Intravenous Injection of Sodium Hyaluronate Diminishes Basal Inflammatory Gene Expression in Equine Skeletal Muscle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Groupings
2.2. Treatment and Exercise
2.3. Muscle Biopsies and Tissue Preparation
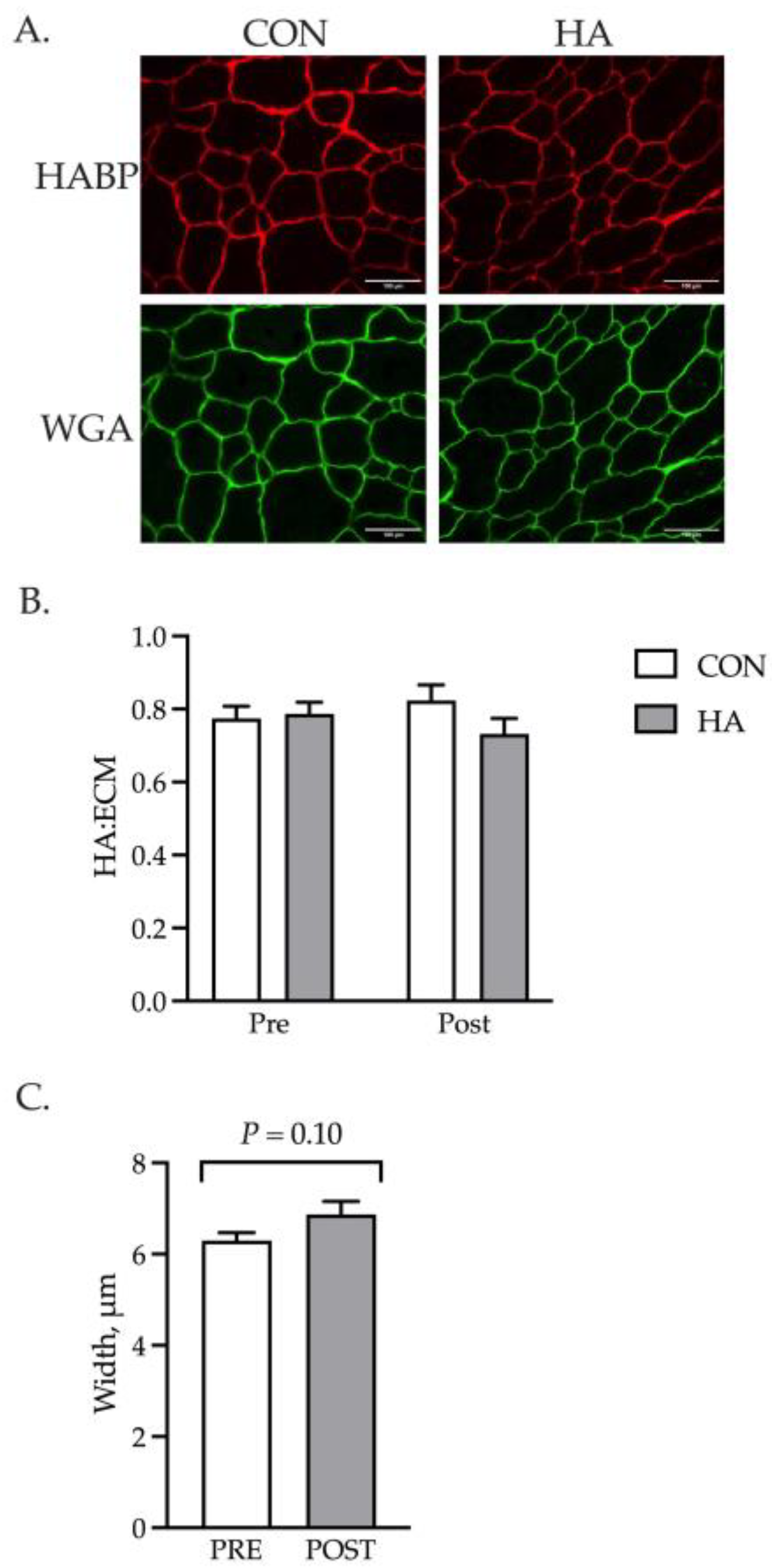
2.4. Muscle Cryosectioning and Staining
2.5. Epifluorescent Imaging
2.6. Total RNA Isolation
2.7. RNA Sequencing and Bioinformatics
2.8. Statistical Analysis
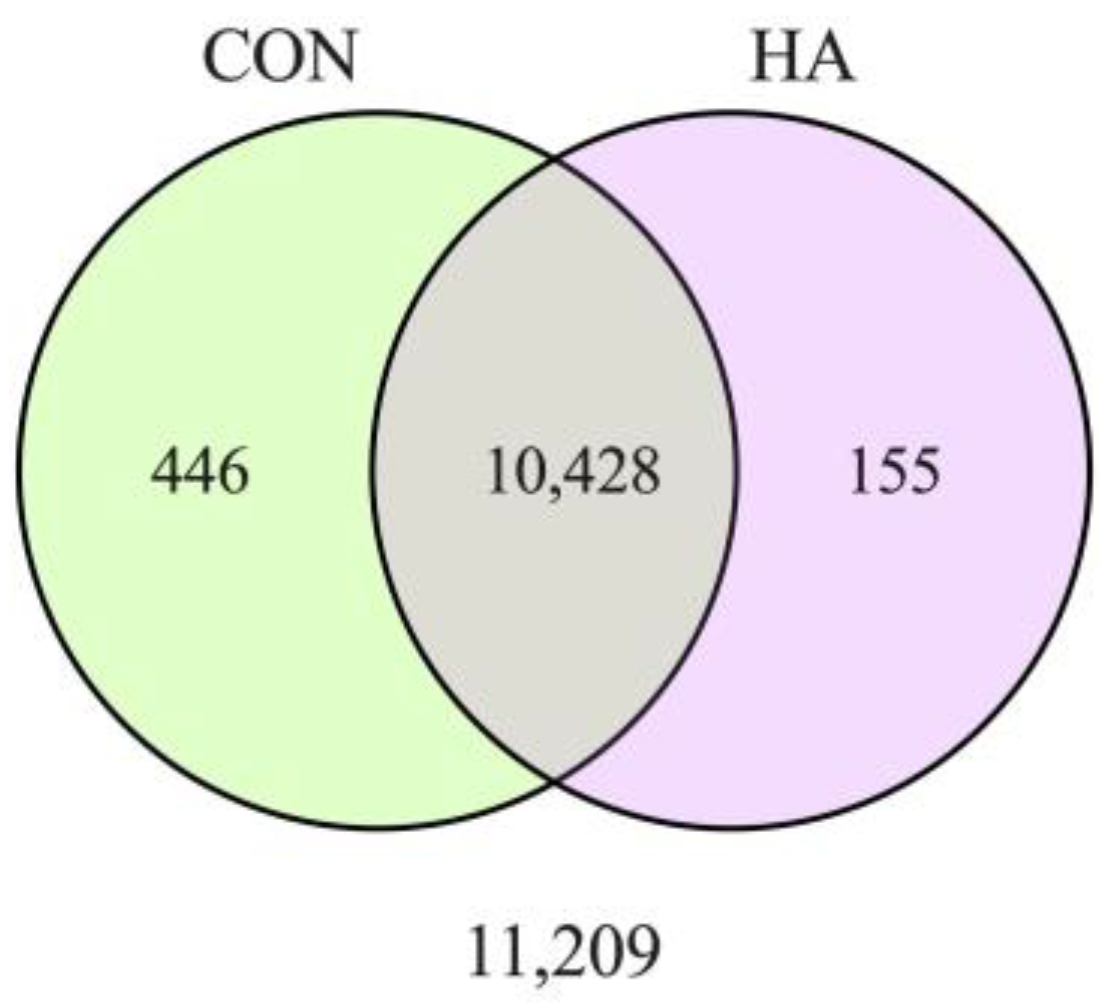
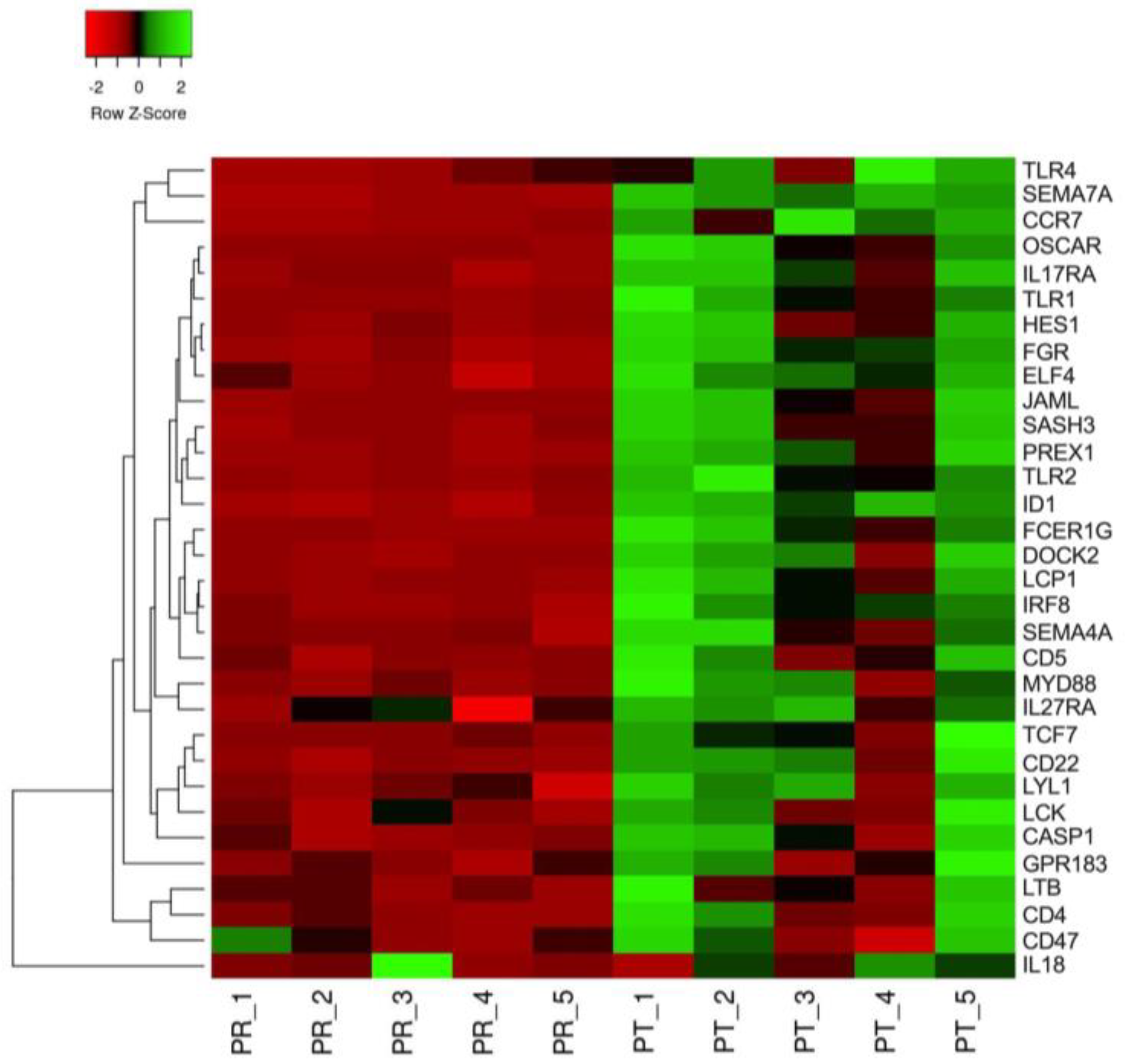
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Salathia, S.; Gigliobianco, M.R.; Casadidio, C.; Martino, P.D.; Censi, R. Hyaluronic Acid-Based Nanosystems for CD44 Mediated Anti-Inflammatory and Antinociceptive Activity. Int. J. Mol. Sci. 2023, 24, 7286. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T. Hyaluronan Inhibits Cytokine Production by Lipopolysaccharide-Stimulated U937 Macrophages through down-Regulation of NF-ΚB via ICAM-1. Inflamm. Res. 2007, 56, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Zheng, S.; Kummarapurugu, A.B. Glycosaminoglycans as Multifunctional Anti-Elastase and Anti-Inflammatory Drugs in Cystic Fibrosis Lung Disease. Front. Pharmacol. 2020, 11, 1011. [Google Scholar] [CrossRef] [PubMed]
- Babasola, O.; Rees-Milton, K.J.; Bebe, S.; Wang, J.; Anastassiades, T.P. Chemically Modified N-Acylated Hyaluronan Fragments Modulate Proinflammatory Cytokine Production by Stimulated Human Macrophages. J. Biol. Chem. 2014, 289, 24779–24791. [Google Scholar] [CrossRef] [PubMed]
- Humaira; Bukhari, S.A.R.; Shakir, H.A.; Khan, M.; Saeed, S.; Ahmad, I.; Muzammil, K.; Franco, M.; Irfan, M.; Li, K. Hyaluronic Acid-Based Nanofibers: Electrospun Synthesis and Their Medical Applications; Recent Developments and Future Perspective. Front. Chem. 2022, 10, 1092123. [Google Scholar] [CrossRef]
- Kyyak, S.; Blatt, S.; Wiesmann, N.; Smeets, R.; Kaemmerer, P.W. Hyaluronic Acid with Bone Substitutes Enhance Angiogenesis In Vivo. Materials 2022, 15, 3839. [Google Scholar] [CrossRef]
