 74 | |||
| BL2 | 58 | 50 | 69 | |||
| 85_2 | 61 | 55 | 67 | |||
| A10 (mm) | Baseline | 35 | 21 | 44 | 21–55 | |
| BL1 | 21 | 1 | 34 | |||
| 85_1 | 36 | 1 | 50 | |||
| BL2 | 27 | 1 | 46 | |||
| 85_2 | 33 | 28 | 46 | |||
| MCF (mm) | Baseline | 43 | 29 | 52 | 32–65 | |
| BL1 | 30 | 1 | 44 | |||
| 85_1 | 45 | 1 | 60 | |||
| BL2 | 36 | 1 | 57 | |||
| 85_2 | 41 | 35 | 57 | |||
| MCE (dynes/cm2) | Baseline | 75 | 41 | 110 | 45–142 | |
| BL1 | 43 | 1 | 79 | |||
| 85_1 | 77 | 1 | 142 | |||
| BL2 | 56 | 1 | 130 | |||
| 85_2 | 69 | 54 | 131 | |||
| G | Baseline | 3714 | 2037 | 5518 | 2253–5928 | |
| BL1 | 2110 | 51 | 3935 | |||
| 85_1 | 3838 | 51 | 7113 | |||
| BL2 | 2795 | 51 | 6504 | |||
| 85_2 | 3442 | 2677 | 6538 | |||
| ML (%) | Baseline | 3 | 1 | 6 | 0–12 | |
| BL1 | 0 | 0 | 1 | |||
| 85_1 | 1 | 0 | 19 | |||
| BL2 | 1 | 0 | 12 | |||
| 85_2 | 1 | 0 | 7 | |||
| In-tem | CT (sec) | Baseline | 178 | 150 | 228 | 133–210 |
| BL1 | 256 | 190 | 3600 | |||
| 85_1 | 252 | 187 | 3600 | |||
| BL2 | 239 | 97 | 3600 | |||
| 85_2 | 223 | 190 | 3600 | |||
| CFT (sec) | Baseline | 58 | 40 | 99 | 59–201 | |
| BL1 | 221 | 85 | 3600 | |||
| 85_1 | 169 | 63 | 3600 | |||
| BL2 | 159 | 78 | 3600 | |||
| 85_2 | 134 | 68 | 3600 | |||
| A (°) | Baseline | 79 | 71 | 82 | 58–78 | |
| BL1 | 65 | 19 | 73 | |||
| 85_1 | 73 | 66 | 77 | |||
| BL2 | 68 | 39 | 74 | |||
| 85_2 | 72 | 53 | 76 | |||
| A10 (mm) | Baseline | 55 | 44 | 66 | 35–61 | |
| BL1 | 30 | 1 | 46 | |||
| 85_1 | 35 | 1 | 55 | |||
| BL2 | 36 | 1 | 47 | |||
| 85_2 | 38 | 1 | 49 | |||
| MCF (mm) | Baseline | 63 | 56 | 72 | 52–71 | |
| BL1 | 41 | 1 | 56 | |||
| 85_1 | 49 | 1 | 64 | |||
| BL2 | 48 | 1 | 58 | |||
| 85_2 | 51 | 1 | 60 | |||
| MCE (dynes/cm2) | Baseline | 173 | 126 | 258 | 108–242 | |
| BL1 | 69 | 1 | 127 | |||
| 85_1 | 89 | 1 | 181 | |||
| BL2 | 91 | 1 | 138 | |||
| 85_2 | 105 | 1 | 150 | |||
| G | Baseline | 8657 | 6288 | 12,911 | 5417–12,119 | |
| BL1 | 3440 | 51 | 6348 | |||
| 85_1 | 4717 | 51 | 9028 | |||
| BL2 | 3929 | 51 | 5854 | |||
| 85_2 | 5247 | 51 | 7490 | |||
| ML (%) | Baseline | 0 | 0 | 1 | 0–3 | |
| BL1 | 0 | 0 | 1 | |||
| 85_1 | 0 | 0 | 0 | |||
| BL2 | 0 | 0 | 0 | |||
| 85_2 | 0 | 0 | 0 | |||
| Fib-tem | CT (sec) | Baseline | 69 | 22 | 442 | 21–112 |
| BL1 | 41 | 27 | 3600 | |||
| 85_1 | 158 | 28 | 3600 | |||
| BL2 | 275 | 32 | 3600 | |||
| 85_2 | 126 | 32 | 2134 | |||
| A10 (mm) | Baseline | 4 | 2 | 13 | 2–9 | |
| BL1 | 4 | 1 | 6 | |||
| 85_1 | 4 | 1 | 7 | |||
| BL2 | 3 | 1 | 6 | |||
| 85_2 | 3 | 2 | 5 | |||
| MCF (mm) | Baseline | 4 | 2 | 13 | 2–9 | |
| BL1 | 4 | 1 | 7 | |||
| 85_1 | 4 | 1 | 7 | |||
| BL2 | 3 | 1 | 7 | |||
| 85_2 | 4 | 2 | 5 | |||
| Ap-tem | CT (sec) | Baseline | 55 | 24 | 80 | 21–75 |
| BL1 | 51 | 19 | 510 | |||
| 85_1 | 64 | 31 | 3600 | |||
| BL2 | 76 | 50 | 111 | |||
| 85_2 | 59 | 27 | 103 | |||