- Kyyak, S.; Pabst, A.; Heimes, D.; Kämmerer, P.W. The Influence of Hyaluronic Acid Biofunctionalization of a Bovine Bone Substitute on Osteoblast Activity In Vitro. Materials 2021, 14, 2885. [Google Scholar] [CrossRef]
- Hunt, L.C.; Gorman, C.; Kintakas, C.; McCulloch, D.R.; Mackie, E.J.; White, J.D. Hyaluronan Synthesis and Myogenesis A requirement for hyaluronan synthesis during myogenic differentiation independent of pericellular MATRIX formation. J. Biol. Chem. 2013, 288, 13006–13021. [Google Scholar] [CrossRef]
- Leng, Y.; Abdullah, A.; Wendt, M.K.; Calve, S. Hyaluronic Acid, CD44 and RHAMM Regulate Myoblast Behavior during Embryogenesis. Matrix Biol. 2019, 78, 236–254. [Google Scholar] [CrossRef]
- Dumont, N.A.; Wang, Y.X.; Rudnicki, M.A. Intrinsic and Extrinsic Mechanisms Regulating Satellite Cell Function. Development 2015, 142, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Calve, S.; Isaac, J.; Gumucio, J.P.; Mendias, C.L. Hyaluronic Acid, HAS1, and HAS2 Are Significantly Upregulated during Muscle Hypertrophy. Am. J. Physiol.-Cell Physiol. 2012, 303, C577–C588. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.S.; Panitch, A.; Calve, S. Functionalization of Hyaluronic Acid Hydrogels with ECM-Derived Peptides to Control Myoblast Behavior. Acta Biomater. 2019, 84, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.; Doma, K.; Sinclair, W.; Connor, J.; Leicht, A. Acute Effects of Training Loads on Muscle Damage Markers and Performance in Semi-Elite and Elite Athletes: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 2181–2207. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Piccione, G.; Trimarchi, F.; Panzera, M.F.; Giannetto, C. Stress, Metabolic and Serum Muscle-Derived Enzymes Response of Horses Employed in Wooded Area and Field Trekking Courses. J. Equine Vet. Sci. 2022, 112, 103919. [Google Scholar] [CrossRef]
- Mami, S.; Khaje, G.; Shahriari, A.; Gooraninejad, S. Evaluation of Biological Indicators of Fatigue and Muscle Damage in Arabian Horses After Race. J. Equine Vet. Sci. 2019, 78, 74–78. [Google Scholar] [CrossRef]
- Yang, W.; Hu, P. Skeletal Muscle Regeneration Is Modulated by Inflammation. J. Orthop. Transl. 2018, 13, 25–32. [Google Scholar] [CrossRef]
- Philippou, A.; Tryfonos, A.; Theos, A.; Nezos, A.; Halapas, A.; Maridaki, M.; Koutsilieris, M. Expression of Tissue Remodelling, Inflammation- and Angiogenesis-Related Factors after Eccentric Exercise in Humans. Mol. Biol. Rep. 2021, 48, 4047–4054. [Google Scholar] [CrossRef]
- Dennis, R.A.; Trappe, T.A.; Simpson, P.; Carroll, C.; Huang, B.E.; Nagarajan, R.; Bearden, E.; Gurley, C.; Duff, G.W.; Evans, W.J.; et al. Interleukin-1 Polymorphisms Are Associated with the Inflammatory Response in Human Muscle to Acute Resistance Exercise. J. Physiol. 2004, 560, 617–626. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory Monocytes Recruited after Skeletal Muscle Injury Switch into Antiinflammatory Macrophages to Support Myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 Triggers Changes in Macrophage Phenotype That Promote Muscle Growth and Regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qu, C.; Li, T.; Cui, W.; Wang, X.; Du, J. Phagocytosis Mediated by Scavenger Receptor Class BI Promotes Macrophage Transition during Skeletal Muscle Regeneration. J. Biol. Chem. 2019, 294, 15672–15685. [Google Scholar] [CrossRef] [PubMed]
- Dort, J.; Fabre, P.; Molina, T.; Dumont, N.A. Macrophages Are Key Regulators of Stem Cells during Skeletal Muscle Regeneration and Diseases. Stem Cells Int. 2019, 2019, 4761427. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-E.; Gerken, E.; Zhang, Y.; Zhan, M.; Mohan, R.K.; Li, A.S.; Reid, M.B.; Li, Y.-P. Role of TNF-α Signaling in Regeneration of Cardiotoxin-Injured Muscle. Am. J. Physiol.-Cell Physiol. 2005, 289, C1179–C1187. [Google Scholar] [CrossRef]
- Summan, M.; Warren, G.L.; Mercer, R.R.; Chapman, R.; Hulderman, T.; Rooijen, N.V.; Simeonova, P.P. Macrophages and Skeletal Muscle Regeneration: A Clodronate-Containing Liposome Depletion Study. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R1488–R1495. [Google Scholar] [CrossRef]