| CFT (sec) | Baseline | 164 | 78 | 639 | 99–485 | |
| BL1 | 1003 | 454 | 3600 | |||
| 85_1 | 348 | 96 | 3600 | |||
| BL2 | 233 | 82 | 3600 | |||
| 85_2 | 197 | 108 | 277 | |||
| A (°) | Baseline | 63 | 63 | 82 | 42–79 | |
| BL1 | 38 | 38 | 73 | |||
| 85_1 | 63 | 63 | 72 | |||
| BL2 | 66 | 66 | 74 | |||
| 85_2 | 63 | 63 | 84 | |||
| A10 (mm) | Baseline | 37 | 19 | 59 | 23–52 | |
| BL1 | 17 | 5 | 22 | |||
| 85_1 | 30 | 1 | 50 | |||
| BL2 | 30 | 9 | 46 | |||
| 85_2 | 32 | 28 | 46 | |||
| MCF (mm) | Baseline | 45 | 28 | 66 | 32–63 | |
| BL1 | 25 | 8 | 32 | |||
| 85_1 | 36 | 1 | 60 | |||
| BL2 | 39 | 12 | 56 | |||
| 85_2 | 40 | 34 | 56 | |||
| MCE (dynes/cm2) | Baseline | 84 | 40 | 199 | 47–175 | |
| BL1 | 34 | 8 | 46 | |||
| 85_1 | 61 | 1 | 152 | |||
| BL2 | 62 | 13 | 125 | |||
| 85_2 | 67 | 52 | 129 | |||
| G | Baseline | 8657 | 6288 | 12,911 | 2368–8755 | |
| BL1 | 3440 | 51 | 6348 | |||
| 85_1 | 4717 | 51 | 9028 | |||
| BL2 | 3929 | 51 | 5854 | |||
| 85_2 | 5247 | 51 | 7490 | |||
| ML (%) | Baseline | 0 | 0 | 1 | 0–10 | |
| BL1 | 0 | 0 | 1 | |||
| 85_1 | 0 | 0 | 0 | |||
| BL2 | 0 | 0 | 0 | |||
| 85_2 | 0 | 0 | 0 | |||
| D-dimers | mg/L | Baseline | 0.01 | 0.01 | 1.70 | 0–0.5 |
| BL1 | 0.15 | 0.01 | 0.60 | |||
| 85_1 | 0.40 | 0.10 | 1.40 | |||
| BL2 | 0.20 | 0.01 | 0.80 | |||
| 85_2 | 0.30 | 0.20 | 0.70 |
References
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intens. Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, H.; Flachsbart, F. Blood coagulation: A powerful bactericidal mechanism of human innate immunity. Int. Rev. Immunol. 2019, 38, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood. Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Kenney, E.M.; Rozanski, E.A.; Rush, J.E.; deLaforcade-Buress, A.M.; Berg, J.R.; Silverstein, D.C.; Montealegre, C.D.; Jutkowitz, L.A.; Adamantos, S.; Ovbey, D.H. Association between outcome and organ system dysfunction in dogs with sepsis: 114 cases (2003–2007). J. Am. Vet. Med. Assoc. 2010, 236, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Chan, D.L. Evaluation of platelet function using multiple electrode platelet aggregometry in dogs with septic peritonitis. J. Vet. Emerg. Crit. Care 2016, 26, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Z.; Ilcol, Y.O.; Torun, S.; Ulus, I.H. Intravenous administration of choline or cdp-choline improves platelet count and platelet closure times in endotoxin-treated dogs. Shock 2006, 25, 73–79. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Ilcol, Y.O.; Ulus, I.H. Investigation of diagnostic importance of platelet closure times measured by Platelet Function Analyzer-PFA 100 in dogs with endotoxemia. Berl. Munch. Tierarztl. 2005, 118, 341–348. [Google Scholar]