- Niemelä, T.M.; Tulamo, R.-M.; Carmona, J.U.; López, C. Evaluation of the Effect of Experimentally Induced Cartilage Defect and Intra-Articular Hyaluronan on Synovial Fluid Biomarkers in Intercarpal Joints of Horses. Acta Vet. Scand. 2019, 61, 24. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Horses, 6th ed.; The National Academies Press: Washington, DC, USA, 2007; ISBN 9780309102124. [Google Scholar]
- White, S.H.; Johnson, S.E.; Bobel, J.M.; Warren, L.K. Dietary Selenium and Prolonged Exercise Alter Gene Expression and Activity of Antioxidant Enzymes in Equine Skeletal Muscle. J. Anim. Sci. 2016, 94, 2867–2878. [Google Scholar] [CrossRef]
- Gonzalez, M.L.; Jacobs, R.D.; Ely, K.M.; Johnson, S.E. Rapid Communication: Dietary Tributyrin Supplementation and Submaximal Exercise Promote Activation of Equine Satellite Cells. J. Anim. Sci. 2019, 97, 4951–4956. [Google Scholar] [CrossRef]
- Fortier, L.A. Systemic Therapies for Joint Disease in Horses. Vet. Clin. N. Am. Equine Pract. 2005, 21, 547–557. [Google Scholar] [CrossRef]
- Neuenschwander, H.M.; Moreira, J.J.; Vendruscolo, C.P.; Fülber, J.; Seidel, S.R.T.; Michelacci, Y.M.; Baccarin, R.Y.A. Hyaluronic Acid Has Chondroprotective and Joint-Preserving Effects on LPS-Induced Synovitis in Horses. J. Vet. Sci. 2019, 20, e67. [Google Scholar] [CrossRef]
- Nakka, K.; Hachmer, S.; Mokhtari, Z.; Kovac, R.; Bandukwala, H.; Bernard, C.; Li, Y.; Xie, G.; Liu, C.; Fallahi, M.; et al. JMJD3 Activated Hyaluronan Synthesis Drives Muscle Regeneration in an Inflammatory Environment. Science 2022, 377, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Keen, J.; McGowan, C. Equine Metabolic Syndrome. Vet. Rec. 2015, 177, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.S.; Kushwaha, K.; Dubey, R.; Gupta, J. Association between Obesity, Inflammation and Insulin Resistance: Insights into Signaling Pathways and Therapeutic Interventions. Diabetes Res. Clin. Pract. 2023, 200, 110691. [Google Scholar] [CrossRef] [PubMed]
- Parnigoni, A.; Viola, M.; Karousou, E.; Rovera, S.; Giaroni, C.; Passi, A.; Vigetti, D. Hyaluronan in Pathophysiology of Vascular Diseases: Specific Roles in Smooth Muscle Cells, Endothelial Cells, and Macrophages. Am. J. Physiol. Physiol. 2022, 323, C505–C519. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.-H. The Effects of the Molecular Weights of Hyaluronic Acid on the Immune Responses. Biomater. Res. 2021, 25, 27. [Google Scholar] [CrossRef]
- Ferris, D.J.; Frisbie, D.D.; McIlwraith, C.W.; Kawcak, C.E. Current Joint Therapy Usage in Equine Practice: A Survey of Veterinarians 2009. Equine Vet. J. 2011, 43, 530–535. [Google Scholar] [CrossRef]
- Popot, M.-A.; Bonnaire, Y.; Guéchot, J.; Toutain, P.-L. Hyaluronan in Horses: Physiological Production Rate, Plasma and Synovial Fluid Concentrations in Control Conditions and Following Sodium Hyaluronate Administration. Equine Vet. J. 2004, 36, 482–487. [Google Scholar] [CrossRef]
- Jadin, L.; Bookbinder, L.H.; Frost, G.I. A Comprehensive Model of Hyaluronan Turnover in the Mouse. Matrix Biol. 2012, 31, 81–89. [Google Scholar] [CrossRef]
- Schreiner, B.; Voss, J.; Wischhusen, J.; Dombrowski, Y.; Steinle, A.; Lochmüller, H.; Dalakas, M.; Melms, A.; Wiendl, H. Expression of Toll-like Receptors by Human Muscle Cells in Vitro and in Vivo: TLR3 Is Highly Expressed in Inflammatory and HIV Myopathies, Mediates IL-8 Release, and Up-regulation of NKG2D-ligands. FASEB J. 2006, 20, 118–120. [Google Scholar] [CrossRef]
- Frost, R.A.; Nystrom, G.J.; Lang, C.H. Multiple Toll-like Receptor Ligands Induce an IL-6 Transcriptional Response in Skeletal Myocytes. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R773–R784. [Google Scholar] [CrossRef]