- Majoy, S.B.; De Laforcade, A.M.; Barnard, M.R.; Shaw, S.P. Platelet activation in a population of critically ill dogs as measured with whole blood flow cytometry and thromboelastography. Am. J. Vet. Res. 2015, 76, 328–337. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Eralp, O.; Ilcol, Y.O. Evaluation of platelet count and its association with plateletcrit, mean platelet volume, and platelet size distribution width in a canine model of endotoxemia. Vet. Clin. Path. 2008, 37, 159–163. [Google Scholar] [CrossRef]
- McMichael, M.A.; Smith, S.A. Viscoelastic coagulation testing: Technology, applications, and limitations. Vet. Clin. Path. 2011, 40, 140–153. [Google Scholar] [CrossRef]
- Eralp, O.; Yilmaz, Z.; Failing, K.; Moritz, A.; Bauer, N. Effect of Experimental Endotoxemia on Thrombelastography Parameters, Secondary and Tertiary Hemostasis in Dogs. J. Vet. Intern. Med. 2011, 25, 524–531. [Google Scholar] [CrossRef]
- Daudel, F.; Kessler, U.; Folly, H.; Lienert, J.S.; Takala, J.; Jakob, S.M. Thromboelastometry for the assessment of coagulation abnormalities in early and established adult sepsis: A prospective cohort study. Crit. Care 2009, 13, R42. [Google Scholar] [CrossRef] [PubMed]
- Bentley, A.M.; Mayhew, P.D.; Culp, W.T.; Otto, C.M. Alterations in the hemostatic profiles of dogs with naturally occurring septic peritonitis. J. Vet. Emerg. 2013, 23, 14–22. [Google Scholar] [CrossRef]
- Steblaj, B.; Kutter, A.P.N.; Stirn, M.; Daminet, S.; Major, A.; Zini, E. Endotoxic kidney injury in Beagle dogs assessed by serum creatinine and symmetric dimethylarginine, and urinary neutrophil gelatinase-associated lipocalin and clusterin. Res. Vet. Sci. 2023, 162, 104966. [Google Scholar] [CrossRef]
- Muehlestein, M.B.; Steblaj, B.; Joerger, F.B.; Briganti, A.; Kutter, A.P.N. Evaluation of the ability of haemodynamic variables obtained with minimally invasive techniques to assess fluid responsiveness in endotoxaemic Beagles. Vet. Anaesth. Analg. 2021, 48, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Heimgartner, L.M.; Stirn, M.; Kutter, A.P.N.; Sigrist, N.E.; Jud Schefer, R. Whole blood platelet impedance aggregometry with the ROTEM platelet device: Comparison of 2 anticoagulants and storage times for the establishment of canine reference intervals. J. Vet. Diagn. Investig. 2022, 34, 15–22. [Google Scholar] [CrossRef]
- Kutter, A.; Kantyka, M.; Meira, C.; Bettschart-Wolfensberger, R.; Sigrist, N. Effect of 50 mg/kg intravenous tranexamic acid on coagulation assessed by rotational thromboelastometry (ROTEM) in healthy Beagle dogs. Schweiz. Arch. Tierheilkd. 2019, 161, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Schefer, R.J.; Heimgartner, L.; Stirn, M.; Sigrist, N.E. Determination of reference intervals for single vial rotational thromboelastometry (ROTEM) parameters and correlation with plasmatic coagulation times in 49 clinically healthy dogs. Res. Vet. Sci. 2020, 129, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Kutter, A.P.N.; Sigrist, N.E. Correlation of rotational thromboelastometry (ROTEM) parameters with platelet count and their ability to predict thrombocytopenia in dogs. Res. Vet. Sci. 2019, 126, 45–50. [Google Scholar] [CrossRef]
- Grozovsky, R.; Giannini, S.; Falet, H.; Hoffmeister, K.M. Novel mechanisms of platelet clearance and thrombopoietin regulation. Curr. Opin. Hematol. 2015, 22, 445–451. [Google Scholar] [CrossRef]
- Hanke, A.A.; Roberg, K.; Monaca, E.; Sellmann, T.; Weber, C.F.; Rahe-Meyer, N.; Gorlinger, K. Impact of platelet count on results obtained from multiple electrode platelet aggregometry (Multiplate). Eur. J. Med. Res. 2010, 15, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Würtz, M.; Hvas, A.-M.; Kristensen, S.D.; Grove, E.L. Platelet aggregation is dependent on platelet count in patients with coronary artery disease. Thromb. Res. 2012, 129, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Stissing, T.; Dridi, N.P.; Ostrowski, S.R.; Bochsen, L.; Johansson, P.I. The influence of low platelet count on whole blood aggregometry assessed by Multiplate. Clin. Appl. Thromb. Hemost. 2011, 17, E211–E217. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.J.; Bacek, L.M.; Christopherson, P.W.; Spangler, E.A. In vitro effect of blood cell counts on multiple-electrode impedance aggregometry in dogs. Am. J. Vet. Res. 2017, 78, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Shropshire, S.B.; Olver, C.S.; Lappin, M.R. Variability of tissue factor—Activated thromboelastography and whole blood impedance platelet aggregometry in healthy dogs. J. Vet. Emerg. Crit. Care 2018, 28, 334–339. [Google Scholar] [CrossRef]
- Blois, S.L.; Lang, S.T.; Wood, R.D.; Monteith, G. Biologic variability and correlation of platelet function testing in healthy dogs. Vet. Clin. Path. 2015, 44, 503–510. [Google Scholar] [CrossRef]
- Mavrommatis, A.C.; Theodoridis, T.; Economou, M.; Kotanidou, A.; El Ali, M.; Christopoulou-Kokkinou, V.; Zakynthinos, S.G. Activation of the fibrinolytic system and utilization of the coagulation inhibitors in sepsis: Comparison with severe sepsis and septic shock. Intens. Care Med 2001, 27, 1853–1859. [Google Scholar] [CrossRef]
- Adamantos, S.; Waters, S.; Boag, A. Coagulation status in dogs with naturally occurring Angiostrongylus vasorum infection. J. Small Anim. Pract. 2015, 56, 485–490. [Google Scholar] [CrossRef]
- Sigrist, N.E.; Schefer, R.J.J.; Kutter, A.P.N. Characteristics of hyperfibrinolysis in dogs and cats demonstrated by rotational thromboelastometry (ROTEM). Vet. J. 2018, 242, 67–73. [Google Scholar] [CrossRef]
- Vuille-Dit-Bille, J.; Weingand, N.; Jud Schefer, R.; Stirn, M.; Adamik, K.N.; Rathmann, J.M.K.; Sigrist, N.E. Comparison of Jugular vs. Saphenous Blood Samples, Intrarater and In-Between Device Reliability of Clinically Used ROTEM S Parameters in Dogs. Animals 2022, 12, 2101. [Google Scholar] [CrossRef]