- Reyna, S.M.; Ghosh, S.; Tantiwong, P.; Meka, C.S.R.; Eagan, P.; Jenkinson, C.P.; Cersosimo, E.; DeFronzo, R.A.; Coletta, D.K.; Sriwijitkamol, A.; et al. Elevated Toll-Like Receptor 4 Expression and Signaling in Muscle from Insulin-Resistant Subjects. Diabetes 2008, 57, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Bivona, J.J.; Crymble, H.M.; Guigni, B.A.; Stapleton, R.D.; Files, D.C.; Toth, M.J.; Poynter, M.E.; Suratt, B.T. Macrophages Augment the Skeletal Muscle Proinflammatory Response through TNFα Following LPS-induced Acute Lung Injury. FASEB J. 2021, 35, e21462. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Shannon, V.; Ochoa, O.; Reyes-Reyna, S.M.; Sun, D.; Michalek, J.E.; Kuziel, W.A.; McManus, L.M.; Shireman, P.K. Fat Accumulation with Altered Inflammation and Regeneration in Skeletal Muscle of CCR2−/− Mice Following Ischemic Injury. Am. J. Physiol. -Cell Physiol. 2007, 292, C953–C967. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.O.; McHale, M.J.; Wells, J.T.; Ochoa, O.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Regulation of Skeletal Muscle Regeneration by CCR2-Activating Chemokines Is Directly Related to Macrophage Recruitment. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 299, R832–R842. [Google Scholar] [CrossRef]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially Activated Macrophages Orchestrate Myogenic Precursor Cell Fate During Human Skeletal Muscle Regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef]
- Olsen, L.A.; Nicoll, J.X.; Fry, A.C. The Skeletal Muscle Fiber: A Mechanically Sensitive Cell. Eur. J. Appl. Physiol. 2019, 119, 333–349. [Google Scholar] [CrossRef]
- Kivelä, R.; Kyröläinen, H.; Selänne, H.; Komi, P.V.; Kainulainen, H.; Vihko, V. A Single Bout of Exercise with High Mechanical Loading Induces the Expression of Cyr61/CCN1 and CTGF/CCN2 in Human Skeletal Muscle. J. Appl. Physiol. 2007, 103, 1395–1401. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Olesen, J.L.; Haddad, F.; Langberg, H.; Kjaer, M.; Baldwin, K.M.; Schjerling, P. Expression of Collagen and Related Growth Factors in Rat Tendon and Skeletal Muscle in Response to Specific Contraction Types. J. Physiol. 2007, 582, 1303–1316. [Google Scholar] [CrossRef]