- Weingand, N.; Vuille-dit-Bille, J.; Jud Schefer, R.; Kutter, A.P.N.; Stirn, M.; Adamik, K.-N.; Sigrist, N.E. Evaluation of the Effect of Storage Time on ROTEM S Parameters in Healthy and Ill Dogs. Animals 2022, 12, 1996. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Martin, L.F.; Chicca, F.D.; Sigrist, N.E.; Kutter, A.P.N. Impact of general anesthesia on rotational thromboelastometry (ROTEM) parameters and standard plasmatic coagulation tests in healthy Beagle dogs. Vet. Anim. Sci. 2021, 14, 100223. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Z.; Chen, K.; Zhang, F.; Peng, M.; Wang, Y. Dexmedetomidine regulates inflammatory molecules contributing to ventilator-induced lung injury in dogs. J. Surg. Res. 2014, 187, 211–218. [Google Scholar] [CrossRef] [PubMed]

| Test | Parameter | Timepoint | Reference Interval | Median | Min | Max | Friedmann Test p-Value | Median % Change to Baseline |
|---|---|---|---|---|---|---|---|---|
| Complete blood count | Platelet count (109/L) | Baseline | 150–399 | 239 | 163 | 312 | <0.001 | 0% |
| BL1 | 71 | 53 | 96 | −69% | ||||
| 85_1 | 136 | 94 | 172 | −43% | ||||
| BL2 | 142 | 79 | 161 | −41% | ||||
| 85_2 | 144 | 83 | 170 | −43% | ||||
| ARA-tem | AUC (Ω × minutes) | Baseline | 55–137 | 84 | 56 | 121 | <0.01 | 0% |
| BL1 | 16 | 0 | 46 | −82% | ||||
| 85_1 | 21 | 0 | 32 | −74% | ||||
| BL2 | 46 | 8 | 50 | −61% | ||||
| 85_2 | 39 | 18 | 62 | −59% | ||||
| A6 (Ω) | Baseline | 15–35 | 23 | 19 | 31 | np | 0% | |
| BL1 | 5 | 0 | 14 | −74% | ||||
| 85_1 | 4 | 0 | 8 | −80% | ||||
| BL2 | 12 | 1 | 14 | −55% | ||||
| 85_2 | 11 | 2 | 17 | −61% | ||||
| Maximal Slope (Ω/minute) | Baseline | 4–12 | 7 | 5 | 12 | np | 0% | |
| BL1 | 2 | 0 | 3 | −83% | ||||
| 85_1 | 3 | 0 | 3 | −65% | ||||
| BL2 | 3 | 1 | 5 | −65% | ||||
| 85_2 | 3 | 2 | 4 | −60% | ||||
| ADP-tem | AUC (Ω × minutes) | Baseline | 49–137 | 83 | 40 | 129 | <0.05 | 0% |
| BL1 | 33 | 8 | 47 | −69% | ||||
| 85_1 | 23 | 0 | 45 | −65% | ||||
| BL2 | 40 | 0 | 53 | −67% | ||||
| 85_2 | 29 | 8 | 63 | −73% | ||||
| A6 (Ω) | Baseline | 12–34 | 24 | 12 | 31 | np | 0% | |
| BL1 | 10 | 3 | 13 | −63% | ||||
| 85_1 | 5 | 0 | 11 | −68% | ||||
| BL2 | 11 | 0 | 13 | −65% | ||||
| 85_2 | 7 | 3 | 16 | −73% | ||||
| Maximal Slope (Ω/minute) | Baseline | 4–13 | 6 | 3 | 13 | np | 0% | |
| BL1 | 2 | 1 | 4 | −75% | ||||
| 85_1 | 2 | 0 | 4 | −55% | ||||
| BL2 | 3 | 0 | 5 | −67% | ||||
| 85_2 | 2 | 1 | 5 | −69% |
| Test | Parameter | Timepoint | Reference Interval | Median | Min | Max | Overall p-Value Friedmann Test | Median % Change to Baseline |
|---|---|---|---|---|---|---|---|---|
| In-tem | CT (sec) | Baseline | 133–210 | 178 | 150 | 228 | <0.05 | 0% |
| BL1 | 256 | 190 | 3600 | 26% | ||||
| 85_1 | 252 | 187 | 3600 | 35% | ||||
| BL2 | 239 | 97 | 3600 | 33% | ||||