- Brightwell, C.R.; Latham, C.M.; Thomas, N.T.; Keeble, A.R.; Murach, K.A.; Fry, C.S. A Glitch in the Matrix: The Pivotal Role for Extracellular Matrix Remodeling during Muscle Hypertrophy. Am. J. Physiol.-Cell Physiol. 2022, 323, C763–C771. [Google Scholar] [CrossRef]
- Peck, B.D.; Murach, K.A.; Walton, R.G.; Simmons, A.J.; Long, D.E.; Kosmac, K.; Dungan, C.M.; Kern, P.A.; Bamman, M.M.; Peterson, C.A. A Muscle Cell-macrophage Axis Involving Matrix Metalloproteinase 14 Facilitates Extracellular Matrix Remodeling with Mechanical Loading. FASEB J. 2022, 36, e22155. [Google Scholar] [CrossRef]
- Du, H.; Shih, C.-H.; Wosczyna, M.N.; Mueller, A.A.; Cho, J.; Aggarwal, A.; Rando, T.A.; Feldman, B.J. Macrophage-Released ADAMTS1 Promotes Muscle Stem Cell Activation. Nat. Commun. 2017, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Bilal, M.; Fujisaka, S.; Kado, T.; Aslam, M.R.; Ahmed, S.; Okabe, K.; Igarashi, Y.; Watanabe, Y.; Kuwano, T.; et al. Depletion of CD206+ M2-like Macrophages Induces Fibro-Adipogenic Progenitors Activation and Muscle Regeneration. Nat. Commun. 2022, 13, 7058. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregg, S.R.; Barshick, M.R.; Johnson, S.E. Intravenous Injection of Sodium Hyaluronate Diminishes Basal Inflammatory Gene Expression in Equine Skeletal Muscle. Animals 2023, 13, 3030. https://doi.org/10.3390/ani13193030
Gregg SR, Barshick MR, Johnson SE. Intravenous Injection of Sodium Hyaluronate Diminishes Basal Inflammatory Gene Expression in Equine Skeletal Muscle. Animals. 2023; 13(19):3030. https://doi.org/10.3390/ani13193030
Chicago/Turabian StyleGregg, Savannah R., Madison R. Barshick, and Sally E. Johnson. 2023. "Intravenous Injection of Sodium Hyaluronate Diminishes Basal Inflammatory Gene Expression in Equine Skeletal Muscle" Animals 13, no. 19: 3030. https://doi.org/10.3390/ani13193030
APA StyleGregg, S. R., Barshick, M. R., & Johnson, S. E. (2023). Intravenous Injection of Sodium Hyaluronate Diminishes Basal Inflammatory Gene Expression in Equine Skeletal Muscle. Animals, 13(19), 3030. https://doi.org/10.3390/ani13193030