| 85_2 | 223 | 190 | 3600 | 22% | ||||
| CFT (sec) | Baseline | 59–201 | 58 | 40 | 99 | <0.01 | 0% | |
| BL1 | 221 | 85 | 3600 | 118% | ||||
| 85_1 | 169 | 63 | 3600 | 90% | ||||
| BL2 | 159 | 78 | 3600 | 135% | ||||
| 85_2 | 134 | 68 | 3600 | 99% | ||||
| MCF (mm) | Baseline | 52–71 | 63 | 56 | 72 | <0.001 | 0% | |
| BL1 | 41 | 1 | 56 | −26% | ||||
| 85_1 | 49 | 1 | 64 | −17% | ||||
| BL2 | 48 | 1 | 58 | −24% | ||||
| 85_2 | 51 | 1 | 60 | −20% | ||||
| Ex-tem | CT (sec) | Baseline | 23–87 | 35 | 26 | 69 | <0.05 | 0% |
| BL1 | 53 | 23 | 3600 | −12% | ||||
| 85_1 | 53 | 31 | 3600 | 38% | ||||
| BL2 | 81 | 31 | 3600 | 73% | ||||
| 85_2 | 65 | 33 | 77 | 47% | ||||
| CFT (sec) | Baseline | 85–357 | 196 | 129 | 519 | <0.05 | 0% | |
| BL1 | 579 | 184 | 3600 | 123% | ||||
| 85_1 | 175 | 78 | 3600 | −15% | ||||
| BL2 | 308 | 103 | 3600 | 21% | ||||
| 85_2 | 189 | 117 | 270 | −17% | ||||
| MCF (mm) | Baseline | 32–65 | 43 | 29 | 52 | <0.05 | 0% | |
| BL1 | 30 | 1 | 44 | −24% | ||||
| 85_1 | 45 | 1 | 60 | 6% | ||||
| BL2 | 36 | 1 | 57 | −13% | ||||
| 85_2 | 41 | 35 | 57 | 5% | ||||
| Fib-tem | MCF (mm) | Baseline | 2–9 | 4 | 2 | 13 | =0.94 | 0% |
| BL1 | 4 | 1 | 7 | 0% | ||||
| 85_1 | 4 | 1 | 7 | −23% | ||||
| BL2 | 3 | 1 | 7 | −31% | ||||
| 85_2 | 4 | 2 | 5 | −13% | ||||
| Ap-tem | MCF (mm) | Baseline | 32–63 | 45 | 28 | 66 | <0.05 | 0% |
| BL1 | 25 | 8 | 32 | −54% | ||||
| 85_1 | 36 | 1 | 60 | −10% | ||||
| BL2 | 39 | 12 | 56 | −12% | ||||
| 85_2 | 40 | 34 | 56 | −6% | ||||
| D-dimers | mg/L | Baseline | 0–0.5 | 0.01 | 0.01 | 0.10 | <0.01 | 0% |
| BL1 | 0.15 | 0.01 | 0.60 | 900% | ||||
| 85_1 | 0.40 | 0.10 | 1.40 | 2100% | ||||
| BL2 | 0.20 | 0.01 | 0.80 | 1300% | ||||
| 85_2 | 0.30 | 0.20 | 1.70 | 1900% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutter, A.P.N.; Joerger, F.B.; Riond, B.; Steblaj, B. Evaluation of the Effect of Induced Endotoxemia on ROTEM S® and Platelet Parameters in Beagle Dogs Anaesthetized with Sevoflurane. Animals 2023, 13, 2997. https://doi.org/10.3390/ani13192997
Kutter APN, Joerger FB, Riond B, Steblaj B. Evaluation of the Effect of Induced Endotoxemia on ROTEM S® and Platelet Parameters in Beagle Dogs Anaesthetized with Sevoflurane. Animals. 2023; 13(19):2997. https://doi.org/10.3390/ani13192997
Chicago/Turabian StyleKutter, Annette P. N., Fabiola B. Joerger, Barbara Riond, and Barbara Steblaj. 2023. "Evaluation of the Effect of Induced Endotoxemia on ROTEM S® and Platelet Parameters in Beagle Dogs Anaesthetized with Sevoflurane" Animals 13, no. 19: 2997. https://doi.org/10.3390/ani13192997
APA StyleKutter, A. P. N., Joerger, F. B., Riond, B., & Steblaj, B. (2023). Evaluation of the Effect of Induced Endotoxemia on ROTEM S® and Platelet Parameters in Beagle Dogs Anaesthetized with Sevoflurane. Animals, 13(19), 2997. https://doi.org/10.3390/ani13192997







